To the Editor—The intensive care units (ICUs) in most hospitals are high-risk settings for hospital-acquired diarrhea. Patients in the ICU are likely to have numerous comorbidities, to be of older age, and to have concomitant antibiotic use—all major risk factors for Clostridium difficile infection (CDI).Reference Evans and Safdar 1 Human gut flora is composed of trillions of microbes working in a symbiotic relationship with the human immune system to prevent colonization of opportunistic bacteria, often occurring with antibiotic usage and other illnesses. Probiotics, or oral preparations of live microorganisms, can stabilize the gut flora and might prevent CDI.Reference Shen, Maw and Tmanova 2 , Reference Silva, Carneiro and dos Anjos Pultz 3 Though multiple studies and meta-analyses have demonstrated the efficacy of probiotics toward CDI primary prevention,Reference Shen, Maw and Tmanova 2 , Reference Johnston, Ma and Goldenberg 4 – Reference Lau and Chamberlain 6 guidelines of major societies, such as the American College of Gastroenterology (ACG), the Society for Healthcare Epidemiology of America (SHEA), and the Infectious Disease Society of America (IDSA), have not formally recommended probiotic use for primary prevention of CDI in any setting or for any patient demographic.Reference Cohen, Gerding and Johnson 7 , Reference Surawicz, Brandt and Binion 8 Although recent evidence has suggested that probiotics administered close to antibiotic administration in hospitalized patients can reduce the risk of CDI, these studies had numerous exclusion criteria and did not include the vulnerable ICU patient population.Reference Shen, Maw and Tmanova 2
The paradigm seems to be shifting toward probiotic administration for primary CDI prevention in certain populations, as guideline committees are likely calling for further analysis for their next formal recommendations. When the time comes, new recommendations reach physicians in various ways, but formally implementing changes in practice likely requires hospital policy and support by all healthcare providers and personnel. We aimed to determine the proportion of physician providers reluctant to place ICU patients on probiotics, even after educational intervention, support, and endorsement from the hospital medical executive committee (MEC) to do so.
The study was approved by our institutional review board and was conducted as a quality improvement analysis at a 300-bed tertiary community hospital, Wheaton Franciscan Healthcare in Milwaukee, Wisconsin. The MEC endorsed an intervention to place all ICU patients on VSL#3 probiotic (The Living Shield, VSL Pharmaceuticals, Covington, LA) upon admission to the ICU. Three attending intensive care physicians in a 20-bed ICU were champions for the project, and they held an informal verbal discussion with all the remaining ICU attending physicians. No checklist was implemented to monitor individual physician utilization. A dedicated pharmacist was on service for the ICU at all times and was instructed to approach attending physicians requesting a VSL#3 order if one had not been placed. Nurses were also instructed to request probiotic orders if the physician had not done so.
A 9-month period from January 2015 to September 2015 served as the preintervention baseline. A 1-month period of staff education occurred prior to formal tracking starting January 2016 through September 2016. For months 1 through 4, a standard printed check-box order set allowed ordering physicians to select for VSL#3 use. Months 5 through 9 required the ordering physician to electronically enter VSL#3 because the hospital switched to an electronic order set. Rates of probiotic utilization after MEC and pharmacy probiotic intervention were compared to preintervention rates. Month-by-month utilization rates were also compared.
A retrospective review for the 9-month period prior to educational intervention and probiotic recommendation was performed. Daily hospital notes for this 9-month period demonstrated that ~30% of the hospital ICU patients having diarrhea on any given day. In addition, nearly 30% of the CDI cases in the hospital were associated with ICU admissions, whereas <10% of admissions involved an ICU encounter.
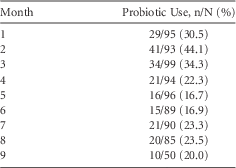
The aggregate physician probiotic utilization rate for the first 9 months after the MEC endorsement and intervention was 26.2%; a total of 207 of 791 ICU patients received the VSL#3 probiotic as outlined in the policy guideline (Table 1A). For the same 9 months the year prior to implementation, the number of ICU patients receiving probiotics was 8.6%, or 71 of 837 patients (Table 1A). Month-by-month percentages from month 1 to month 9 are shown in Table 1B.
TABLE 1A Probiotic Utilization Pre and Post Intervention

NOTE. ICU, intensive care unit.
TABLE 1B Probiotic Utilization Month-by-Month Post Intervention
Our study has several limitations. First, it was performed in a single medical center and during a period of paper and electronic medical record modification. We did not stratify utilization rates based on patient illness or prior history of antibiotic-associated diarrhea (AAD) or CDI. Because we did not measure AAD and CDI rates prior to and after recommended probiotic utilization, it is not possible to generalize the substantive effects of this intervention on patient outcomes.
Our study results suggest that practicing physicians remain reluctant to utilize probiotics to all ICU patients, even after formal recommendation by the MEC, educational intervention, and continuing pharmacy support. Policy can be made, but it does not guarantee clinical support practice. Because utilization rates progressively declined after the intervention, it can be hypothesized that education is not always a lasting process and that attitudes toward policy change may fade over time and after initial project backing. Our analysis also suggests that the route of probiotic ordering may affect utilization rates. Thus, the best method of ensuring physician utilization may entail making it an automatic system order and having it ordered unless it is individually removed by the physician involved for that patient.
Potential barriers to implementation may include lack of incorporation into formal hospital order sets, fear of active patient infection, paucity of society guidelines on probiotics, or just sheer lack of knowledge on probiotics and gut microbiome pathophysiology. Further prospective studies investigating both the safety profile and efficacy of probiotics for microbiome dynamics and primary prevention of AAD and CDI are needed, making sure to incorporate minimal exclusion criteria and, thus, to represent day-to-day clinical encounters.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.




