The unique characteristics of ultra-marathon events have enticed endurance enthusiasts to attempt such challenges, as evidenced by the substantial growth of ultra-endurance sports over the past decade( Reference Knoth, Knechtle and Rust 1 ), and an increased number of single- and multi-stage ultra-endurance running events conducted worldwide (http://www.racingtheplanet.com). Even though the popularity of ultra-endurance running has risen, research into the physiological and metabolic demands of such an extreme sport is scarce( Reference Murray and Costa 2 ), with limited studies investigating the dietary habits, alongside nutritional and hydration status of ultra-runners during these events. To date, only one observational study has comprehensively assessed nutritional and hydration status along with the dietary intake of ultra-runners during a multi-stage ultra-marathon competition( Reference Costa, Swancott and Gill 3 , Reference Costa, Teixiera and Rama 4 ), while other studies have mainly focused on the assessment of specific nutrient or hydration variables during shorter single-stage events( Reference Kruseman, Bucher and Bovard 5 , Reference Hoffman, Hew-Butler and Stuempfle 6 ).
Previously, studies investigating the dietary habits of endurance runners during a mountain marathon( Reference Kruseman, Bucher and Bovard 5 ) and a 225 km multi-stage ultra-marathon( Reference Costa, Swancott and Gill 3 ) have reported suboptimal carbohydrate intake, with the latter also reporting compromised energy intake throughout the competition. In both studies, the participants were not able to consume sufficient carbohydrates during running (31 and 24 g/h, respectively) to meet the benchmark recommendations( 7 , Reference Jeukendrup 8 ). Indeed, carbohydrate intakes of 30–60 g/h are advised for endurance sports( 7 ), with even higher levels (i.e. up to 90 g/h and a glucose:fructose ratio of 2:1) being advocated for exercise bouts lasting more than 3 h( Reference Jeukendrup and McLaughlin 9 , Reference Burke, Hawley and Wong 10 ). Such recommendations have been associated with improved oxidation efficiency, reduced fatigue and enhanced exercise performance( Reference Jeukendrup 11 ). Moreover, the compromised total daily intakes of energy and carbohydrate observed in all ultra-runners during the multi-stage ultra-marathon event were found to be accompanied by pronounced levels of urinary ketones as the competition progressed and to be positively associated with the quality of recovery in between the stages( Reference Costa, Swancott and Gill 3 ). Such findings suggest that difficulties in meeting the benchmark recommendations may be associated with a variety of symptoms and practical barrier factors. These may include the lack of nutrition education, cultural norms within ultra-endurance sports, development of unintentional symptoms (e.g. appetite suppression, gastrointestinal symptoms and injury) and/or practical–logistical issues (e.g. lack of food preparation facilities, equipment, time and/or motivation), which may limit the total intake of foods and fluids during periods of increased requirements( Reference Costa, Swancott and Gill 3 , Reference Broad and Cox 12 – Reference Zalcman, Guarita and Juzwiak 14 ).
In comparison with those conducted on nutritional intake and status, a greater but still limited number of studies have investigated the fluid intake habits and hydration status of ultra-runners during single- and multi-stage ultra-marathon competitions in both thermoneutral and hot environmental conditions( Reference Costa, Teixiera and Rama 4 , Reference Kruseman, Bucher and Bovard 5 , Reference Noakes, Sharwood and Speedy 15 ). Dehydration is a common feature of endurance running( Reference Sawka and Burke 16 – Reference Goulet 18 ), with mild-to-severe hypohydration being observed after single-stage (up to 6·0 % exercise-induced body mass (BM) loss)( Reference Hoffman, Hew-Butler and Stuempfle 6 ) and multi-stage (up to 5·5 % exercise-induced BM loss)( Reference Costa, Teixiera and Rama 4 ) ultra-marathon events and with faster runners generally exhibiting the greatest exercise-induced BM losses( Reference Costa, Teixiera and Rama 4 , Reference Noakes 19 ). On a concerning note, a large number of endurance running studies have also reported evidence of fluid overload, with both asymptomatic and symptomatic hyponatraemia being documented( Reference Costa, Teixiera and Rama 4 , Reference Hoffman, Hew-Butler and Stuempfle 6 , Reference Noakes, Sharwood and Speedy 15 , Reference Hoffman, Fogard and Winger 20 ), especially in slower runners( Reference Noakes, Sharwood and Speedy 15 , Reference Hoffman, Fogard and Winger 20 , Reference Hew-Butler, Ayus and Kipps 21 ). From a medical perspective, hyperhydration (and potential incidence of hyponatraemia) appears to be more of a clinically significant issue than acute episodes of hypohydration in such events( Reference Hew-Butler, Ayus and Kipps 21 ). In most cases, medical attention and intervention are required, and an increasing concern is currently being felt in the field. Indeed, of the total number of ultra-runners sampled during a multi-stage ultra-marathon competition conducted in a hot ambient environment, 42 % demonstrated plasma Na concentrations indicative of hyponatraemia during the course of the competition( Reference Costa, Teixiera and Rama 4 ). Such outcomes were achieved by ultra-runners consuming a daily average intake of 114 ml/kg BM per d of water (from foods and fluids), of which 732 ml/h was consumed, on average, during running. Interestingly, of all the fluids consumed, 72 % was plain water. These are similar to observations recorded during a single-stage ultra-marathon competition( Reference Hoffman, Hew-Butler and Stuempfle 6 , Reference Hoffman, Fogard and Winger 20 ) and are classical drinking behaviours for the development of clinically significant incidence of hyponatraemia( Reference Noakes, Sharwood and Speedy 15 , Reference Hew-Butler, Ayus and Kipps 21 ).
Despite a limited number of studies investigating nutritional and hydration status during the course of an ultra-marathon competition, no study has previously investigated the dietary habits of ultra-runners during a 24 h continuous ultra-marathon or the impact on nutritional and/or hydration status. The multitude of stressors encountered by ultra-runners (e.g. physical exertion, phases of food and fluid rationing, sleep deprivation and/or environmental extremes) that accompany such an event, individually or in combination, have the potential to substantially increase nutritional requirements and/or exacerbate factors that would limit overall food and fluid ingestion( Reference Rehrer, Brouns and Beckers 13 , Reference de Graaf, Blom and Smeets 22 , Reference Park and Bloom 23 ). Information on the impact of such an event on nutritional and hydration status would provide valuable insights to field practitioners, supporting this unique athlete population in preparation for and during actual events. Moreover, considering that most of the nutritional recommendations for endurance exercise are derived from controlled laboratory settings, generally among highly trained elite athletes, and over shorter exercise durations, it is plausible that the current recommendations may need adjusting to cater for the unintentional symptomology, real-life practical barriers and specific race characteristics (e.g. degree of self-sufficiency, environmental conditions and/or course topography) experienced by ultra-endurance competitors. With this in mind, in the present study, we aimed to assess the adequacy of energy, macronutrient and water intakes of ultra-runners competing in a 24 h ultra-marathon as well as monitor their gastrointestinal symptoms and appetite during the event.
Methods
Setting and participants
The present study was conducted during the 2011 and 2012 Glenmore24 Trail Race (http://www.glenmore24.com), held during the first week of September, in the Cairngorms National Park, Scottish Highlands, UK (ambient temperature range: 0–20°C in 2011 and 3–19°C in 2012; relative humidity range: 54–82 %). This ultra-marathon event consisted of a continuous 24 h period, whereby the participants attempted to achieve the greatest distance possible within the time frame. The event was routed over a 6 km looped course on a variety of off-road terrains, including trails, paths and grasslands, at an altitude averaging 342 (sd 303) m above the sea level. The distance covered by the study cohort ranged from 122 to 208 km.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Coventry University Ethics Committee. Written informed consent was obtained from all the subjects before the commencement of the study. A convenience sampling observational cohort was studied, whereby twenty-five (male: n 19 and female: n 6) of the forty-eight ultra-endurance runners (UER) entering the event volunteered to participate in the study (age 39 (sd 7) years, height 177 (sd 8) cm and BM 78 (sd 11) kg). Additionally, seventeen (male n 6 and female n 11) individuals who did not compete (absence of exercise stress) but were present during the event programme (i.e. event spectators) volunteered to participate in the study, after giving informed consent, as part of the control (CON) group (age 32 (sd 11) years, height 170 (sd 10) cm and BM 69 (sd 13) kg). The CON group was included for comparative purposes only, thus allowing to determine the impact of participating in a 24 h ultra-marathon on dietary intake and nutritional and hydration status against those of free-living healthy individuals. Additionally, for the purpose of data analysis, besides standard sex comparisons, the participants were also divided into two groups based on the overall distance covered( Reference Costa, Teixiera and Rama 4 ): a slow-running (SR) group, which completed the entire time period through a mixture of walking and running with occasional feeding breaks and/or sleep ( < 160 km), and a fast-running (FR) group, which completed the majority of the time period running with minimal breaks ( ≥ 160 km). This criterion was predetermined, and the participants were grouped according to their overall race time, before data analysis.
Study design and data collection
The present ultra-marathon was self-sufficient, whereby the participants (both UER and CON) planned and made arrangements for their own ad libitum foods and fluids during the course of the competition. Only plain water and electrolyte supplementation (Active Hydration, Nuun & Company, Inc.) were provided by the race organisers ad libitum every 3 km. The participants were advised to adhere to their programmed habitual dietary practices throughout the ultra-marathon competition. Total energy expenditure, metabolic equivalents (MET) and sleep activity during the ultra-marathon were measured by triaxial accelerometry, which also included measurements of heat flux, skin temperature and galvanic skin responses (SenseWear 7.0, BodyMedia, Inc.), as has been used previously to aid in nutritional intervention during a mountain-based multi-stage ultra-marathon( Reference Britton, Dempster and Moore 24 ). The device was attached firmly to the upper arm of the participants, over the mid-point of the triceps muscle, during the measurement period. Data were processed using the latest proprietary algorithms available in the software (version 7.0, algorithm version 2.2.4).
The height of the participants was measured 1 h before the commencement of the competition (11.00–12.00 hours) using a stadiometer, and then the participants were asked to provide a mid-flow urine sample in 30 ml universal tubes (HR 120-EC, A&D Instruments), before BM measurements using calibrated electronic scales (BF510, Omron Healthcare) placed on a hard levelled surface. The participants were then required to sit for 10 min before blood sampling. Whole-blood samples were collected into one lithium heparin vacutainer tube (6 ml, 1·5 IU/ml heparin; Becton Dickinson) by venepuncture without venestasis from an antecubital vein using a 21G butterfly syringe. BM was re-measured in those participants who needed to urinate before the start of the competition. Immediately after the competition (12.00 hours the following day) and before any foods or fluids could be consumed, BM was measured, followed by blood sampling, and then the participants were asked to provide a mid-flow urine sample at their earliest convenience.
To determine total food and fluid intakes during the 24 h period of the competition, trained researchers conducted a standardised structured interview among the participants and their support crews 1 h after the competition. However, before the competition, the participants and their support crews were educated and instructed to record in detail all foods and fluids ingested during the competition period in real time, which included specified food and beverage quantities (e.g. grams, millilitres, litres and/or portions) and qualities (e.g. type of foods and beverages and brands of foods and beverages). Additionally, the participants and their support crews were instructed to keep the food and beverage packages of all the consumed foods and fluids, which were collected by researchers. The addition of carbohydrate, protein and/or mixed macronutrient supplementations to foods and fluids was also recorded, and their intakes were combined with nutritional intake. Subjective appetite sensation (increase in or loss of appetite) and gastrointestinal symptoms during running were evaluated by trained researchers using a research generated symptomology tool( Reference Pfeiffer, Stellingwerff and Hodgson 25 ). Additionally, to monitor carbohydrate adequacy( Reference Robson-Ansley, Gleeson and Ansley 26 ), urine reagent strips (Multistix® 10SG Urinalysis strips, Siemens Healthcare Diagnostic) were used to detect urinary ketones (i.e. acetoacetic acid) in the pre- and post-competition urine samples.
Dietary analysis
Total energy, macronutrient and water intakes from foods and fluids during the ultra-marathon were calculated by a trained researcher using the Dietplan6 dietary analysis software (version 6.60, Forestfield Software). To improve the validity of the dietary analysis, all the nutritional information gathered from the food and beverage packages during the interview process was entered into the dietary analysis software program. In addition, to improve the reliability of the dietary analysis, all the completed dietary interview logs were blindly analysed by a second trained researcher. The overall mean inter-observer CV for energy, macronutrient and water variables analysed were 0·8, 1·4 and 0·5 %, respectively.
Hydration status assessment
Exercise-induced BM change (pre- to post-competition BM difference) was determined from the pre- and post-competition BM values. The pre- and post-competition plasma osmolality (POsmol) values were determined from 50 μl lithium heparin plasma samples in duplicate by freezing point osmometry (Osmomat 030, Gonotec), as recommended previously( Reference Seifarth, Miertschischk and Hahn 27 ). The CV for POsmol was 3·5 %. Whole-blood Hb concentration and haematocrit were used to estimate changes in plasma volume (PV) relative to the pre-competition values( Reference Dill and Costill 28 , Reference Maughan, Leipers, Greaves, Eston and Reilly 29 ), as reported previously( Reference Costa, Crockford and Moore 30 , Reference James and Shirreffs 31 ).
Data analysis
Data reported in the text, tables and figures are presented as means and standard deviations, unless otherwise specified. Data were processed and analysed in SPSS for Windows (version 17.0.2; SPSS Inc.). Considering the potential influence of individual BM differences (especially in relation to sex and training status) on dietary intake variables, data analysis was carried out on total values and corrected for BM, as reported previously( Reference Costa, Swancott and Gill 3 , Reference Costa, Teixiera and Rama 4 , Reference Cox, Snow and Burke 32 ). Diagnostic checks (Shapiro–Wilk test of normality and Levene's homogeneity of variance test) were performed before applying parametric statistics. Where data violated the assumption of normality, data were analysed using non-parametric equivalents. Paired-sample t tests were used to determine energy balance (intake v. expenditure) and pre- to post-competition variable differences, while independent-sample t tests were used for group comparisons (UER v. CON) and subgroup comparisons (sex and distance covered). Additionally, Spearman's correlation coefficient was used to determine relationships between the variables. The acceptance level of significance was set at P <0·05. Descriptive statistics were used to evaluate the quality of fluids ingested, urinary ketones, gastrointestinal symptomology and appetite.
Results
Body mass
Significant BM loss occurred at pre- to post-competition time points (P =0·001) in the UER (pre-competition: 78·2 (sd 11·5) kg; post-competition: 77·0 (sd 11·7) kg). Exercise-induced BM loss in the UER ranged from − 2·4 to 4·4 % (Fig. 1). No difference in exercise-induced BM loss was observed between the sexes (male: 1·7 (sd 2·0) %; female: 1·2 (sd 1·9) %) and distance covered (SR: 1·7 (sd 1·1) %; FR: 2·1 (sd 2·1) %). No significant change in BM was observed in the CON group at pre- to post-competition time points.
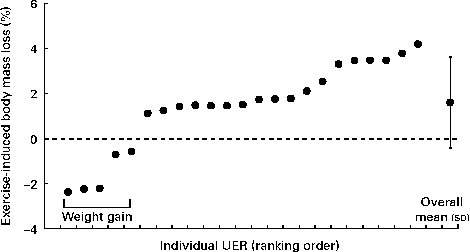
Fig. 1 Exercise-induced body mass loss of ultra-endurance runners (UER, ●) participating in a 24 h ultra-marathon competition. Individual responses: UER.
Metabolic equivalents and energy balance
The UER exhibited greater average MET compared with the CON group (7·2 (sd 1·2) v. 2·1 (sd 0·4) MET, respectively; P <0·001). During the course of the 24 h ultra-marathon, 73 and 28 % of the time, the UER were active at >6 and >9 MET, respectively. No difference was observed in MET activity between the sexes. However, the FR group exhibited greater activity at >6 MET (85 % of the time) compared with the SR group (64 % of the time; P =0·005). Sleep activities were detected in only five UER (1 h 37 min (sd 0 h 32 min)), with no difference being observed between the sexes and distance covered.
The total energy expenditure and intake of the UER were 55 (sd 11) MJ (equivalent to 2·3 MJ/h and fluctuated along the 24 h measurement period) and 20 (sd 11) MJ, respectively (P <0·001; Fig. 2(a) and (b)), while those of the CON group were 12 (sd 1) and 14 (sd 5) MJ (P <0·001 v. UER), respectively. Significantly greater energy deficits were observed in the UER than in the CON group (P <0·001). Male ultra-runners exhibited greater energy expenditure (58 (sd 10) MJ (equivalent to 2·4 MJ/h); P =0·036) and energy intake (23 (sd 12) MJ; P =0·05) compared with the female ultra-runners (45 (sd 8) MJ (equivalent to 1·9 MJ/h) and 13 (sd 5) MJ, respectively), but no difference in energy deficit was observed. When corrected for BM, no differences in energy expenditure, energy intake and energy deficit were observed between the sexes. No differences in total and corrected energy expenditure, energy intake and energy deficit were observed for the distance covered.
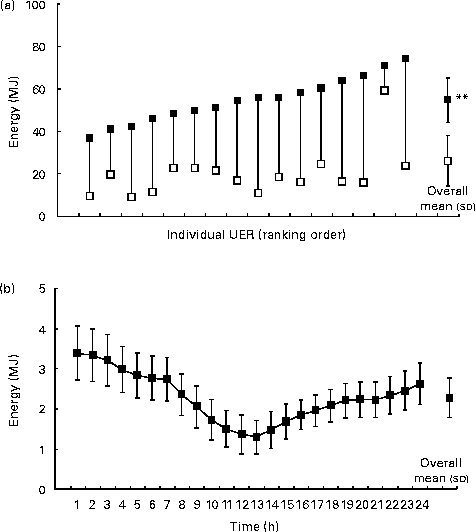
Fig. 2 (a) Energy balance and (b) distribution of energy expenditure of ultra-endurance runners (UER) participating in a 24 h ultra-marathon competition. Individual responses: ■, energy expenditure; □, energy intake. Values are means, with standard deviations represented by vertical bars. ** Mean value was significantly different from that of energy intake (P <0·01).
Macronutrient intakes
The total and corrected macronutrient intakes of the UER are reported in Table 1, and the values depict large individual variation in protein, carbohydrate and fat intakes. Additionally, of the total amount of carbohydrates consumed by the UER, 69 % was from mono/di/oligosaccharide sources, while 31 % was from polysaccharide sources. Male ultra-runners exhibited higher total protein (P =0·039) and carbohydrate (P =0·05) intakes compared with the female ultra-runners, but when these were corrected for BM, no significant differences were observed. No differences in total and corrected macronutrient intakes were observed for the distance covered.
Table 1 Macronutrient (total and corrected for body mass (BM) and intake rate of carbohydrate) intake from foods and fluids recorded in ultra-endurance runners (UER) participating in a 24 h ultra-marathon competition (Mean values and standard deviations)
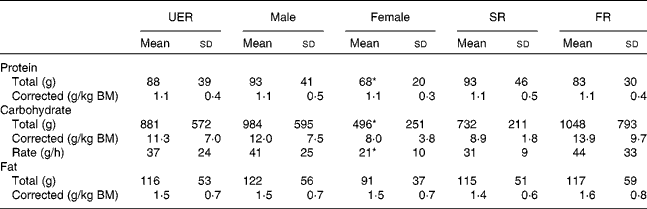
SR, slow running group ( < 160 km distance covered); FR, fast running group ( ≥ 160 km distance covered).
* Mean value was significantly different from that of the male runners (P <0·05).
No acetoacetic acid was detected in the pre-competition urine samples of the UER and the CON group. However, the presence of acetoacetic acid (range: 0·5–8·0 mmol/l) in the post-competition urine samples was evident in 90 % of the UER. A significant Spearman's correlation was observed between total carbohydrate (r − 0·471, n 18, P =0·048) intake and the presence of urinary acetoacetic acid in the post-competition urine samples.
Fluid intake
The fluid intake of the UER during the course of the ultra-marathon is reported in Table 2, and the values depict large individual variation. Plain water accounted for 62 % of the total daily fluids consumed by the UER, with the remaining fluid intake (38 %) being from nutrient-rich sources, which included the following (in the order of predominance type): glucose solutions (consumed by 68 % of the UER); soft drinks (47 %); milk and alternatives (38 %); fruit juices (16 %); carbohydrate–protein mixes (16 %); protein solutions (5 %). In addition, 11 % of the UER were found to consume only plain water during the entire ultra-marathon.
Table 2 Water (total, corrected for body mass (BM) and intake rate) intake from foods and fluids recorded in ultra-endurance runners (UER) participating in a 24 h ultra-marathon competition (Mean values and standard deviations)
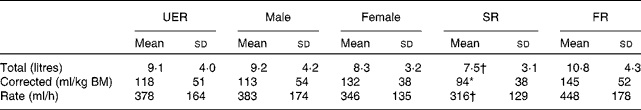
SR, slow running group ( < 160 km distance covered); FR, fast running group ( ≥ 160 km distance covered).
* Mean value was significantly different from that of the FR group (P <0·05).
† Mean values tended to be different from those of the FR group (P =0·077).
No differences in total and corrected water intakes from foods and fluids were observed between the sexes. However, of the total amount of fluids ingested, female ultra-runners were found to consume a greater proportion of plain water (83 %) compared with the male ultra-runners (52 %; P =0·017). Moreover, the FR group exhibited a tendency for higher total (P =0·077) water intake from foods and fluids compared with the SR group. When corrected for BM, the FR group exhibited significantly higher water intake compared with the SR group (P =0·024). No difference in the quality of fluids ingested was observed between the SR group (61 % plain water and 39 % nutrient-rich fluids) and the FR group (59 % plain water and 41 % nutrient-rich fluids).
Hydration status
No significant changes in the POsmol values of the UER were observed at pre- to post-competition time points (Fig. 3(a)) and remained within the normal clinical reference range (280–300 mOsmol/kg)( Reference Thomas, Cote and Lawhorne 33 ). In addition, pre- to post-competition decreases (11 (sd 9) mOsmol/kg) in POsmol values were observed in 44 % of the UER. Compared with those of the CON group, the pre- and post-competition POsmol values of the UER were lower (P =0·05 and P <0·001, respectively). No differences in pre- and post-competition POsmol values were observed between the sexes and distance covered. A significant increase in PV values was observed at post-competition time points in the UER (P <0·001; with 80 % of the UER exhibiting PV increases >5 %), while no change in PV values was observed in the CON group (P <0·001 v. UER; Fig. 3(b)). Male ultra-runners exhibited lower increases in PV values (7·2 %) at post-competition time points compared with the female ultra-runners (13·5 %; P <0·05). No difference in PV values was observed for the distance covered.
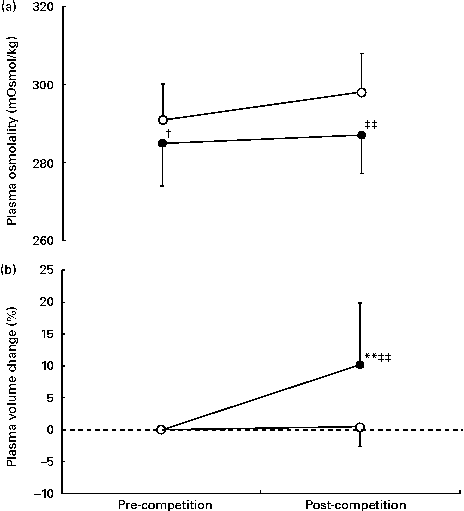
Fig. 3 Change in (a) plasma osmolality and (b) plasma volume of ultra-endurance runners (UER, ●) participating in a 24 h ultra-marathon competition. Values are means, with standard deviations represented by vertical bars. ** Mean value was significantly different from that recorded at pre-competition time points (P <0·01). Mean value was significantly different from that of the control group (○): † P <0·05; †† P <0·01.
Gastrointestinal symptomology and appetite
Gastrointestinal symptoms were a common feature, with 65 % of the UER reporting at least one severe gastrointestinal symptom during the competition. These included the following: nausea; gastrointestinal pain; vomiting; indigestion; bloating; abnormal bowel movements (e.g. urgency to defecate). No difference in the reported rates of gastrointestinal symptoms was observed between the sexes. However, a 2·5-fold greater occurrence of gastrointestinal symptoms was observed in the FR group (P =0·001 v. SR). No associations between gastrointestinal symptoms and energy and carbohydrate intakes were evident. Suppressed appetite was reported by 61 % of the UER during the competition. No difference in the reported rates of appetite suppression was observed between the sexes and distance covered. Additionally, no associations were observed between appetite and energy and carbohydrate intakes. No gastrointestinal symptoms or suppressed appetite were reported by the CON group.
Discussion
The aims of the present study were to assess the adequacy of energy, nutrient and water intakes of ultra-runners competing in a 24 h continuous ultra-marathon and to monitor their gastrointestinal symptoms and appetite during the competition. The findings highlight a substantial energy deficit in all the UER during the ultra-marathon, despite ad libitum intake. Additionally, with the exception of one UER, all other UER failed to meet the benchmark recommendations for carbohydrate intake during endurance exercise, with reports of gastrointestinal symptoms and suppressed appetite generally being high and likely contributing factors. The presence of urinary ketones, albeit in the samples of 90 % of the UER, further highlights the inability of the UER to meet the carbohydrate energy demands of such an extreme event, despite competing at a modest exercise intensity. The findings also highlight increases in PV values at post-competition time points in the majority (80 %) of the UER (with the maintenance of POsmol values also being observed at post-competition time points). Therefore, participation in a 24 h ultra-marathon competition is more likely to promote a situation of hyperhydration rather than hypohydration.
The present study is the first to determine total energy expenditure during a 24 h continuous ultra-marathon using triaxial accelerometry (with adjunct heat flux, skin temperature and galvanic skin response measurements), in which its validity and reliability were comparable to those of doubly labelled water techniques and practically ideal for determining energy expenditure in field-based settings( Reference Britton, Dempster and Moore 24 , Reference Johannsen, Calabro and Stewart 34 ). The results revealed energy expenditure in the UER to range from 36·7 to 74·2 MJ and to be the highest recorded daily energy expenditure in the literature in comparison with that recorded in a multi-stage bicycle competition( Reference Saris, van Erp-Baart and Brouns 35 ), 2 d of continuous stationary cycling( Reference Stewart and Stewart 36 ), a multi-sport ultra-endurance race( Reference Colombani, Mannhart and Wenk 37 ) and multi-day expeditions in both mountainous and arctic terrains( Reference Koehler, Huelsemann and de Marees 38 – Reference Stroud, Coward and Sawyer 41 ). Moreover, the distribution of expended energy varied along the 24 h period in three distinct sections associated with running, feeding and sleeping activities (Fig. 2(b)). These included the following: predominantly running periods (the 1st to the 7th hour (12.00–19.00 hours) and the 15th to the 24th hour (03.00–12.00 hours)); a resting meal period for the majority of the UER (the 8th to the 10th hour (19.00–22.00 hours)); a sleep period, but only in five UER (the 11th to the 14th hour (22.00–03.00 hours)). However, it must be acknowledged that the UER who achieved the furthest distances spent the majority of the 24 h running, with no distinct changes in energy expenditure being observed throughout the ultra-marathon. Even though the energy expenditure per hour was not exceptionally large (2·3 MJ/h), the continuity over the 24 h resulted in an exaggerated expense. A novel finding in the present study was the substantial energy deficit in all the UER (ranging from 11·3 to 50·4 MJ), despite ad libitum intake. Previously, a 225 km multi-stage ultra-marathon resulted in an inability of the ultra-runners to consistently meet the daily energy demands, possibly because of unintentional symptoms and/or real-life factors associated with limiting total food and fluid intakes( Reference Costa, Swancott and Gill 3 ). Similarly, in the present study, irrespective of the sex or distance covered, it was found that the UER inevitably were unlikely to meet such high energy demands due to similar factors (65 % reporting gastrointestinal symptoms and 61 % reporting appetite suppression) and in addition the difficulties in consuming the volumes of food and beverage required to meet such extreme energy targets.
Despite the large energy deficit, BM losses in the UER were modest and lower than those reported after a shorter one-stage competition (2·5 %)( Reference Knechtle, Knechtle and Wirth 42 ) and a multi-stage ultra-marathon competition (2·4 %)( Reference Costa, Teixiera and Rama 4 ). Although acute changes in BM have previously been reported to be associated with acute changes in body water( Reference Sawka and Burke 16 ), the observed reductions in BM at post-competition time points are more likely due to energy substrate losses (solid losses resulting from a substantial negative glycaemic and N balance) on this occasion and not indicative of body water losses( Reference Maughan, Shirreffs and Leiper 43 ). This observation is similar to the outcomes of a 100 km one-stage ultra-marathon competition( Reference Knechtle, Knechtle and Wirth 42 ) and a 48 h period of energy restriction without fluid restriction( Reference Costa, Harper-Smith and Oliver 44 ), whereby substantial BM loss has been observed to occur without changes in hydration status. Taking into account the increases in PV values (10·2 %) observed in the present study, it is suggested that the true solid losses may have been masked by extracellular fluid gains and/or retention and thus underdetermined( Reference Costa, Swancott and Gill 3 ). Future studies could potentially assess changes in endogenous substrate oxidation and body composition during ultra-endurance events, with and without nutritional intervention, to determine the impact of acute energy deficits on glucose and lipid metabolism, muscle protein balance and bone density, with the aim of identifying potential clinically significant issues (e.g. short- and long-term impact on muscle wasting and bone density of UER).
Forward strategies to support ultra-runners meeting high energy requirements could possibly focus on a combination of appropriate carbo-loading protocols in the days leading up to the competition, gut training and meal planning, aimed at maximising carbohydrate stores before the competition and providing exogenous energy substrate during the competition. Unfortunately, on this occasion, the determination of pre-competition nutritional intake was not established due to practical difficulties in obtaining an accurate self-reported dietary log by participants in the days leading up to the ultra-marathon( Reference Burke, Cox and Culmmings 45 ). However, a particular strength of the present study was the methodological approach used to collect dietary intake data during the ultra-marathon event. Provisions of dietary assessment education and training to the participants and their support crews, real-time intake logging, collection of food and beverage packages and structured interview were strategies applied to reduced the error risk in dietary assessment. Additionally, use of a validated dietary analysis software program, inclusion of packaging information and reliability check by a second trained researcher showing low CV were strategies applied to reduce the error risk in dietary analysis.
In the present study, the macronutrient intakes of the UER ranged from 0·5 to 2·0 g/kg BM of protein, 4·4 to 16·3 g/kg BM of carbohydrate (with one UER consuming 37·7 g/kg BM) and 0·7–3·1 g/kg BM fat, on average, corresponding to 8, 70 and 22 % of the total energy intake, respectively. Even though this macronutrient energy distribution constitutes a high-carbohydrate diet and a greater proportion of carbohydrate was consumed compared with that consumed in previous ultra-marathon studies (7·8 g/kg BM per d)( Reference Costa, Swancott and Gill 3 ), they still appear insufficient to meet the carbohydrate needs of the UER, as evidenced by the presence of urinary ketones in the post-competition samples of the majority of the UER, despite generally meeting the benchmark daily carbohydrate intakes (10–12 g/kg BM per d) for endurance exercise( Reference Burke, Hawley and Wong 10 ). This highlights the need for further investigations into the carbohydrate absorption and oxidation rates of ultra-endurance athletes during ultra-endurance sports and subsequent development of population-specific guidelines and recommendations. Moreover, carbohydrate intake rates during the competition ranged from 16 to 53 g/h (with one UER managing to consume 126 g/h), which are similar to those recorded during previous triathlon, mountain marathon and multi-stage ultra-marathon studies( Reference Costa, Teixiera and Rama 4 , Reference Kruseman, Bucher and Bovard 5 , Reference Cox, Snow and Burke 32 ) and below the recommendations( Reference Burke, Hawley and Wong 10 ). Alongside practical factors influencing total food and fluid ingestion, exercise-induced splanchnic hypoperfusion, splanchnic ischaemia, and running impact on the gastrointestinal integrity and splanchnic areas are all likely factors to explain the high rates of gastrointestinal symptoms and appetite suppression reported by the majority of the UER and probably contributed to a suboptimal carbohydrate intake( Reference Rehrer, Brouns and Beckers 13 , Reference French and Cecil 46 – Reference van Wijck, Lenaerts and Grootjans 49 ).
Recently, updates made in the benchmark recommendations for carbohydrate intake during exercise have indicated carbohydrate intakes >90 g/h of different carbohydrate blends (glucose:fructose ratio of 2:1) for exercise lasting more than 3 h( Reference Burke, Hawley and Wong 10 , Reference Jeukendrup 11 ). Even though such high recommendations have been based on a limited number of observational and laboratory studies in highly trained individuals, such advice would benefit the ultra-marathon population, as tolerance to intakes greater than the current carbohydrate intakes per hour over a 24 h period would contribute towards the reduction of the overall energy (and glycaemic) deficit burden. However, tolerance to such high rates of carbohydrate intake during strenuous exercise would require gut adaptations. Therefore, investigation and development of ‘gut training’ protocols are warranted and predicted to be substantially nutritionally beneficial to the ultra-endurance population. For example, similar to the present findings, it has previously been established that faster runners (generally with higher training levels and accustomed to feeding during running) tolerate more food and fluid ingestion rates during running stress compared with slower runners( Reference Costa, Swancott and Gill 3 , Reference Kruseman, Bucher and Bovard 5 , Reference Peters and Goetzsche 50 , Reference Rehrer 51 ). Alternatively, meal planning during the 24 h event (i.e. ingestion of programmed meals and snacks) instead of consistent intake on an hourly rate may lower the gastrointestinal burden and taste fatigue, but this requires further investigation and substantiation.
Water intake from foods and fluids during the ultra-marathon in the UER ranged from 3·2 to 16·2 litres, equivalent to 152–673 ml/h, while plain water was predominantly consumed fluid. Despite the relatively low water intake rates, ingested volumes appeared to be sufficient to prevent significant degrees of hypohydration in the majority of the UER, but interestingly were substantial enough to promote increases in extracellular water, as evidenced by PV increases at pre- to post-competition time points in 80 % of the UER. This observation is probably due to water intake being above the overall losses and/or retention mechanisms (i.e. metabolic water production, exercise-induced increase in plasma albumin levels and subsequent increased circulatory osmotic pressure, and up-regulation of the levels of fluid regulatory hormones known to increase with exercise stress)( Reference Wendt, van Loon and Lichtenbelt 52 – Reference Hew-Butler, Noakes and Soldin 55 ). Contrary to the commonly reported increases in POsmol values (with adjunct reductions in PV values) after prolonged physical exertion due to extracellular water losses( Reference Costa, Teixiera and Rama 4 ), POsmol values did not change at pre- to post-competition time points in the UER, with 44 % of the UER actually exhibiting reductions in POsmol values. These findings possibly indicate that hypohydration is not necessarily a key issue in such an extreme event, but potentially hyperhydration may be of greater concern. Taking into account the limitations of POsmol as a hydration marker and the body's drive to maintain normal values even in extreme circumstances (e.g. exercise stress) through multiple mechanisms (i.e. fluid shifts between cellular compartments, renal function, and thermoregulatory and thirst responses)( Reference Maughan, Shirreffs and Leiper 43 , Reference Armstrong, Maughan and Senay 56 ), it is plausible that the degree of POsmol change in those UER who exhibited increases in PV values may have been masked by transient concentrating haemorheologic effects in responses to extreme stress and pronounced energy deficiency (i.e. circulatory release of energy substrates, metabolic by-products and immune factors)( Reference Frayne 57 – Reference Walsh, Gleeson and Shephard 59 ). Indeed, the UER who exhibited weight gains at post-competition time points also exhibited reductions (or maintenance) in POsmol values and the greatest increases in PV values, providing clear evidence for the presence of fluid overload.
Similar fluid intake behaviours have previously been observed during a multi-stage ultra-marathon competition in the heat and been reported to have resulted in 42 % of the participants developing asymptomatic hyponatraemia( Reference Costa, Teixiera and Rama 4 ). Interestingly, on this occasion, the UER who covered the furthest distances consumed greater amounts of water from foods and fluids, but did not necessarily exhibit greater hyperhydration status (i.e. possibly due to greater losses). In accordance with the marathon, single-stage ultra-marathon, multi-stage ultra-marathon and now a 24 h continuous ultra-marathon, such events have clearly demonstrated a high risk of hyperhydration during a competition( Reference Costa, Teixiera and Rama 4 , Reference Hoffman, Hew-Butler and Stuempfle 6 , 7 , Reference Noakes, Sharwood and Speedy 15 , Reference Hew-Butler, Ayus and Kipps 21 , Reference Speedy, Noakes and Rogers 60 ). In the present study, plasma Na concentrations were not measured; therefore, the incidence of hyponatraemia was not evaluated. However, in accordance with the study of Costa et al. ( Reference Costa, Teixiera and Rama 4 ), it is highly likely that the UER who participated in the present study had asymptomatic hyponatraemia. High rates of plain water consumption and increases in extracellular water at pre- to post-competition time points are key factors that have been implicated in the pathophysiological mechanisms of exercise-associated hyponatraemia( Reference Noakes, Sharwood and Speedy 15 , Reference Hew-Butler, Ayus and Kipps 21 , Reference Vrijens and Rehrer 61 ). The results clearly demonstrate the need for appropriate education to ultra-endurance athletes and race organisers, based on environmental conditions and course topography, aimed at maximising energy provision and avoiding any potential clinically significant episodes of too much water intake above the requirements.
Conclusion
The food and fluid intakes of ultra-runners recorded in the present study during a 24 h ultra-marathon appear to be substantially insufficient to meet the energy, carbohydrate and potentially protein needs of the UER, but promote drinking behaviours that may induce a state of water overconsumption. The findings of the present study indicate that nutritional education and intervention by qualified sport and exercise nutritional professionals focused at recreational ultra-runners are warranted. Reflecting on the results of the present study, nutritional education and intervention may include developing strategies and promoting dietary changes aimed at the following: (1) appropriate carbo-loading protocols in the days leading up to the competition; (2) gut training; (3) meal planning during the competition; (4) modifying eating behaviours during periods of food and fluid disinterest, suppressed appetite and gastrointestinal symptoms; (5) appropriate quantity and quality of fluid intake to maintain euhydration, mitigating the development of clinically significant episodes arising from both hypohydration and hyperhydration. A follow-up study should be conducted to evaluate the outcomes of such nutritional education and intervention with regard to dietary practices, nutritional and hydration status, and changes in the body composition indices of ultra-runners in proceeding events.
Acknowledgements
The authors thank all the UER who volunteered to participate in the present study. They acknowledge the Glenmore24 Trail Race (http://www.glenmore24.com) directors Bill Heirs and Mike Adams. They also thank the members of the Coventry University Sport & Exercise Nutritional Support & Research Team (Emily Freeth, Edel Barrett, Katie Wardle, Evelyn Toner, Rhiannon Britton and Yolander Snyder) for their support during the process of sample and data collection; Jane Sheehy and Susie Wilson from Coventry University for their technical support during the course of the study; and Dr Andrew Murray from the SportsScotland Institute of Sport for his medical support.
The study was funded by the Coventry University Sport & Exercise Science Applied Research Group and the Department of Health Professionals.
The authors' contributions are as follows: R. J. S. C. was responsible for the original research idea and overall supervision and management of the research project; R. J. S. C. and S. K. G. contributed to the development of the experimental design; R. J. S. C. and S. K. G. contributed to the analysis of the raw data. All authors contributed to the various aspects of data collection and sample analysis, and the preparation and review of the manuscript. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.







