The American Society for Clinical Oncology in 2016 recommended that cancer patients maintain a normal weight, increase physical activity and eat a diet low in fat and refined carbohydrates and high in vegetables and fruit, as a potential aid to some aspects of prognosis, acknowledging the lack of definitive data(Reference Runowicz, Leach and Henry1). If lifestyle factors could be confirmed as important in breast cancer prognosis, they may provide additional options for breast cancer survivors to maximise health and well-being.
This paper reviews the literature on associations between weight, physical activity and prognosis variables (cancer-specific mortality, all-cause mortality, recurrence and second primary cancers) in breast cancer in women. Referenced are comprehensive reviews of weight status, physical activity and breast cancer prognosis(Reference Chan, Vieira and Aune2–Reference Zhong, Jiang and Ma11). Many observational studies and one randomised clinical trial have investigated the associations of weight, weight change or physical activity with prognosis among women diagnosed with breast cancer, as discussed in the sections following.
Weight, BMI and breast cancer prognosis
Overweight, obesity or underweight may increase the risk of breast cancer recurrence or death, although the effect of weight change on prognosis is not established. Overweight and obesity also increase the risk of some complications from breast cancer treatment and increase the risk of several co-morbidities.
A recent meta-analysis on weight status and breast cancer survival included 213 075 breast cancer patients from eighty-two prospective cohort studies, with 41 477 deaths (of which 23 182 were breast cancer-specific deaths)(Reference Chan, Vieira and Aune2). Thirty-five studies (114 012 women; 17 894 deaths) provided categorical results on BMI within 12 months after diagnosis, using WHO standard classifications (underweight, BMI <18·5 kg/m2; normal weight, BMI 18·6 to <25·0 kg/m2; overweight, BMI 25·0 to <30·0 kg/m2; obese, BMI ≥30·0 kg/m2).
Compared with normal weight women, the summary relative risks (RR) for total mortality were 1·23 (twenty-four studies, 95 % CI 1·12, 1·33) for obese women, 1·07 (twenty-two studies, 95 % CI 1·02, 1·12) for overweight women and 1·25 (eleven studies, 95 % CI 0·99, 1·57) for underweight women (Table 1). Compared with normal weight women, the summary RR for breast cancer-specific mortality were 1·25 (twelve studies, 95 % CI 1·10, 1·42) for obese women, 1·11 (twelve studies, 95 % CI 1·03, 1·20) for overweight women and 1·53 (five studies, 95 % CI 1·27, 1·83) for underweight women (Table 1).
Table 1. Summary of meta-analyses of BMI (kg/m2) and survival in women with breast cancer
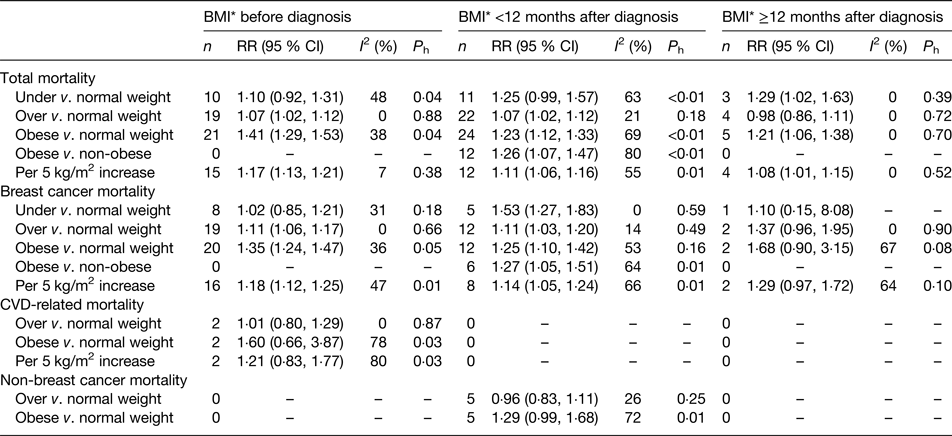
*BMI before and after diagnosis (<12 months after, or ≥12 months after diagnosis) was classified according to the exposure period which the studies referred to in the BMI assessment; the BMI categories were included in the categorical meta-analyses as defined by the studies.
P h, P for heterogeneity between the studies; I 2, percentage of variation across studies that is due to heterogeneity rather than chance.
From: Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer – systematic literature review and meta-analysis of 82 follow-up studies. Annals of Oncology, 2014, Volume 25, Issue 10, 1 October 2014, Pages 1901–1914, by permission of Oxford University Press.
The meta-analysis did not identify any subgroups of patients that were statistically significantly different from other subgroups in terms of weight associations with mortality, including menopausal status, hormone receptor status, sample size (number of deaths), length of follow-up, geographic location, or method of ascertaining height and weight (measured v. self-reported). Adjustment for potential confounders also did not substantially influence effect size, including tumour stage, cancer treatment, presence of diabetes or other co-morbidities, physical activity or smoking.
Dose–response curves were estimated, and showed a non-linear J-shaped curve for total mortality, with increasing RR for underweight, overweight and obese breast cancer survivors (Fig. 1). However, the dose–response relationship for breast cancer-specific mortality revealed only a linear response.
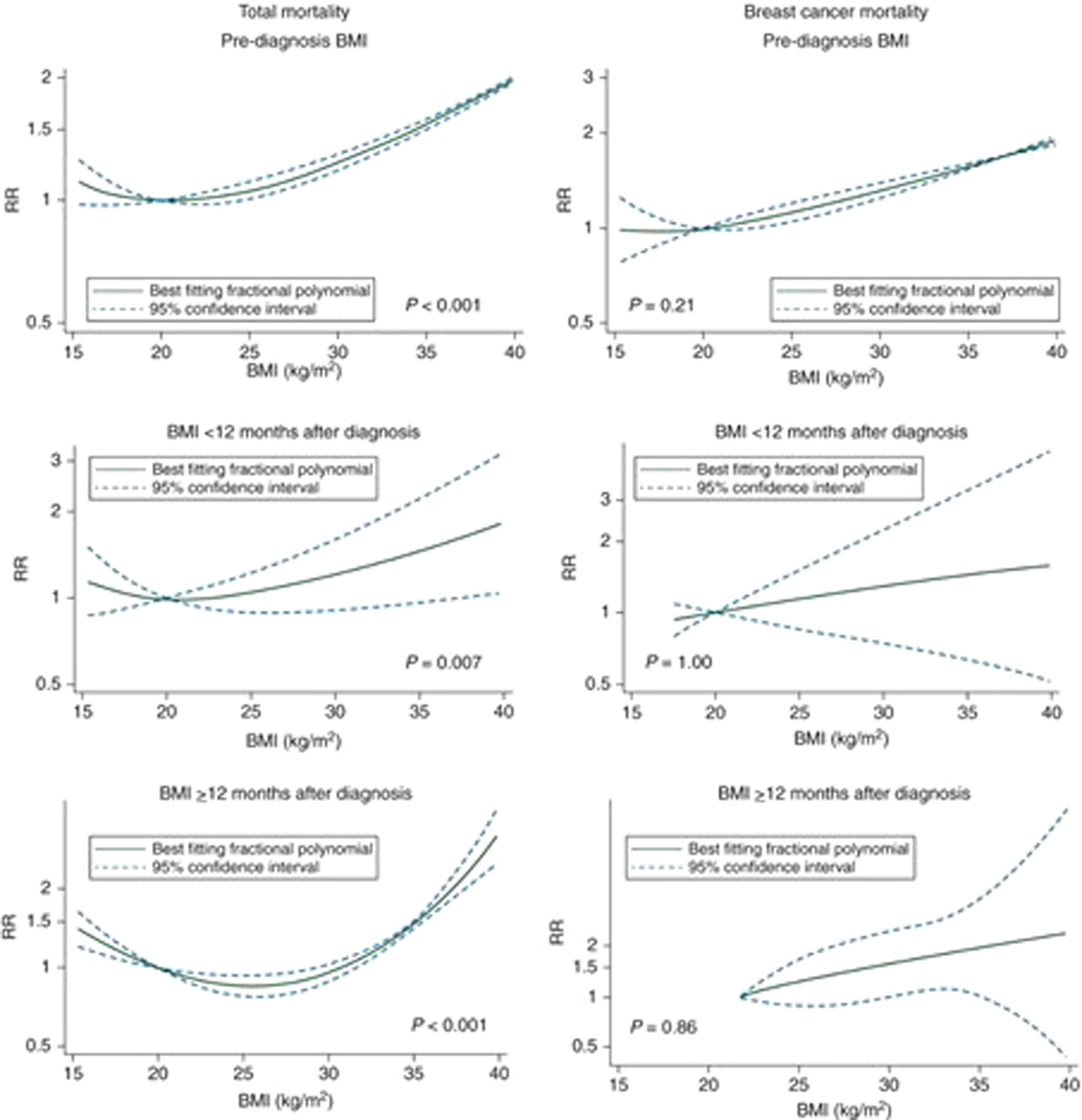
Fig. 1. (Colour online) Non-linear dose–response curves of BMI and mortality in women with breast cancer.
Several cohort studies have investigated whether the association of overweight/obesity with survival differs by molecular subtype of breast cancer. A 2012 meta-analysis with twenty-one studies found that all-cause mortality was higher in patients with highest v. lowest category of BMI for patients with both oestrogen receptor-positive and oestrogen receptor-negative tumours(Reference Niraula, Ocana and Ennis4). The pooled hazard ratio (HR) for all-cause mortality in heavier v. lighter women was 1·31 (95 % CI 1·17, 1·46) for those with oestrogen receptor/progesterone receptor-positive cancers; 1·18 (95 % CI 1·06, 1·31) for those with oestrogen receptor/progesterone receptor-negative cancers; and the difference between the two groups was NS (P = 0·31). The same meta-analysis found a statistically significant association between heavier v. lighter weight and risk of breast cancer-specific mortality in patients with oestrogen receptor/progesterone receptor-positive tumours (HR 1·36 95 % CI 1·20, 1·54). For women with oestrogen receptor/progesterone receptor-negative breast cancer, heavier v. lighter weight was associated with a non-statistically significant increase in breast cancer-specific mortality (HR 1·46; 95 % CI 0·98, 2·19). A combined analysis of 15 538 breast cancer patients from the National Surgical Adjuvant Breast and Bowel Project trials found some variability in the association of BMI with survival, with the suggestion that the effect of obesity on survival was limited to women with oestrogen receptor-positive disease(Reference Cecchini, Swain and Costantino12).
A smaller number of studies have looked at the association of obesity with survival by other classifications of tumour type and patients, including tumour genetics, locally advanced or inflammatory breast cancer and young patients(Reference Ligibel, Cirrincione and Liu13–Reference Mazzarella, Disalvatore and Bagnardi17). In general, obesity increases the risk for poor survival, although some types may be more affected than others.
Several studies have investigated interactions between overweight/obesity and adjuvant therapy effectiveness. A National Surgical Adjuvant Breast and Bowel Project analysis of 3385 clinical trial patients from a randomised, placebo-controlled trial evaluating tamoxifen for lymph node-negative, oestrogen receptor–positive breast cancer found that obese women benefited from tamoxifen therapy as much as lighter weight women(Reference Dignam, Wieand and Johnson18). Compared with normal weight women, however, obese women had greater all-cause mortality (HR 1·31, 95 % CI 1·12, 1·54) and non-breast cancer mortality (HR 1·49, 95 % CI 1·15, 1·92). Several studies have found evidence that obesity reduces the effectiveness of some, but not all, aromatase inhibitors(Reference Jiralerspong and Goodwin19).
Two studies have reported on body fat distribution in relation to breast cancer prognosis, with suggestions of increased breast cancer mortality risk with android body fat distribution defined as the high waist:hip ratio(Reference Borugian, Sheps and Kim-Sing20) or as high suprailiac:thigh ratio(Reference Kumar, Cantor and Allen21).
Risk of future second primary breast cancer may also be increased with higher levels of adiposity. In a 2014 meta-analysis, highest v. lowest BMI increased the risk for the development of a second primary breast cancer (RR 1·30, 95 % CI 1·14, 1·48)(22). In this same meta-analysis, each five unit increase in BMI was associated with a 13 % increased risk for a second primary breast cancer (RR 1·13, 95 % CI 1·06, 1·21).
Weight change after breast cancer diagnosis and risk of mortality
Weight gain after diagnosis has been frequently reported for breast cancer patients, especially among women receiving systemic adjuvant chemotherapy(Reference Chlebowski, Aiello and McTiernan23, Reference Goodwin, Ennis and Pritchard24); with weight gains averaging between 2 and 4 kg, following some chemotherapy exercises such as cyclophosphamide, methotrexate and 5-fluorouracil(Reference Demark-Wahnefried, Winer and Rimer25, Reference Shepherd, Parulekar and Day26). Tamoxifen treatment does not appear to influence body weight(Reference Goodwin, Ennis and Pritchard24), and the effect of aromatase inhibitors on weight is not established. A 2015 meta-analysis including twelve studies found a 23 % increase in all-cause mortality in breast cancer survivors who had a >10 % weight gain after diagnosis(Reference Playdon, Bracken and Sanft3). Lesser amounts of weight gain were not associated with increased mortality, however.
Weight loss after a diagnosis of breast cancer is less common, but may be a sign of disease progression. In a meta-analysis of five studies, weight loss after diagnosis was associated with increases in both all-cause and breast cancer-specific mortality, both in women who at diagnosis were non-overweight and in those who were overweight or obese(Reference Jackson, Heinrich and Beeken5).
Obesity and development of co-morbidities in breast cancer survivors
Obese breast cancer patients are at increased risk for morbidities including surgical wound complications, lymphoedema and possibly, congestive heart failure if treated with doxorubicin(Reference Chlebowski27). Obesity also may increase the risk for development of endometrial cancer among women with breast cancer treated with tamoxifen(Reference Bernstein, Deapen and Cerhan28). Obesity increases the risk of several cancers in addition to breast and endometrium, including kidney, oesophageal adenocarcinoma, colon and others(29). Finally, overweight or obese breast cancer survivors may suffer from obesity-related co-morbidities including type II diabetes, hypertension, CVD, osteoarthritis and pulmonary disease(Reference Everett, Tamimi and Greer30, Reference von Gruenigen, Courneya and Gibbons31).
Physical activity and breast cancer prognosis
Evidence from several systematic reviews and meta-analyses shows a consistent inverse association between physical activity level after diagnosis and cancer-specific and all-cause mortality among breast cancer survivors(Reference Friedenreich, Neilson and Farris6–Reference Zhong, Jiang and Ma11, Reference Ballard-Barbash, Friedenreich and Courneya32). A 2015 meta-analysis of eight cohorts found that highest v. lowest levels of physical activity were associated with a 48 % reduction in the risk for all-cause mortality (RR 0·52, 95 % CI 0·43, 0·64)(Reference Lahart, Metsios and Nevill9). A 2016 meta-analysis of ten cohorts found that highest v. lowest levels of post-diagnosis physical activity were associated with a 38 % reduction in the risk of breast cancer-specific mortality (RR 0·62, 95 % CI 0·48, 0·80)(Reference Friedenreich, Neilson and Farris6). This latter study found that the risk of recurrence was significantly reduced in four cohorts and one trial that collected recurrence data (RR 0·68, 95 % CI 0·58, 0·80)(Reference Friedenreich, Neilson and Farris6). It should be noted that various studies used quite different definitions of recurrence, so it is difficult to interpret the combined effect of these results.
A pooling project addressed the association between meeting the 2008 US Physical Activity Guidelines recommended activity levels and breast cancer survival(33). The project found that engaging in ≥10 metabolic equivalent of task-hours/week was associated with a 27 % reduction in all-cause mortality (HR 0·73, 95 % CI 0·66, 0·82) and a 25 % reduction in breast cancer-specific mortality (HR 0·75, 95 % CI 0·65, 0·85)(Reference Beasley, Kwan and Chen34).
Little information is available on the dose–response association of physical activity with breast cancer survival. A meta-analysis of four cohort studies found that each 5, 10 or 15 metabolic equivalent of task-hours/week increase in post-diagnosis physical activity was associated with a 6 % (95 % CI 3, 8 %), 11 % (95 % CI 6, 15 %) and 16 % (95 % CI 9, 2 %) reduction in the risk of breast cancer mortality, respectively(Reference Schmid and Leitzmann10). Furthermore, each 5, 10 or 15 metabolic equivalent of task-hours/week increase in post-diagnosis physical activity was associated with a 13 % (95 % CI 6, 20 %), 24 % (95 % CI 11, 36 %) and 34 % (95 % CI 16, 38 %) decreased risk of all-cause mortality, respectively(Reference Schmid and Leitzmann10).
Increasingly, research is focusing on the role of sedentary behaviour in health and disease. While several studies have looked at the associations of indices of sedentary lifestyle (e.g. sitting, television watching) and the risk for cancer(Reference Biswas, Oh and Faulkner35), only one study has focused on the association of sedentary behaviour and breast cancer survival(Reference George, Smith and Alfano36). In this study, 687 breast cancer survivors in the HEAL cohort were queried about the time spent watching television approximately 30 months after diagnosis, and were followed for a mean 7 years. Women in the top v. bottom tertile of television time had almost double the risk of all-cause mortality compared with women in the lowest tertile (HR 1·94, 95 % CI 1·02, 3·66, P trend = 0·024). However, the association was attenuated and no longer statistically significant after adjusting for potential confounders such as physical activity level.
Randomised controlled trials of weight loss and breast cancer prognosis
In the women's intervention nutrition study, 2437 women with breast cancer were randomly assigned between 1994 and 2001 in a ratio of 40 : 60 to a low-fat dietary intervention (n 975) or control (n 1462) group and followed for a median of 60 months(Reference Chlebowski, Blackburn and Thomson37). While the goals of the intervention did not include weight loss, at 12 months, women in the intervention group weighed a mean 6 pounds less than controls despite identical mean baseline weights in the groups (160 lbs). A total of 277 relapse events (local, regional, distant or ipsilateral breast cancer recurrence or new contralateral breast cancer) were reported in ninety-six of 975 (9·8 %) women in the dietary intervention group and 181 of 1462 (12·4 %) women in the control group. The HR of relapse events in the intervention group compared with the control group was 0·76 (95 % CI 0·60, 0·98, P = 0·077 for stratified log-rank and P = 0·034 for adjusted Cox model analysis). Exploratory analyses suggested a greater effect of the dietary intervention among women with hormone receptor-negative tumours.
There are several ongoing randomised clinical trials testing the effects of weight loss on breast cancer survival and related variables(Reference Chlebowski and Reeves38). The DIANA-5 trial is randomly assigning 1208 pre- and postmenopausal women with early-stage breast cancer at increased risk for recurrence (oestrogen receptor-negative, metabolic syndrome, high testosterone or high insulin) to the Mediterranean and macrobiotic diet intervention or control(Reference Villarini, Pasanisi and Traina39). The intervention is delivered via group face-to-face session including cooking classes. Recruited patients are a mean 1·8 years post-diagnosis. The primary outcome is breast cancer recurrence.
The SUCCESS-C trial is testing a 2-year reduced energy, reduced fat intervention with physical activity v. controls. The trial is a 2 × 2 factorial with chemotherapy exercises as the other two conditions(Reference Rack, Andergassen and Neugebauer40). A total of 3547 pre- and postmenopausal patients will be enrolled in the trial. Women with BMI between 24·0 and 40·0 kg/m2 will be randomised into the weight loss intervention or control arms of the trial. Patients are recruited within 6 weeks after surgery for human epidermal receptor-2-negative, node-positive or high-risk node-negative breast cancer. The intervention, delivered via phone, includes a 2092–4184 kJ/d deficit, fat intake reduction to 20–25 % of energy and 150–200 min/week of moderate-intensity physical activity. The primary outcome is disease-free survival.
In the UK, a small randomised controlled trial, the B-AHEAD3 trial, is testing energy reduction via 2 d fasting/5 d usual diet, v. control, on progression-free survival in 134 pre- and postmenopausal women with advanced breast cancer (locally advanced or distant metastases)(Reference Chlebowski and Reeves38). Patients are eligible if they have BMI ≥24·0 kg/m2.
A randomised trial in Spain (PREDICOP) will evaluate a 12-month lifestyle intervention v. usual care on breast cancer recurrence over 5 years’ follow-up(41). A total of 2108 pre- and postmenopausal women diagnosed with stage I–IIIa breast cancer (BMI 18–40 kg/m2) will be recruited. The reduced energy intervention is delivered in person, with once-weekly nutrition classes and twice-weekly supervised moderate- to high-intensity exercise sessions, with reduced frequency in the second 6 months. The primary endpoint is progression-free survival.
The Breast Cancer Weight Loss Study will enrol 3136 pre- and postmenopausal women with stage II or III human epidermal receptor-2-negative breast cancer (BMI ≥27 kg/m2) in the USA and Canada to determine whether weight loss will improve breast cancer outcome(Reference Ligibel, Barry and Alfano42). Participants will be randomly assigned to a 2-year weight loss intervention or control group. The phone-based intervention has goals to increase exercise and reduce energy, with the inclusion of a variety of exercise recording devices and programmes. The primary outcome is invasive disease-free survival, with follow-up over 10 years.
These ongoing trials are well powered to determine the effects of various dietary weight loss interventions on breast cancer prognosis endpoints, although their success will depend on patient recruitment, adherence to interventions and ability to retain participants long term.
Potential mechanisms for the effects of obesity and physical activity on breast cancer prognosis
Several mechanisms have been proposed to explain the relationships between overweight/obesity, physical activity and breast cancer prognosis, as graphically depicted in Fig. 2(Reference Jiralerspong and Goodwin19, Reference Chlebowski, Aiello and McTiernan23, Reference McTiernan43–Reference Griggs, Mangu and Anderson46). These mechanisms include increased circulating or tissue levels of sex and metabolic hormones; reduced levels of hormone-binding proteins; increased levels of inflammation and other adipocytokines; and chemotherapy underdosing in obese patients.
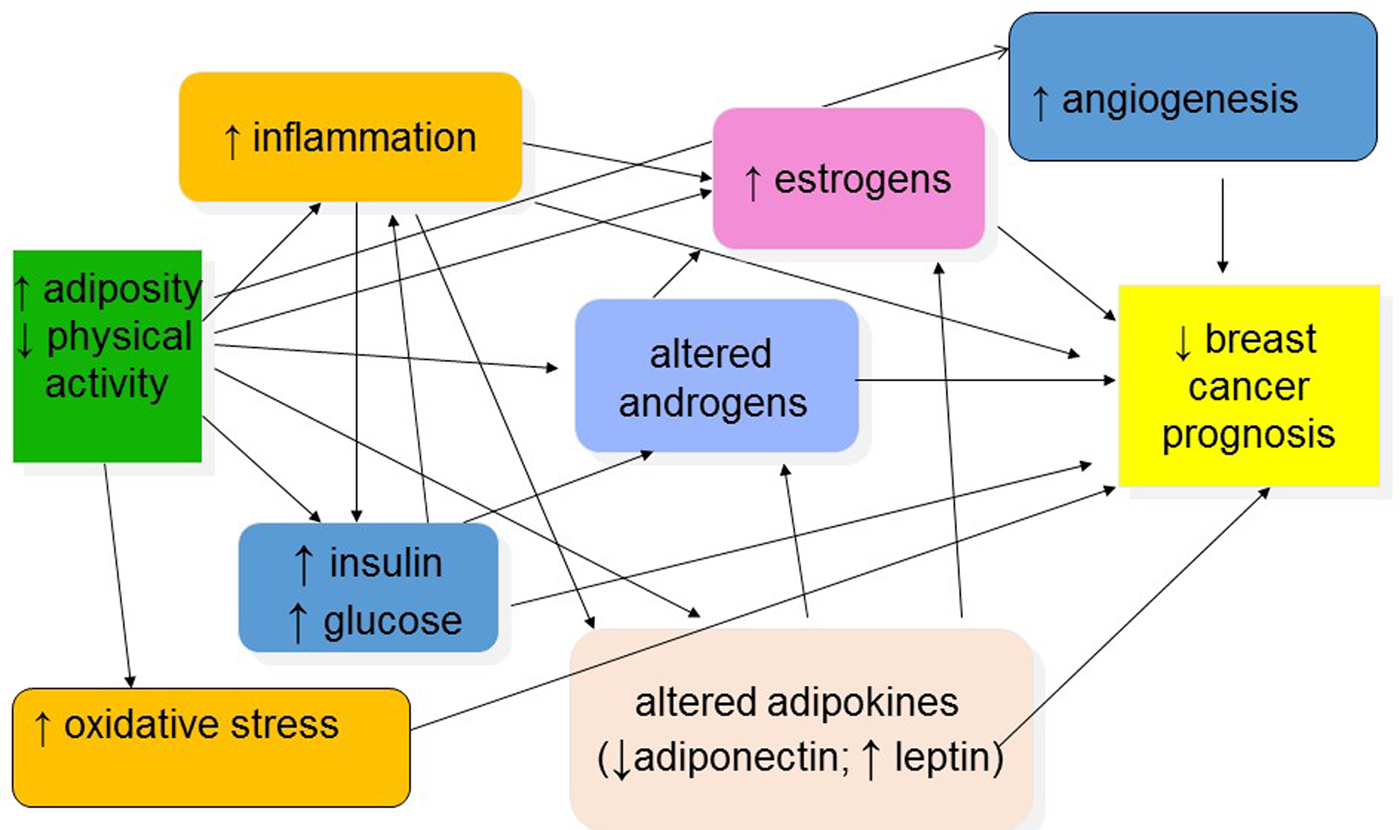
Fig. 2. (Colour online) Potential mechanisms explaining associations between obesity, physical activity and breast cancer prognosis.
Excess oestrogens and androgens are related to reduced prognosis as supported by some(Reference Rock, Flatt and Laughlin47, Reference Micheli, Meneghini and Secreto48) but not all observational data(Reference Goodwin, Ennis and Pritchard49), and by the effect of anti-oestrogens or withdrawal of endogenous oestrogens as adjuvant treatments for breast cancer(Reference Howell50). Overweight and obese breast cancer patients and survivors have elevated blood levels of oestrogens and androgens, and some hormonal therapies have lower efficacy in obese patients(Reference Dignam, Wieand and Johnson18, Reference McTiernan, Rajan and Tworoger51, Reference Ioannides, Barlow and Elwood52).
High levels of insulin and C-peptide are elevated in overweight/obese and sedentary individuals and are reduced with either weight loss or regular exercise(Reference Frank, Sorensen and Yasui53). In a study of 535 women with early-stage breast cancer, women in the highest quartile of fasting insulin levels had a 2·1 times increased risk of distant recurrence compared with those in the lowest quartile (95 % CI 1·2, 3·6, P = 0·01) and a 3·3 times greater risk of death (95 % CI 1·5, 7·0, P = 0·002; adjusted for age, nodal stage, tumour stage, tumour grade, hormone receptor status, adjuvant chemotherapy, adjuvant tamoxifen)(Reference Goodwin, Ennis and Pritchard49). The effect of insulin on survival was independent of BMI. In a cohort of 571 breast cancer survivors diagnosed with stage I–IIIa breast cancer and followed for 5–8 years (median 6 years), fasting C-peptide levels at approximately 3 years post-diagnosis among women without type 2 diabetes were associated with an increased risk of death due to all causes or breast cancer(Reference Irwin, Duggan and Smith54). A 1 ng/ml increase in C-peptide was associated with a 34 % increased risk of death (HR 1·34; 95 % CI 1·07, 1·69; P = 0·013) and a 58 % increased risk of breast cancer death (HR 1·58; 95 % CI 1·15, 2·16, P = 0·0048) after adjustment for confounding variables. Women with C-peptide levels >2·5 ng/ml had an approximate 4-fold increased risk of breast cancer death compared with women with a C-peptide level <1·7 ng/ml (HR 3·90, 95 % CI 1·16, 13·13; P for trend = 0·028). Another report from this study found that decreased adiponectin levels and greater insulin resistance were associated with both increased breast cancer-specific and all-cause mortality(Reference Duggan, Irwin and Xiao55).
Overweight, obesity and lack of physical activity are associated with elevated inflammatory markers including C-reactive protein, serum amyloid A, IL-6, IL-1 and TNF-α, some of which have been shown to be higher in patients with metastatic breast cancer compared with women without breast cancer and with women with early breast cancer(Reference Chlebowski, Aiello and McTiernan23, Reference Pierce, Neuhouser and Wener56). In 731 breast cancer patients diagnosed with early-stage breast cancer, elevated inflammation markers C-reactive protein and serum amyloid A at approximately 31 months post-diagnosis were associated with reduced overall survival (P trend <0·002 and <0·0001, respectively)(Reference Pierce, Ballard-Barbash and Bernstein57). The HR for C-reactive protein and serum amyloid A tertiles suggested a threshold effect on death, rather than a dose–response relationship (HR for highest v lowest tertile: 2·27 and 3·15, respectively). Elevated C-reactive protein and serum amyloid A were also marginally associated with reduced disease-free survival.
Results of small randomised controlled trials suggest that weight loss or exercise interventions can alter circulating levels of obesity-related biomarkers in women with breast cancer. A 2014 systematic review reported statistically significant decreases in both insulin and C-reactive protein for dietary weight loss interventions that produced sizeable weight loss, such as >5 %(Reference Reeves, Terranova and Eakin58). That review also concluded that weight loss in breast cancer survivors reduces leptin and increases adiponectin. A meta-analysis found an overall statistically significant reduction in insulin with exercise interventions, and non-statistically significant reductions in C-reactive protein(Reference Kang, Lee and Suh59). The exercise intervention programmes varied in type (aerobic exercise, resistance exercise, aerobic plus resistance exercise, yoga or Tai Chi), setting (supervised, home-based or combined), intensity (moderate, moderate to vigorous or vigorous) and session length (from 15 to 90 min per session).
All the trials presented in these reviews of exercise or weight loss were of small size (N ≤ 102), and all were of short duration (range from 6 to 24 weeks). Given the small sizes, there is a considerable chance of publication bias.
Clinical impact
Over 297 million women worldwide were estimated to be obese in 2008, and the prevalence of overweight and obesity continue to rise(Reference Finucane, Stevens and Cowan60, 61). Globally, approximately 1·7 million women develop new breast cancer each year(62), of whom an estimated 40 % are obese(Reference Jiralerspong and Goodwin19). In developed countries such as the UK and US, the prevalence of overweight or obesity in postmenopausal breast cancer survivors may approach 65 %(Reference Reeves, Pirie and Beral63, Reference Engmann, Golmakani and Miglioretti64). After diagnosis, breast cancer patients on average decrease their levels of physical activity, and many remain less active for a decade or longer(Reference Irwin and Ainsworth65, Reference Mason, Alfano and Smith66). A US population-based cohort study of women diagnosed with breast cancer showed that by 10 years post-diagnosis, only about one-fifth of survivors engaged in recommended levels of physical activity (≥150 min/week moderate or ≥75 min/week vigorous activity)(33, Reference Mason, Alfano and Smith66). Therefore, overweight/obesity and lack of physical activity are common in breast cancer survivors and may counteract some of the benefits of modern treatments for this disease.
Clinical guidelines
Several organisations have developed guidelines for weight and physical activity for cancer survivors, including breast cancer survivors.
The American Cancer Society/American Society for Clinical Oncology guidelines(Reference Runowicz, Leach and Henry1, Reference Rock, Doyle and Demark-Wahnefried67) are based on expert opinion, and include recommendations that clinicians counsel survivors to: (1) achieve and maintain healthy weight, and if overweight or obese, limit consumption of high-energy foods and beverages and increase physical activity to promote and maintain weight loss; (2) engage in regular physical activity and specifically avoid inactivity and return to normal daily activities as soon as possible after diagnosis, aim for ≥150 min of moderate or 75 min vigorous aerobic exercise per week, and include strength training exercises ≥2 d per week and emphasise strength training for women treated with adjuvant chemotherapy or hormone therapy; and (3) achieve a dietary pattern that is high in vegetables, fruit, whole grains and legumes; low in saturated fats; and limited in alcohol consumption.
The 2008 United States Department of Health and Human Services Physical Activity Guidelines included recommendations for physical activity for individuals who have had a diagnosis of cancer, which was to follow guidelines for adults, if possible(33). In 2018, these guidelines will be updated based on the 2018 United States Department of Health and Human Services Physical Activity Guidelines Advisory Committee Report. The American College of Sports Medicine published recommendations for physical activity for cancer survivors, with detailed guidance for specific types of cancer patients and survivors including the stage of disease, the phase of treatment and co-morbidities(Reference Schmitz, Courneya and Matthews68). The World Cancer Research Fund guidance for breast cancer survivors is to follow cancer prevention guidelines(69). For weight and physical activity, this includes: maintain body weight within the normal range, avoid weight gain, be moderately physically active (such as brisk walking) for at least 30 min every day; as fitness improves, aim for 60 min or more of moderate, or for 30 min or more of vigorous, physical activity every day; limit sedentary habits such as watching television; limit consumption of energy-dense foods (foods high in fats and/or added sugars and/or low in fibre); and avoid sugary drinks.
Research needs
While a large number of prospective observational studies have investigated the association between BMI or other weight indicators and breast cancer prognosis, none have completely controlled for potential confounding effects of disease spread, treatment type and completion of treatment. Therefore, more rigorous sources of data are needed. In particular, randomised clinical trials are needed to determine the effects and safety of weight loss interventions on breast cancer survival and related variables. There is a need to test diets in addition to standard reduced energy/reduced fat, such as reduced carbohydrate diets and alternate fasting diet. The effects on survival of weight loss medications in addition to behavioural weight loss interventions need to be tested. The effect of physical activity interventions on survival needs to be tested. Trials in patients with advanced cancer/metastatic disease are needed, as are intervention trials in patients with specific types of breast cancers and particular treatment exercises.
Given the long-term survival of many breast cancer patients, but with continuing risk of recurrence, present prospective cohort studies should be extended for follow-up for at least 20 years. The role of sedentary behaviours on breast cancer survival should be investigated in cohort studies. Finally, the associations of weight, physical activity and sedentary lifestyles on breast cancer prognosis in understudied, underserved populations should be investigated, such as women from racial/ethnic minorities, young and aged women, and women with co-morbidities.
Conclusion
Observational data show strong evidence that increased adiposity and insufficient physical activity are associated with decreased prognosis in women with breast cancer. There is also a suggestion of reduced prognosis in underweight breast cancer survivors. Both weight gain and unexplained weight loss are associated with lower survival in women with breast cancer. The worldwide trends in increasing overweight and obesity and decreased physical activity may lead to an increasing proportion of women with breast cancer being overweight, obese and sedentary, thus the clinical implications are significant.
There have been no randomised clinical trials testing the effect of weight loss or physical activity interventions on recurrence or survival in overweight or obese breast cancer patients. In the absence of clinical trial data, most individual patients should be advised to avoid weight gain through the cancer treatment process. In addition, weight loss through dietary change is probably safe, and perhaps helpful, for overweight and obese breast cancer survivors who are otherwise healthy. Finally, most breast cancer survivors should be able to perform regular physical activity with the goal of achieving recommended levels.
Financial support
Grants from the Breast Cancer Research Foundation (BCRF-16-106 and BCRF-17-105). The Breast Cancer Research Foundation had no role in the design, analysis or writing of this article.
Conflicts of interest
None.
Authorship
A. McT. was responsible for the concept, writing and review of this manuscript.





