Since oxidative stress is considered to be implicated in the ethiopathology of many chronic diseases, considerable efforts have been made upon using antioxidants to counteract free radicals, and nutritional strategies designed to increase cellular defence systems have been identified as a promising approach to minimize oxidative stress associated disease conditionsReference Rakotovao, Berthonneche, Guiraud, de Lorgeril, Salen, de Leiris and Boucher1, Reference Scalbert, Johnson and Saltmarsh2. In this respect, dietary supplementation with Se could offer protection in preventing free radical-induced diseases.
Se in the form of selenocysteine is part of the active centre of several seleno-enzymes which have antioxidant function, e.g. the glutathione peroxidases (GPx), the deiodinases and the thioredoxine reductasesReference Brenneisen, Steinbrenner and Sies3. It is documented that any significant modification of the Se status would lead to changes in the activity of the seleno-enzymes and have important consequences on the susceptibility of tissues to oxidative stressReference El-Bayoumy4, Reference Serwatka, Stachowska and Chlubek5.
Regarding nutritional supplementation, an important debate is still open about the bioavailability and the effectiveness of nutrients in supplements compared with foods. Foods are complex matrices, and the interaction of one component with other components may either enhance or reduce its availability, and therefore its effectiveness. It is demonstrated that Se activity, particularly in chemoprevention, depends not only on the dose, but also on the chemical form in which it is administeredReference Ganther6.
In this study, we have evaluated in rats the possibility of a nutritional counteraction of an oxidative stress by two different experimental diets in which an adequate Se content was achieved by adding to a standard diet for rats the element in pure chemical form (as sodium selenite) or as component of a lyophilized Se-enriched food. The oxidative stress was induced by intraperitoneal injection of adriamycin (ADR).
Different parameters related to oxidative stress and antioxidant defences were measured in blood and liver of rats receiving the experimental diets or the standard one; furthermore, since it has been reported that the activity of the fatty acid desaturating enzymes is influenced by SeReference Infante7, liver fatty acid composition was evaluated.
Experimental methods
Materials
Diets were prepared by Mucedola (Milano, Italy). ADR was from Pharmacia (Milano, Italy). All chemicals and solvents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and were of the highest analytical grade.
Animals and diets
Thirty male Wistar rats aged 21 d were used. After 4 d on a standard diet, they were randomly divided into three groups, one fed ad libitum on the standard diet (St) and the other two fed on one of the experimental Se-enriched diets (E1 or E2). Se content was 0·05 mg/kg in the St diet, and 0·1 mg/kg in both the experimental ones. In the E1 diet Se was supplemented as sodium selenite, while in the E2 diet by the addition of the lyophilized form of a common food having a high Se content. The food used was a potato commercially available in Italy, in which the Se content is increased by foliar Se supplementation during plant growthReference Poggi, Pifferi, Bordoni and Biagi8.
Food lyophilization was obtained by two 24-h cycles using a Drywinner 3 lyophilizer (Heto-Holten/Jouan Nordic, Hallerød, Denmark). The Se concentration in the lyophilized food was 0·0882 μg/g, as determined by inductively coupled plasma-atomic emission spectrometryReference Navarro-Blasco and Alvarez-Galindo9. Both sodium selenite and lyophilized food were added in appropriate amounts to diets during their preparation to obtain a Se final concentration of 0·1 mg/kg diet. Protein, lipid and carbohydrate content was in the normal range of adequacy for rats (g/100 g diet): proteins about 21; lipids about 8; carbohydrates about 61·5, and contained appropriate amounts of vitamins and other minerals.
Animals were housed in individual cages in strictly controlled conditions of temperature (20 ± 2°C) and humidity (60–70 %), with a 12-h dark–light cycle. Water and food were provided ad libitum; food consumption was measured every day, and rat body weight every week.
After 60 d dietary treatment, rats of each group were divided into two subgroups, one receiving intraperitoneally ADR (10 mg/kg body weight), and the other a similar volume of physiological solution. ADR is an anthracycline antibiotic widely used in the treatment of human malignancies; similar to most of the anticancer drugs, it causes various toxic effects, and its cytotoxicity is mediated by the formation of an iron anthracycline complex that generates free radicalsReference Mukherjee, Banerjee, Maulik, Dinda, Talwar and Maulik10.
After 48 h animals were sacrificed with anaesthetic ether. Blood was sampled, and the liver was quickly excised, washed in PBS, weighed, and immediately frozen at − 80°C. The Animal Care Committee of the University of Bologna approved the study.
Methods
Concentration of reactive oxygen metabolites (ROM)
ROM were measured by the d-ROM test (Diacron, Grosseto, Italy)Reference Erba, Riso, Bordoni, Foti, Biagi and Testolin11, which is based on the ability of transition metals to react with peroxides by the Fenton reaction. The reaction produces free radicals which, trapped by an alchilamine, form a coloured compound detectable at 505 nm. The test was applied directly on plasma samples and on samples obtained from about 1 g liver after lipid extraction according to Folch et al. Reference Folch, Lees and Sloane Stanley12.
Total antioxidant activity (TAA)
TAA was measured using the method of Re et al. Reference Re, Pellegrini, Proteggente, Pannala, Yang and Rice-Evans13, on the basis of the ability of the antioxidant molecules in the sample to reduce the radical cation of 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), determined by the decolourization of ABTS°+, and measured as the quenching of the absorbance at 734 nm. Values obtained for each sample were compared to the concentration–response curve of the standard trolox solution and expressed as trolox equivalent (TE). TAA was measured directly in plasma, while liver was homogenized in cold 5 mm potassium phosphate buffer (pH 7·4), filtered, and the resulting filtrate was used for TAA assay.
Glutathione peroxidise activity
GPx activity was assayed spectrophotometrically at 25°C according to Flohe & GunzlerReference Flohe and Gunzler14, following at 340 nm the disappearance of NADPH due to the reduction of oxidized glutathione (GSH) coupled to the oxidation of NADPH. The assay was performed on both plasma and aliquots of the filtrate obtained after liver homogenization in cold buffer (50 mmTris-HCl, 0·5 mm EDTA, pH 8·0). One unit of GPx activity is defined as the amount of enzyme that catalyses the reduction of 1 μmol NADPH/min.
Glutathione concentration
Concentration of total GSH was determined by the rate of formation of 5,5′-dithio-bis(2-nitro benzoic acid) at 412 nm as described by Akerboom & SiesReference Akerboom and Sies15. The liver was homogenized in 0·3 m potassium phosphate buffer (pH 8·4), centrifuged at 3000 g for 10 min at 0–4°C, and the resulting supernatant was used for GSH assay. Results were expressed as μg GSH/mg protein.
Conjugated diene-containing lipids
The appearance of conjugated diene-containing lipids was evaluated as an index of lipid peroxidation using the method of Burton et al. Reference Burton, McCord and Ghai16. Lipids were extracted from liver aliquots in chloroform–methanol–water (2:1:1 by volume). The chloroform layers from two extractions were combined and then dried under N2. Samples were resuspended in a known volume of acetonitrile and absorbance determined at 235 nm.
Fatty acid composition
Total lipids were extracted from liver according to Folch et al. Reference Folch, Lees and Sloane Stanley12, and fatty acid methyl esters were prepared from all samples according to Stoffel et al. Reference Stoffel, Chu and Ahrens17. The fatty acid composition of liver total lipids was determined by gas chromatography (Carlo Erba model 4160, Milan, Italy) using a capillary column (30 m × 0·25 mm internal diameter) filled with a thermostable stationary phase (SP 2340, 0·10–0·15 μm film thickness), at a programmed temperature (160–210°C, with a 8°C/min gradient), as previously reportedReference Bordoni, Lopez-Jimenez, Spano, Biagi, Horrobin and Hrelia18.
All data are means with their standard deviation. Statistical analysis was by one-way analysis of variance for comparison of the different dietary treatments in basal conditions or after ADR administration, and by Student's t test for the analysis of ADR effects in the same dietary group.
Results
During the 12 weeks of the dietary treatment, food consumption was similar in all rats and no differences were detected in body and liver weight among the different groups (data not shown).
In basal conditions plasma ROM level, TAA and GPx activity were similar in all dietary groups (Table 1). ADR administration did not modify plasma GPx activity, while it significantly decreased TAA and significantly increased ROM level, independent of the dietary treatment.
Table 1 Reactive oxygen metabolite (ROM) level, total antioxidant activity (TAA) and glutathione peroxidase (GPx) activity in plasma of rats fed on the different diets (St, standard diet; E1 and E2, Se-enriched diets) in basal condition and after adriamycin (ADR) administration†‡
(Mean values and standard deviations for five rats)
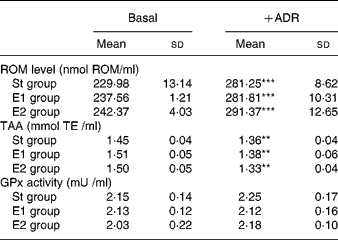
† ROM level, TAA and GPx activity were measured as reported in Methods, and are expressed as nmol/ml, mmol trolox equivalent (TE)/ml, and mU/ml, respectively.
‡ Statistical analysis was by one-way analysis of variance comparing the effects of the different dietary treatments in basal conditions (NS) or after ADR administration (NS), and by Student's t test to evaluate the effect of ADR administration in the same dietary group (**P < 0·01; ***P < 0·001).
In basal conditions, no differences in liver ROM level were detected among the dietary groups; ADR administration caused a significant increase in ROM level in all rats, but higher in St group (P < 0·01) than in the two experimental ones (P < 0·05). As a result, ROM level was significantly different (P < 0·001) after ADR administration among the three dietary groups (Fig. 1(A)). Lipid peroxidation, evaluated as conjugated diene containing lipids, was significantly influenced by the dietary treatments in both basal condition (P < 0·001) and after ADR administration (P < 0·05), being lower in E1 and E2 rats (Fig. 1(B)). Comparing the same dietary group, ADR administration did not cause modification in conjugated diene level compared to basal condition. In basal condition TAA was similar in the three dietary groups, and it significantly decreased after ADR administration in St group only (P < 0·01). Consequently, after the oxidative stress, TAA was significantly different among the dietary groups (P < 0·01), being higher in E1 and E2 rats (Fig. 1(C)).
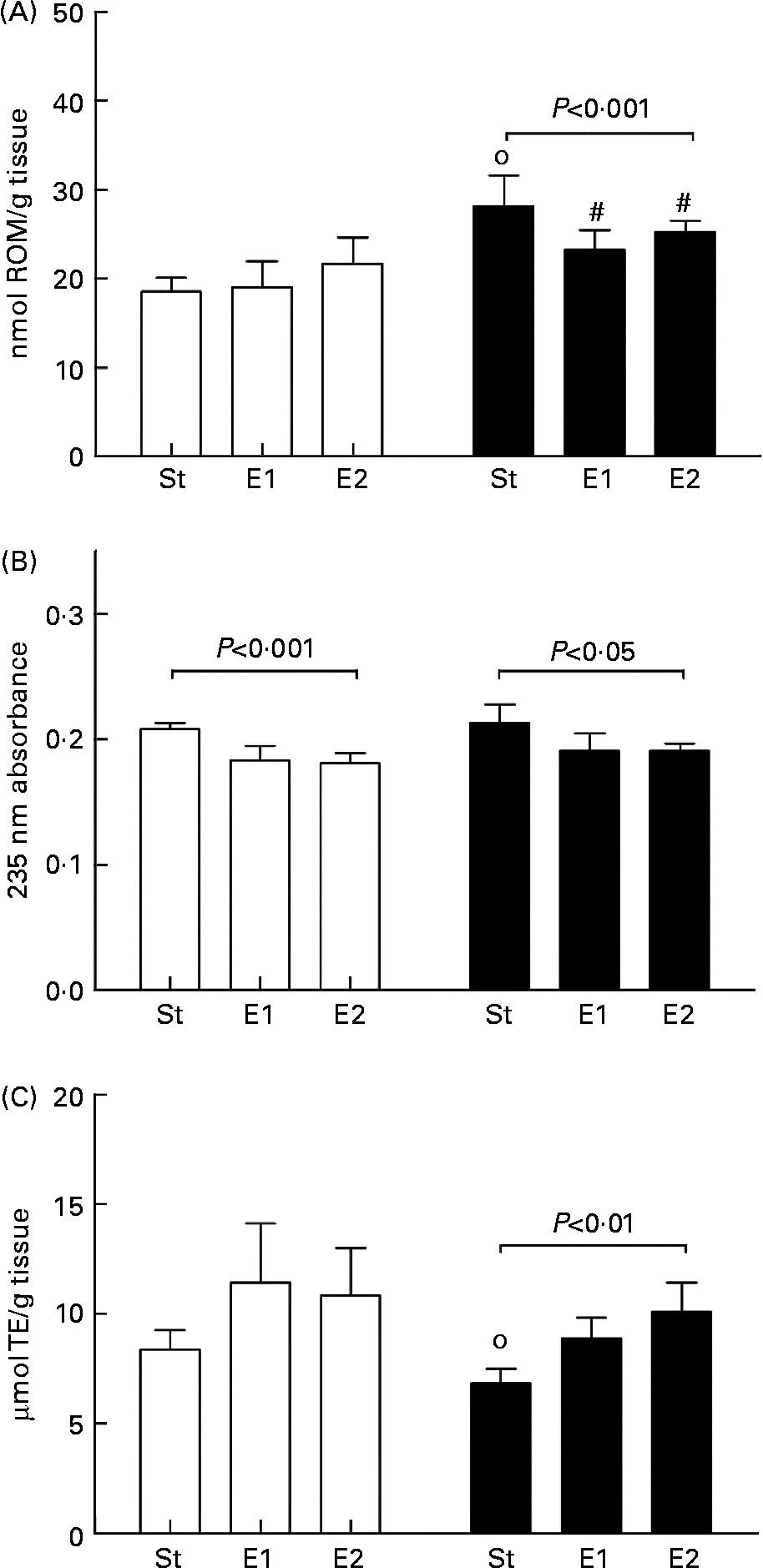
Fig. 1 Reactive oxygen metabolite (ROM) level (A), conjugated diene-containing lipids (B), and total antioxidant activity (TAA; (C)) in liver of rats fed on the different diets (St, standard diet; E1 and E2, Se-enriched diets) in basal condition (□) and after ADR administration (■). ROM level, conjugated dienes and TAA were measured as reported in Methods, and are expressed as nmol ROM/g tissue, 235 nm absorbance (arbitrary units), and μmol trolox equivalent (TE)/g tissue, respectively. Data are means for five rats with standard deviations indicated by vertical bars. Statistical analysis was by one-way analysis of variance comparing the effects of the different dietary treatments in basal conditions (ROM level, NS; conjugated dienes, P < 0·001; TAA, NS) or after ADR administration (ROM level, P < 0·001; conjugated dienes, P < 0·05; TAA, P < 0·01), and by Student's t test to evaluate the effect of ADR administration in the same dietary group (# P < 0·05; ○ P < 0·01).
In both basal condition and after ADR administration Se enrichment of the diets significantly influenced liver GPx activity (P < 0·05 and P < 0·01, respectively); this appeared significantly higher in the experimental groups. Comparing the same dietary group in basal condition and after ADR administration no differences were detected (Fig. 2(A)). Similarly, liver GSH content appeared influenced by Se dietary content in both basal and oxidative conditions, being lower in E1 and E2 rats than in standard ones, and ADR administration did not influence it in the same dietary group (Fig. 2(B)).
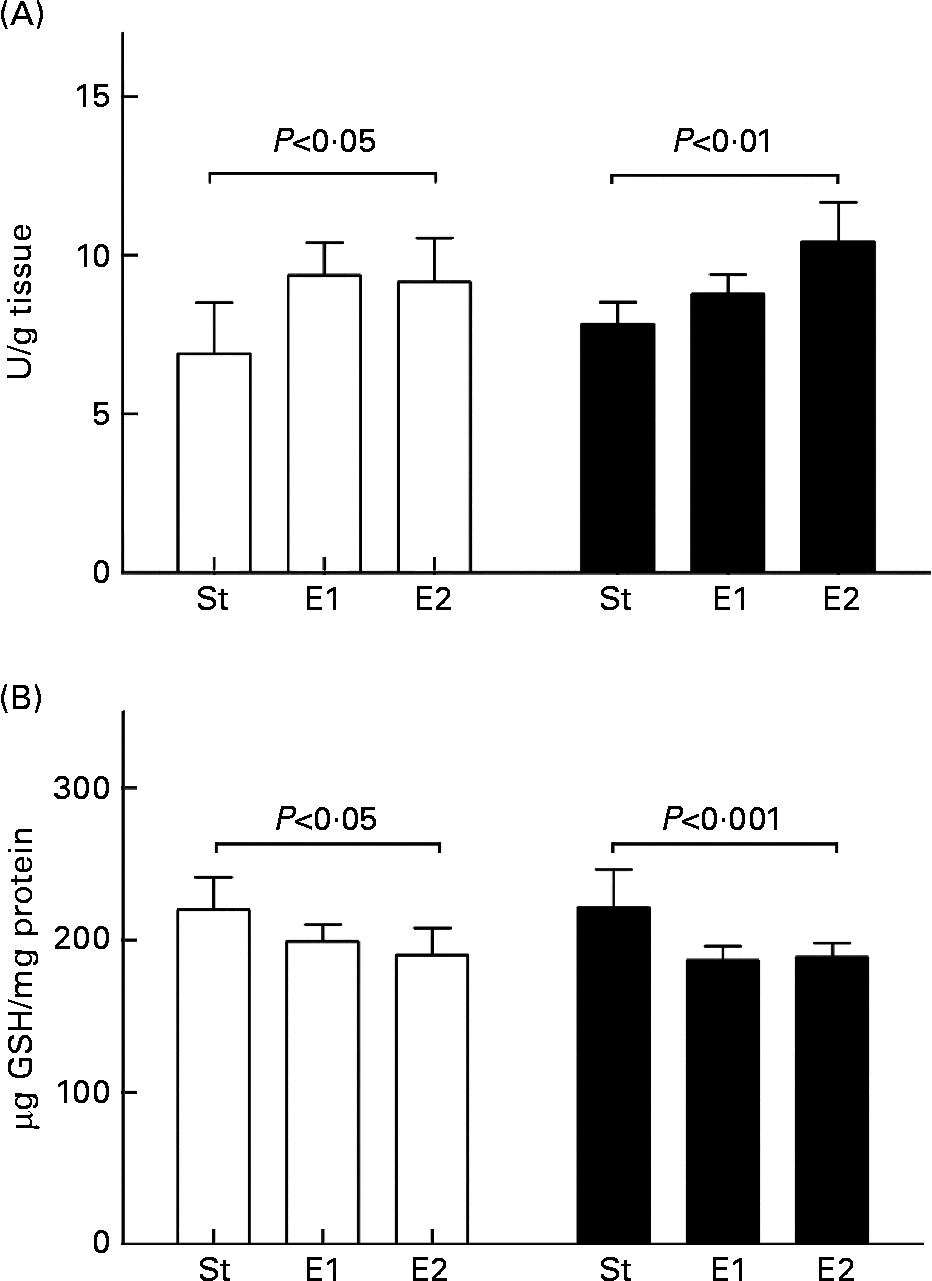
Fig. 2 Glutathione peroxidase (GPx) activity (A) and glutathione (GSH) content (B) in liver of rats fed on the different diets (St, standard diet; E1 and E2, Se-enriched diets) in basal condition (□) and after ADR administration (■). GPx activity and GSH content were measured as reported in Methods and expressed as U/g tissue and μg GSH/mg protein, respectively. Data are means for five rats with standard deviations indicated by vertical bars. Statistical analysis was by one-way analysis of variance comparing the effects of the different dietary treatments in basal conditions (GPx activity, P < 0·05; GSH content, P < 0·05) or after ADR administration (GPx activity, P < 0·01; GSH content, P < 0·001), and by Student's t test to evaluate the effect of ADR administration in the same dietary group (NS).
Total liver fatty acid composition of rats fed on the different diets, in basal conditions and after ADR administration, is shown in Fig. 3. No differences in fatty acid content were detected among the different dietary groups in either basal or oxidative conditions, apart from arachidonic acid relative molar content, which was higher in E1 and E2 rats than in St ones. ADR administration did not cause any modification in fatty acid composition considering the same dietary group. No differences were detected in the unsaturation index (data not shown), while the 18:2/20:4 ratio appeared significantly lower in E1 and E2 rats than in St ones, in both basal (St 0·99 (sd 0·08), E1 0·86 (sd 0·04), E2 0·90 (sd 0·07), P < 0·05) and oxidative (St 0·98 (sd 0·06), E1 0·77 (sd 0·02), E2 0·95 (sd 0·05), P < 0·001) condition.

Fig. 3 Fatty acid composition of liver total lipids of rats fed on the different diets (St, standard diet; E1 and E2, Se-enriched diets), in basal condition and after adriamycin (ADR) administration (St – ADR □ ; E1 – ADR ![]() ; E2 – ADR ■ ; St + ADR
; E2 – ADR ■ ; St + ADR ![]() ; E1+ ADR
; E1+ ADR ![]() ; E2+ ADR
; E2+ ADR ![]() ). Fatty acid composition (as methyl esters) was determined by gas chromatography, and expressed as mol/100 mol. Data are means for five rats with standard deviations indicated by vertical bars. Statistical analysis was by one-way analysis of variance comparing the effects of the different dietary treatments in basal conditions (20:4 n-6, P < 0·05) or after ADR administration (20:4, n-6 P < 0·05), and by Student's t test to evaluate the effect of ADR administration in the same dietary group (NS).
). Fatty acid composition (as methyl esters) was determined by gas chromatography, and expressed as mol/100 mol. Data are means for five rats with standard deviations indicated by vertical bars. Statistical analysis was by one-way analysis of variance comparing the effects of the different dietary treatments in basal conditions (20:4 n-6, P < 0·05) or after ADR administration (20:4, n-6 P < 0·05), and by Student's t test to evaluate the effect of ADR administration in the same dietary group (NS).
Discussion
Se is an essential trace element which must be supplied by daily diet; its main role is that of an antioxidant in the GPx enzyme, and Se depletion results in a decrease in both GPx activity and protein levels. GPx, the main intracellular antioxidant, and other Se-dependent systems such as selenoprotein P and thioredoxin reductase have been reported to be critical antioxidant defencesReference Aboul-Fadl19, Reference de Lorgeril, Salen, Accominotti, Cadau, Steghens, Boucher and de Leiris20.
Increased oxidative stress may be involved in the pathogenesis of many chronic diseases, and there is an obvious link between diet and oxidative stress, since the human body derives its main antioxidant defences from essential nutrients. Several studies have shown that Se deficiency adversely affects the susceptibility to different diseases, in particular cancersReference Aboul-Fadl19 and chronic heart failureReference de Lorgeril, Salen, Accominotti, Cadau, Steghens, Boucher and de Leiris20. The positive correlation between dietary Se and GPx expression/activity is well recognized, while the relationship between dietary Se and other selenoproteins appears ambiguous, depending on Se concentration in the diet and on the tissue considered. In fact, during dietary Se depletion there is a ‘prioritization’ of available Se so that synthesis of some selenoproteins is maintained more than others, and this prioritization is tissue-specific. Although in the liver thioredoxin reductase is more sensitive to Se depletion than in other tissue, in a very recent paper Crosley et al. Reference Crosley, Meplan, Nicol, Rundlof, Arner, Hesketh and Arthur21 demonstrated the greatest response of GPx mRNA than other selenoprotein mRNA to decreased dietary Se in marginally deficient animals, confirming the main implication of GPx in the alteration of antioxidant defences consequent to Se depletion.
The chemical form of ingested Se partially determines the physiologic outcome in animals. Salts such selenite and selenate and the amino acid selenocysteine easily incorporate into selenoproteins, but because selenoprotein expression is tightly regulated, Se from these sources will not accumulate beyond a certain point. Because selenomethionine substitutes for methionine, it will accumulate in large protein masses such as muscle, and total Se body burden is much higher for selenomethionine than for selenocysteine or inorganic Se saltsReference Beilstein and Whanger22.
Plants are able to synthesize seleno-aminoacids from selenite and selenateReference Whanger23, and several Se-containing compounds present in foods have been identifiedReference Whanger24. In the Se-enriched potato used as Se source in our study the majority of Se was allocated to soluble and insoluble (the residual fraction) protein fractions, according to Turakainen et al. Reference Turakainen, Hartikainen, Ekholm and Seppanen25. Organic Se forms are considered to be more efficient than inorganic Se forms in increasing the Se content and the activity of GPx in plasma and muscle tissueReference Whanger23.
Although overall absorption of all forms of Se appears relatively high (70 % to 95 %), the bioavailability of the different forms of this trace element appears very important in the light of a dietary supplementation. Unlike other trace minerals (e.g. Fe and Zn), the covalent nature of Se bonding precludes estimation of bioavailability of selenocompounds by simple measurements of absorption. Instead, bioavailability also must address metabolic transformation to biologically active metabolites. In this study we have compared the effects due to the dietary supplementation of the same amount of Se but in two different forms, i.e. a sodium salt, as often found in supplements, and the lyophilized form of a Se-rich potato obtained by foliar fertilization, on the counteraction of an oxidative stress induced in rats by ADR administration.
In plasma, different Se intake did not influence GPx activity, TAA and ROM level. It is demonstrated that plasma GPx activity is not strictly related to Se statusReference Whitin, Tham, Bhamre, Ornt, Scandling, Tune, Salvatierra, Avissar and Cohen26, and although we did not measure the activity of other seleno-enzymes with antioxidant function such as selenoprotein P, the invariance of plasma TAA allows speculation also of an invariance of these antioxidant proteins. This could explain the lack of protection against oxidative stress observed in plasma of E1 and E2 rats, as indicated by the similar increase of ROM level after ADR administration in all the dietary groups. This increase in plasma ROM level and the decrease in TAA observed in all rats after the anthracycline injection clearly demonstrate the onset of an oxidative condition, ROM concentration being recently indicated as a potent marker of oxidative injuryReference Samouilidou, Grapsa, Karpouza and Lagouranis27.
Similarly, the significant increase in liver ROM level observed in all dietary groups after ADR administration confirmed an oxidative condition even in this organ; nevertheless, the increase was lower in Se-supplemented rats (about 120 % in both groups) than in St ones (>150 %). Since the oxidative stress was the same, it is plausible that the lower increase in ROM level was due to a partial protection by dietary Se, independent from the use of sodium selenite or lyophilized food for the supplementation.
The decrease in conjugated diene levels observed in E1 and E2 rats in both basal and oxidative conditions confirmed the protective effect of Se enrichment of the diets, independent from the form of Se. It is plausible that the increase in liver TAA contributed to the protective effect against oxidative stress; in basal condition TAA appeared significantly higher in E1 and E2 rats compared to St ones by Student's t test (P < 0·05 in both cases), and after ADR administration significant differences were detected among the three groups even by one-way ANOVA.
TAA represents the overall level of defence system against oxidative stress, and both endogenous and exogenous antioxidants contribute to it. In basal condition and after ADR administration Se, both as sodium selenite and lyophilized food, significantly increased GPx activity which, in turn, greatly influenced the entity of global defences against oxidative stress, in agreement with our previous data in Se-supplemented cultured cardiomyocytesReference Bordoni, Biagi, Angeloni, Leoncini, Muccinelli and Hrelia28, Reference Bordoni, Biagi, Angeloni, Leoncini, Danesi and Hrelia29, and with data obtained in erythrocytesReference Zawadzka-Bartczak30 and liverReference Vali, Taba, Szentmihalyi, Febel, Kurucz, Pallai, Kupcsulik and Blazovics31. A linear correlation was found between GPx activity and TAA in both basal (r 0·912, P < 0·001) and oxidative condition (r 0·964, P < 0·001).
The increase in GPx activity can explain the reduced ROM and conjugated diene levels observed in E1 and E2 rats, since it is demonstrated that this Se-containing enzyme, which is ubiquitously present but predominantly in liver, functions to detoxify both hydrogen and lipid peroxidesReference Arthur32. Although both experimental diets increased GPx activity, in an oxidative condition the supplementation with the lyophilized food appeared more efficient than the one with sodium selenite (P < 0·05).
In St rats GPx activity appeared lower and GSH content higher than in E1 and E2 rats, in agreement with Matsumoto et al. Reference Matsumoto, Suzuki, Washimi, Hisamatsu, Okajo, Ui and Endo33, who reported that a low-Se diet causes a decrease in GPx activity and an increase in GSH content in the liver. In E1 and E2 rats the decrease in liver GSH concentration paralleled the increase in GPx activity, probably due to an increased GSH utilization as a cofactor by GPx.
Regarding liver total lipid fatty acid composition, it has been suggested that Se could play a role in the desaturation of n-3 and n-6 PUFAReference Infante7, Reference Celik, Yilmaz, Asan, Naziroglu, Cay and Aksakal34, and Quilliot et al. Reference Quilliot, Walters, Bohme, Lacroix, Bonte, Fruchart, Drouin, Duriez and Ziegler35 found a negative correlation between Se level and PUFA content in diabetic patients, suggesting that Se could affect desaturase activity. Schafer et al. Reference Schafer, Kyriakopoulos, Gessner, Grune and Behne36 reported a decreased desaturase activity in Se-deficient rats fed on a high n-3 PUFA diet, but since it is known that a high-PUFA diet may inhibit desaturation, a direct effect of Se itself on the desaturase enzyme was not surely established. In this work, the lower arachidonic acid relative molar content observed in St rats compared with E1 and E2 ones, together with the higher 18:2/20:4 ratio, allow speculation of a direct effect of Se in linoleic acid conversion. Since St diet was marginally Se deficient, our data suggest the need of adequate Se intake to maintain PUFA metabolism.
In conclusion, our data indicate that an adequate Se dietary intake, in pure chemical form or as a lyophilized, Se-enriched, vegetable food, is able to protect against exogenous pro-oxidants. Our results are in agreement with those obtained by Kocdor et al. Reference Kocdor, Cehreli, Kocdor, Sis, Yilmaz, Canda, Demirkan, Resmi, Alakavuklar and Harmancioglu37 in rats exposed to 7,12-dimethylbenz[a]anthracene, and confirm that Se plays a major and synergistic role as exogenous antioxidants in the regulation of the endogenous antioxidant defence systemReference Goldfarb38.
According to Crosley et al. Reference Crosley, Meplan, Nicol, Rundlof, Arner, Hesketh and Arthur21, our St diet, containing 50 μg Se/kg, can be considered as marginally deficient, while E1 and E2 diets, containing 100 μg Se/kg, can be considered as adequate. In several EU countries Se intakes are less than half the UK reference nutrient intakes of 60 and 75 μg Se/d for females and males respectively, and Se intake in the UK has declined from >60 μg Se/d in 1974 to 29–39 μg Se/d (for review, see RaymanReference Rayman39). Although there is substantial evidence that Se is a potent anti-carcinogen when it is present at levels above those required for the maximal expression of selenoproteins, at present we think that the main nutritional goal is to guarantee adequate Se intake. This result may be reached either using foods or supplements, and it is important to understand if these two strategies give similar functional results.
No dramatic differences due to the different form of Se supplementation were detected, but the increase in GPx activity appeared more evident in rats fed on the diet enriched with the lyophilized food, according to our previous data comparing the efficacy of sodium selenite and selenomethionine in cultured cardiomyocytesReference Bordoni, Biagi, Angeloni, Leoncini, Muccinelli and Hrelia28, Reference Bordoni, Biagi, Angeloni, Leoncini, Danesi and Hrelia29. This could be due to the different chemical forms of Se, the majority of which was allocated to soluble and insoluble protein fractions in the Se-enriched potato used as Se source, or to other components of the food itself. In fact, protection is mediated by the ideal combination of nutrients and phytochemicals in foods and not by a single molecule; some other components of lyophilized potatoes, such as methionine could have also acted as positive modulators of GPx activityReference Seneviratne, Li, Khaper and Singal40.
Although further studies are needed, data herein presented may contribute to the characterization of the effectiveness of Se from different sources, foods or supplements, in the light of dietary advice concerning improvement of Se intake within the population.
Acknowledgements
This work was partially supported by grants from Italian MIUR 60 % and PRIN 2005, and from ‘Consorzio delle Buone Idee’ (Bologna, Italy). The authors thanks Dr. Valeria Poggi, Dr. Melissa Mazzoni and Dr. Giorgia Colinucci for their skillful technical assistance.






