Introduction
Now, more than ever, academic institutions are at the forefront of scientific initiatives to improve health care through innovative medical products, including new drugs, biologics, and medical devices. To accomplish this goal, academic medical center (AMC) clinical researchers must successfully navigate the United States (U.S.) Food and Drug Administration (FDA) regulatory pathways from discovery to clinical implementation, including the so-called “valley of death” where federal- and foundation-funded medical innovations struggle to transition to early clinical development and later-stage industry-funded clinical development and commercialization [1]. Regulatory affairs professionals play a critical role by providing expertise to guide and help manage the process, increase efficiencies, and ensure the conduct of safe and effective research [2]. Access to these professionals is a critical need at academic institutions. To address this need, the Investigational New Drug/Investigational Device Exemption (IND/IDE) Taskforce of the Clinical and Translational Science Award (CTSA) Consortium recommended that institutions provide either central consultative or full-service regulatory support for sponsor-investigators [Reference Berro, Burnett and Fromell3] as well as develop and implement training programs to ensure their readiness to fulfill increasingly complex regulatory requirements [Reference Holbein, Berglund and O’Reilly4]. Despite progress in this area, highly trained regulatory professionals are not always available for AMC researchers to engage or are limited to specialized subject matter areas, thus creating a barrier to the timely translation of scientific findings into new medical products [Reference Drago, Shire and Ekmekci5].
To improve the accessibility of regulatory expertise for AMC clinical researchers, the regulatory affairs professionals from four National Institutes of Health (NIH) CTSA Program-funded North Carolina institutions [the University of North Carolina at Chapel Hill (UNC-CH) and their partner Research Triangle Institute (RTI) International, Duke University, and Wake Forest School of Medicine (WFU)] developed ReGARDD – an innovative platform to share FDA regulatory expertise and resources across institutions. ReGARDD built on pre-2015 collaborative relations (e.g., Duke/UNC co-sponsored IND/IDE workshops and shared templates) by combining the regulatory talents from each CTSA institution in the region to enable sharing of ideas, best practices, and lessons learned, and to foster the development of successful strategies to assist AMC researchers in navigating a dynamic and complex regulatory environment. The ReGARDD program mission is twofold: (1) create and sustain a regional regulatory forum to enhance the expertise of regulatory professionals and clinical researchers at our respective institutions and (2) develop and maintain a shared best practice regulatory website geared toward the needs of AMC researchers. Here, we describe ReGARDD as a model platform to improve the regulatory practice and overall translational research enterprise of CTSA-funded institutions.
ReGARDD Forum
Created in 2015, the ReGARDD forum provides a platform for regulatory professionals from ReGARDD member institutions to meet in person and build interpersonal connections, share best practices, discuss complex regulatory issues, and learn from one another and from external speakers invited to address select topics. Remote attendance is available to facilitate participation by members for whom long distance travel is problematic – a feature that has helped expand and sustain the forum during the COVID-19 pandemic. The forum host rotates among the member institutions, offering the opportunity for each site to showcase their unique regulatory strength. The forum also offers opportunities for investigators to bring forward complex regulatory issues to benefit from the collective expertise of ReGARDD members with the goal of strengthening the investigator’s regulatory plan. A confidential disclosure agreement (CDA) signed by the member institutions adds an extra layer of protection and sense of security when discussing confidential information.
The ReGARDD forum has featured external speakers (e.g., FDA, industry, contract research organizations) who addressed topics such as aligning clinical trials to achieve both regulatory and reimbursement goals, risk-based monitoring and the critical elements of an effective strategy for monitoring investigator-initiated studies, and the regulatory pathway for gene therapies. Internal speakers presented on mobile apps as medical devices, intellectual property, drug discovery, regenerative medicine, data and safety monitoring boards (DSMB), and other topics. Lessons learned have fostered process improvements as evidenced, for example, when the WFU team adopted the UNC-CH team’s approach to managing DSMB reviews via a shared working space/shared drive that made communication between members more efficient and significantly improved DSMB workflow. In addition to the quarterly forum meetings, ReGARDD sponsors recurring IND and IDE Best Practice Workshops that are open to anyone at the member institutions. These workshops provide an overview of how to determine when an IND or IDE is needed for a clinical study and how to prepare and maintain these applications. One ReGARDD member reported increased confidence in her regulatory knowledge after participating in the forums, which helped her successfully obtain the Regulatory Affairs Certification. In 2019, the forum expanded to include the Medical University of South Carolina (MUSC), uniting CTSA institutions across the Carolinas, and provided mentoring for Virginia Commonwealth University (VCU) as they sought to establish a ReGARDD-like forum for the Virginia area. With COVID-19 restrictions limiting in-person meetings, it was challenging for VCU to establish collaborative relationships with other Virginia institutions; thus, in 2020, VCU officially joined our ReGARDD forum.
ReGARDD Website
Early on, the ReGARDD forum developed a publicly available regulatory best practices website (www.regardd.org). The ReGARDD members generated content for the website based on FDA regulations, FDA guidance, and member experience, which was reviewed and edited by the team to reflect a consensus of best practices amongst the group. A website designer from RTI International created an easy to navigate design to showcase the regulatory content. Released in May 2016, the first version of the website contained many helpful articles and illustrative graphics designed to provide guidance on FDA regulatory submissions for AMC researchers. Using 2017 CTSA administrative supplement funds, we improved the website by adapting content and format based on user feedback. A project manager ensured delegation and completion of action items and managed updates to the website. Key ReGARDD members met weekly to reorganize the website structure and create additional instructional materials and regulatory templates to provide a more useful experience for site visitors. Each institution compiled their existing regulatory resources/materials, and then subgroups were assigned to develop regulatory instructional materials to address identified gaps. Templates eventually placed on the website were chosen after reviewing differences in the templates from each site (including initial IND/IDE applications, cover letters, annual progress reports, and IDE supplements) and incorporating nuanced differences that were deemed useful. Instructional materials on the website include videos, recordings of past ReGARDD forum presentations, documents, checklists, weblinks and FAQs. Some examples include instructions on how to fill out forms focusing on sponsor-investigator responsibilities, ClinicalTrials.gov requirements, and safety monitoring. New pages and graphics were added to showcase FDA regulatory review timelines and decision trees to provide step-by-step directions to navigate IND/IDE exemption determinations, FDA interactions, and regulatory requirements (e.g., Fig. 1). Over time, we developed 39 instructional and educational videos on a wide variety of topics to supplement the written text and to provide explanations for commonly asked questions (e.g., see Table 1). Collectively, the user-friendly tools available on www.regardd.org provide up-to-date information to support best practices across the regulatory landscape and to help academic researchers prepare and submit regulatory applications (e.g., IND applications) to the FDA, develop and write clinical protocols, and keep pace with changing federal regulations and guidance such as the new FDA eCopy requirements and new FDA enforcement policy for device software functions and mobile applications in 2020.

Fig. 1. Example of decision tree on the ReGARDD website.
Table 1. Examples of content on the ReGARDD website

Abbreviations: CTSA, Clinical and Translational Awards; FDA, US Food and Drug Administration; IDE, Investigational Device Exemption; IND, investigational new drug.
Following major revisions and upgrades to the website in 2017, we conducted a survey of the target audience to raise awareness of the website, gain a better understanding of the utility of the website, and inform website improvements. Survey professionals at RTI programmed a brief, anonymous, user-friendly survey that enabled qualitative follow-up responses to obtain deeper understanding of quantitative answers. Respondents who reported having never used the website saw an additional initial screen asking them to review the website and then respond to the survey. The survey was sent via email to key contacts involved to varying degrees in FDA regulatory submissions at each member institution (e.g., junior investigators who had requested assistance with FDA submissions, regulatory specialists associated with study teams, and personnel in the clinical trials office).
The survey respondents (N = 20; 60% with prior FDA submission experience) rated their experience with the website favorably: 100% agreed or strongly agreed the website was relevant to their job, and the majority (75% or more) agreed they could easily find the information needed and that the website had increased their understanding of the FDA submission process and would help them prepare for FDA submissions and be useful to others who require regulatory guidance. We learned that experienced respondents found the decision trees, templates, and FAQs particularly useful whereas “novice” respondents found that access to the relevant templates in a single location was helpful. Open text responses provided helpful suggestions that informed the next iteration of the website, including additional information on IDEs and adding links to federal guidances.
Awareness of ReGARDD grew beyond our local institutions, prompting us to expand the website into a centralized resource where other academic institutions could share and advertise regulatory educational opportunities, seminars, and trainings for academic researchers thereby extending the benefits of ReGARDD to other institutions by reducing duplication of efforts and strengthening the regulatory workforce. To inform this effort, in 2019, we surveyed regulatory professionals from over 40 academic institutions in the U.S. (i.e., members of the IND/IDE Taskforce of the CTSA) and received 30 responses from individuals representing 21 CTSA institutions. This survey assessed current institutional training opportunities, interest in advertising trainings on the ReGARDD website, and opinions on the utility of the ReGARDD website. Training opportunities ranged from less than one (10%) to three or more (60%) per year and were available for remote participation to varying degrees (never/almost never, 39.3%; always/almost always, 17.9%). Nearly all (93.3%) respondents endorsed making the website a centralized resource for regulatory educational opportunities, seminars, and trainings for academic researchers; 73.4% of respondents indicated they would be interested in advertising trainings that take place at their institution on the website; 95.4% indicated they were very likely or somewhat likely to use the website as a resource; and 90.9% indicated they were very likely or somewhat likely to recommend the website to study teams. Based on these results, in 2020, we upscaled the ReGARDD website as a centralized resource where other institutions can share and advertise events, such as regulatory trainings and educational seminars.
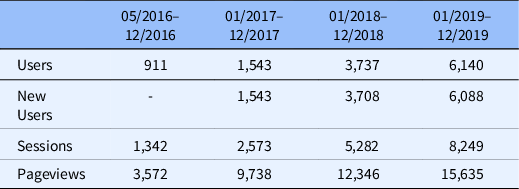
Website traffic has steadily increased from approximately 900 users in 2016 to over 6000 users in 2019 (Table 2), reflecting over 13,000 unique users and 33,000 unique pageviews. New visitors comprised the majority of users, with 10%–15% return visitors each year. Website traffic was primarily driven by direct domain and organic search engine clicks. Over time, website traffic has increased dramatically across multiple domains – a positive indicator that it is a beneficial resource that is gaining traction with our key audience groups. The bounce rate was initially high but declined over time (range, 50%–70%). We continue to curate the website content and resources such that visitors find the information more readily. We ensure that the resources are easily accessible to research teams, reducing the need to search other pages on the website.
Table 2. Yearly website metrics
In 2019–2020, the top seven visited pages were associated with drug development, IND submissions, devices, and training videos, with the top five pages representing approximately 60% of the pageviews on the website (Table 3).
Table 3. Top Pageviews
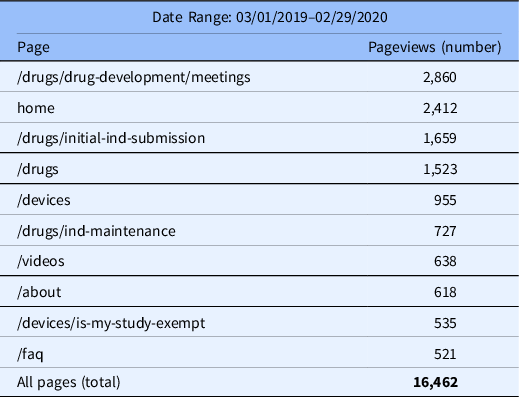
Abbreviations: ind, investigational new drug; faq, frequently asked questions
Dissemination of the ReGARDD program
With the support of the North Carolina Translational and Clinical Sciences Institute (NC TraCS) Communications Core, we developed a promotion strategy to achieve the following goals:
-
Drive traffic to the ReGARDD website so visitors can benefit from the resources available
-
Boost awareness and recognition of the ReGARDD website amongst regulatory professionals, clinical researchers, graduate students, and postdocs at the ReGARDD member institutions
-
Disseminate information about the ReGARDD program broadly to the CTSA consortium sites
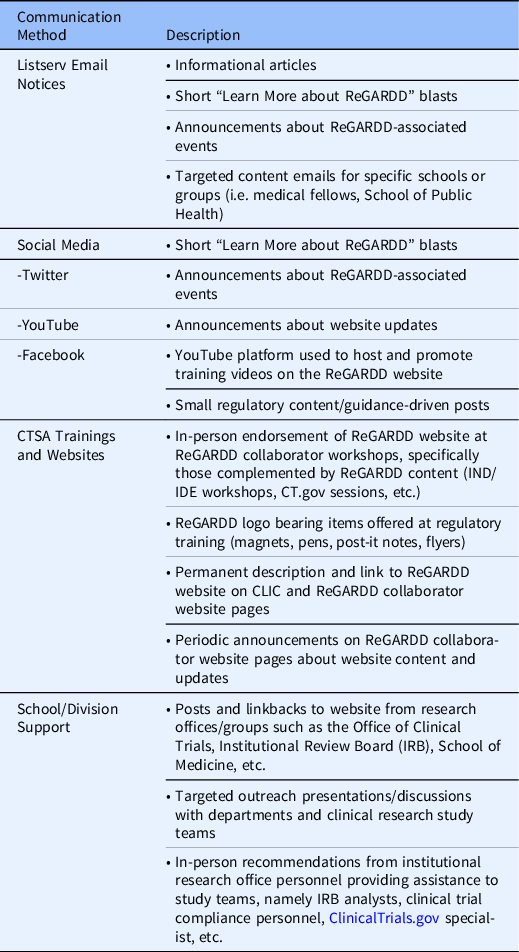
Key ReGARDD members continue to periodically meet with the NC TraCS Communication experts to evaluate communication methods and to refine strategies to target specific audiences to broaden the impact of the dissemination plan. Outreach efforts incorporate frequent announcements about the ReGARDD program, resources available on the website, and educational and training opportunities; and they use outlets such as established professional and institutional listservs, social media platforms, CTSA-affiliated workshops and websites, and school/division supported announcements.
On a quarterly basis, the NC TraCS Communications Core collects and reviews web-based analytics data on (1) Website Traffic (e.g., unique and total number of pageviews, average time users spent viewing a specified page, top 10 pages viewed, and bounce rate); (2) Users (e.g., new and returning visitors, total number and average duration of sessions, and average number of pages viewed per session); and (3) Acquisition (e.g., total number of direct sessions by typing URL into the browser [domain clicks], organic sessions via search engines, referral sessions that are generated from another website, and social sessions generated via Facebook, YouTube, and Twitter).
Marketing efforts for the ReGARDD forum and website have taken on many forms and evolved over time, including targeted emails to listserves within our institutions, presentations at CTSA consortium-sponsored events, promotional stories in various newsletters, merchandizing and distribution of the ReGARDD logo and website address to enhance name recognition, and social media blasts (Table 4). In 2018, the CTSA Center for Leading Innovation & Collaboration (CLIC) published a story about ReGARDD and included the link to the ReGARDD website on the CTSA consortium website (https://clic-ctsa.org). These various forms of publicity boosted our dissemination efforts, leading to large spikes in visitors to the website following these announcements.
Table 4. Communication & dissemination methods
Abbreviations: CTSA, Clinical and Translational Awards; IDE, Investigational Device Exemption; IND, investigational new drug.
Discussion
A review of the ReGARDD forums identified four major areas of impact. First, the discussions with colleagues and presenters strengthen the regulatory workforce by providing knowledge that will translate into better service to investigators. Increased knowledge about regulatory pathways, more efficacious problem solving, and a broader network of experts to reach out to with questions is a tangible benefit of ReGARDD and particularly the forums. Second, the increased knowledge produces increased regulatory team efficiencies at each of the CTSA collaborating institutions. Third, the opportunity for a collaborating institution to learn about organizational and operational aspects of other regulatory offices sparks ideas for process improvement as evidenced, for example, by the DSMB shared drive platform adopted by WFU. Fourth, these events help attendees build confidence about communicating with regulatory officials. The evolution of forum topics over time ensures that learning keeps pace with the ever-changing regulatory landscape.
The ReGARDD forums enhance workforce development by providing members with continuing education through extramural speakers who address emerging or less well-known content areas and through other members who speak on their own content area expertise. Additionally, sharing lessons learned at ReGARDD forums helps regulatory personnel with professional development, including instrumental support to achieve the Regulatory Affairs Certification credential. On occasion, those lessons stem from frank discussions of sensitive, real-time regulatory issues that members would not be free to share outside their home institution without having a CDA in place. The pan-institutional forums, such as the IND and IDE Best Practice Workshops offered by Duke University’s Office of Regulatory Affairs and Quality, attract and serve a wider audience of regulatory and research professionals, thus reducing duplicate, siloed institution-specific trainings.
Streamlining the processes underlying FDA regulatory approval is key to the efficient start-up of clinical trials involving FDA-regulated medical products. To help meet this need, www.regardd.org has evolved into a comprehensive, user-friendly, regulatory website that is uniquely geared toward the community of AMC researchers conducting FDA-regulated clinical research. Building on the collective experiences of the ReGARDD members, the website content is designed to answer the most pertinent and common questions AMC investigators confront. In addition, www.regardd.org provides templates and decision trees that facilitate self-guided access to basic information, as well as contact information for regulatory experts to allow follow-up for more detailed, complicated issues. While much of the website content can be found on other regulatory websites, the ReGARDD platform houses information that is most relevant to academic and translational researchers in one place and in a format that is easily understood and adaptable to their needs. Not only does the website allow researchers without a regulatory support network to better understand FDA regulatory requirements, it also provides templates and instructions to help them comply with these requirements. User feedback obtained through surveys indicates success. The website training videos and modules also support regulatory workforce development. For example, regulatory personnel can use these resources to supplement their formal training, investigators can draw upon them to build on existing regulatory knowledge and to onboard new regulatory staff, IRB members and staff can refer to them when making regulatory determinations, and advanced regulatory professionals can reference them during regulatory consultations.
ReGARDD members have derived several valuable lessons throughout the process of establishing and maintaining www.regardd.org. First, and foremost, a collaboration of this magnitude benefits greatly by having a project manager to keep everyone on track with next steps, to coordinate and monitor survey activity and incoming data, and to ensure action items are delegated and completed on time. Having a designated point-person was instrumental, particularly in managing updates and revisions to the website over time. Second, during website creation, it is important to have processes in place to ensure agreement on best practices and to allow review for accuracy. Third, creating, filming, and editing videos was a time- and resource-intensive undertaking that we underestimated despite having access to expertise and resources for video production at our institutions. Fourth, our low response rate (n = 20 completed surveys) from the fall 2017 survey limited our opportunity to modify the website based on user feedback. We believe our survey response rate would have improved had we asked for feedback from users seeking answers to real-time questions rather than from users invited to explore the website. Lastly, there are challenges associated with maintaining the website to ensure the content stays current and meets the needs of the academic research community. It is important that the ReGARDD members stay attentive to current regulatory trends and topics and have processes in place to update the website when changes are needed. We are currently utilizing a model where a single person has access to update the website, and we communicate any needed changes with that point person. Each quarter, a different ReGARDD member institution takes a turn at reviewing the website carefully for revised regulations, needed updates to content, and links that are broken.
The CTSA institutions involved in ReGARDD are committed to staying current with changes in FDA regulations and guidance, creating and disseminating new and innovative tools, and hosting and updating the website. Future efforts will include creating a ‘blog-style’ component of the ReGARDD website and improving website analytics to track number of downloads per resource and popularity. The blog will include frequent updates with commentary on timely issues in the regulatory space; spotlights and descriptions of new updates from FDA, NIH, and other impactful bodies; and announcements of online seminars/workshops from CTSA sites across the country. Offering frequent updates of this nature aligns with the overall ReGARDD goals of providing regulatory guidance and educational materials for academic investigators and study teams.
The lack of appropriate regulatory support is an important translational problem facing not only academic researchers but all clinical researchers. A first step in alleviating this problem is to export the ReGARDD model broadly to the CTSA consortium and research centers, with the end goal that other institutions can also develop their own regional version of a ReGARDD program. Expertise will grow at each site as regulatory professionals learn from one another. Despite its best efforts, the VCU CTSA was unable to entice partner institutions from the Virginia area to form a regional forum. Analysis of the VCU experience (e.g., lack of available partner institutions) coupled with discussions about accepting other institutions into the current ReGARDD forum highlight that our model program is not a one-size fits all but, rather, will require transformation and alignment with regional needs and resources. For example, regional forums of the future will need to determine their own sense of balance between the benefits of face-to-face versus virtual meetings (e.g., increased networking and interpersonal connection vs. increased accessibility, respectively). Institutions in close proximity to each other may choose a hybrid model such as ours including both face-to-face and virtual meetings; whereas, those that are geographically dispersed may be limited to virtual meetings only. Ultimately, broader adoption and adaptation of our model across the U.S. will allow more regional programs to take advantage of expertise spread across several collaborating CTSA institutions or other kinds of partnerships, healthcare systems, or institutional collaborators, leading to reduced duplication and increased efficiencies.
Acknowledgments
The ReGARDD program is funded in part by the Clinical and Translational Science Awards (CTSA) Program from the National Center for Advancing Translational Sciences, National Institutes of Health, to each of the member institutions: University of North Carolina at Chapel Hill and RTI International (grant UL1TR002489); Duke University (grant UL1TR002553 and UL1TR001117); Wake Forest University (grant UL1TR001420); The Medical University of South Carolina (grant UL1TR001450).
Disclosures
The authors have no conflicts of interest to declare.







