Vitamin D insufficiency is a major health problem in developed as well as developing countries(Reference Calvo, Whiting and Barton1), including Iran(Reference Hashemipour, Larijani and Adibi2, Reference Salek, Hashemipour and Aminorroaya3). Vitamin D is formed in the skin from 7-dehydrocholesterol under the influence of UV radiation. To be fully activated, however, vitamin D needs to undergo two hydroxylation reactions in the liver and kidney to form 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D), respectively(Reference Heaney4). Apart from calcaemic actions, vitamin D has many non-calcaemic functions, among which are immune function(Reference Maruotti and Cantatore5), cellular differentiation(Reference Piek, Sleumer and van Someren6) and anti-cancer actions(Reference Krishnan, Trump and Johnson7–Reference Khan, Kimler and Fabian11). Vitamin D insufficiency in the long term may not only lead to metabolic bone disorders such as osteomalacia and osteoporosis but may also have a role in the development of many chronic diseases such as auto-immune disorders(Reference Arnson, Amital and Shoenfeld12) (e.g. type 1 diabetes mellitus (T1DM)(Reference Zipitis and Akobeng13), lupus(Reference Amital, Szekanecz and Szucs14) and multiple sclerosis (MS)(Reference Ascherio, Munger and Simon15)) and malignancies(Reference Trump, Deeb and Johnson16). Wherever sun exposure is insufficient for any reason, dietary intake of vitamin D becomes a necessity(Reference Bischoff-Ferrari, Giovannucci and Willett17). For this reason, food fortification with vitamin D has been implemented as a prophylactic measure in many countries for several years(Reference Keane, Rochfort and Cox18, Reference Keane, Healy and O'Moore19). However, foods are not commonly fortified in Iran at the moment in spite of scattered reports of a remarkably high prevalence of various degrees of vitamin D deficiency among those 20–69 years of age in the Tehran population (79·6 %)(Reference Hashemipour, Larijani and Adibi2) and among 14–18-year-old adolescents in Isfahan (46·2 %)(Reference Moussavi, Heidarpour and Aminorroaya20).
Children in accelerated growth spurts have increased requirements for many nutrients, including vitamin D. Although a well-balanced diet may provide almost all the required nutrients, vitamin D is an exception since it has no appreciable food source, especially in the Iranian diet(Reference Kalantari and Ghafarpour21). The present study was therefore undertaken to assess vitamin D status of 9–12-year-old primary-school children in Tehran during autumn and winter 2007–2008.
Materials and methods
To estimate the prevalence of vitamin D insufficiency, a two-stage sampling was performed. In the first stage, sixty primary schools were selected through systematic random sampling from all nineteen districts of the Ministry of Education in Tehran. In the second phase, from each school sixteen to twenty children from grades 4 and 5 were enrolled in the study. The parents of all the children were invited to visit the school. The objectives of the study were fully explained to the parents in an open session, after which an informed written consent was signed by the parents who wished their child to participate in the study. After making arrangements with the schools, 3 ml of non-fasting venous blood was taken from the children on pre-determined days. Those children who were unwilling to donate blood for any reason were excluded. Finally, 1111 children (573 boys and 538 girls) of 1120 selected (99·2 % response) were enrolled in the study. The present study was scientifically and ethically approved by the Research Council and the Ethical Committee of the National Nutrition and Food Technology Research Institute, respectively.
Assessment of Ca intake
Ca intake was assessed in a subsample of the study population (n 503) using a sixty-item quantitative FFQ (qFFQ) specifically designed for dietary sources of Ca. The validity and reproducibility of the questionnaire were evaluated before the study(Reference Omidvar, Zaini-Nezhad and Eshraghian22). The questionnaires were completed by face-to-face interviews with the children at the schools. A food album and measuring cups were used to ensure accuracy of the reported servings.
Anthropometric assessments
Weight was measured using a digital scale to the nearest of 0·1 kg (model 840; Seca, Hamburg, Germany) with the child wearing light clothes and no shoes. Height was measured by a measuring tape to the nearest 0·1 cm. BMI was calculated as weight in kilograms divided by the square of height in metres (kg/m2).
Laboratory investigations
All blood samples were stored in the dark until serum separation. After 2 h at room temperature (RT), blood samples were centrifuged at 2500g at RT for 20 min. Sera thus recovered were transferred to fresh clean microtubes in aliquots and kept at −80°C until the day of analysis.
Serum 25(OH)D was determined using a competitive protein-binding assay (Immunodiagnostik, Bensheim, Germany). In the present study, two sets of criteria were used to categorize various degrees of vitamin D deficiency, both based on serum levels of 25(OH)D. The first set was: sufficient ≥37·0 nmol/l; 25·0 nmol/l ≤ mild deficiency<37·0 nmol/l; 12·5 nmol/l ≤ moderate deficiency<25·0 nmol/l; and severe deficiency <12·5 nmol/l. This set of criteria was used by the Iranian Ministry of Health in a national survey on micronutrient status in both 15–23-month-old children and pregnant women(23) and also in a pilot study on Iranian adults(Reference Neyestani, Gharavi and Kalayi24). The second set of criteria, which is more recent(Reference Saintonge, Bang and Gerber25), was: sufficient ≥50·0 nmol/l; 27·5 nmol/l ≤ insufficiency <50·0 nmol/l; and deficiency <27·5 nmol/l.
Serum levels of osteocalcin (OST; Biosource Europe SA, Nivellles, Belgium), intact parathyroid hormone (iPTH; DRG Instruments GmbH, Marburg, Germany) and bone-specific alkaline phosphatase (BAP; Immunodiagnostic Systems Ltd, Boldon, UK) were all determined using enzyme immunoassay. Concentrations were calculated from the absorbances using an automatic system (StatFax 3200 microplate ELISA reader; Awareness Technology, Inc., Palm City, FL, USA).
Statistical analyses
Data were expressed as mean and sd. Normality of distribution was evaluated using the Kolmogrov–Smirnov test. Comparison of data between two groups was carried out using Student's t test (for normal distribution) or the Mann–Whitney U test (for non-normal distribution). Correlations were evaluated using Pearson's (for normal distribution) or Spearman's (for non-normal distribution) coefficients. The χ 2 test was used to compare qualitative data between groups. Differences were considered statistically significant at α < 0·05. All statistical analyses were performed using the Statistical Package for the Social Sciences statistical software package version 16·0 (SPSS Inc., Chicago, IL, USA).
Results
Data on daily Ca intake, serum levels of 25(OH)D, OST, iPTH and duration of sun exposure did not show normal distribution and they were not normalized even after data transformation (including logarithm). The data on other factors had a normal distribution.
Anthropometrics
Although girls were significantly taller than boys (P = 0·010), no significant difference was observed between the BMI of boys and girls (Table 1).
Table 1 Comparison of variables by sex: 9–12-year-old primary-school children, Tehran, autumn and winter 2007–2008
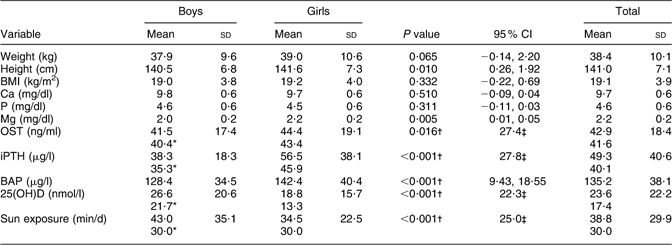
OST, osteocalcin; iPTH, intact parathyroid hormone; BAP, bone-specific alkaline phosphatase; 25(OH)D, 25-hydroxyvitamin D.
*Data distribution was not normal: values given are medians.
†Mann–Whitney U test.
‡Values given are interquartile range.
Ca intake
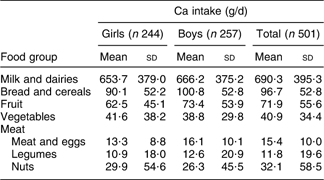
Daily intakes of Ca in boys and girls were not significantly different (929·6 (sd 436·7) mg and 909·5 (sd 465·5) mg, respectively). Dietary sources of Ca in the study population are shown in Table 2.
Table 2 Dietary sources of Ca in 9–12-year-old primary-school children, Tehran, autumn and winter 2007–2008
Serum levels of Ca, P and Mg
Concentrations of Ca and P did not differ significantly between boys and girls; however, those of Mg were slightly, but significantly, higher in girls than in boys (P = 0·005). Boys had significantly lower serum levels of OST (P = 0·016), iPTH and BAP (P < 0·001 for both) but higher 25(OH)D (P < 0·001). Duration of sun exposure was reported longer in boys than in girls (P < 0·001; Table 1).
Table 3 shows the prevalence of various degrees of vitamin D deficiency, according to the first set of criteria, among the whole study population and in both sexes. Approximately 86 % of the children were vitamin D deficient, of whom 38·3 % were severely deficient. Although the percentage of children with moderate and mild deficiency did not differ significantly between the two sexes, severe deficiency was more prevalent in girls than in boys (χ 2 test; P < 0·001).
Table 3 Frequency of different degrees of vitamin D deficiency according to criteria 1Footnote * in 9–12-year-old primary-school children, Tehran, autumn and winter 2007–2008

Frequencies were compared using the χ 2 test.
* Severe deficiency, 25-hydroxyvitamin D (25(OH)D) < 12·5 nmol/l; moderate deficiency, 12·5 ≤ 25(OH)D < 25·0 nmol/l; mild deficiency, 25·0 ≤ 25(OH)D < 37·0 nmol/l; adequate, 25(OH)D ≥ 37·0 nmol/l.
However, when the second set of criteria was used to define vitamin D status, the prevalence of various degrees of vitamin D deficiency rose to 91·7 %. Again in this situation the frequency of boys with adequate vitamin D status was significantly higher than that of girls (χ 2 test; P < 0·001; Table 4).
Table 4 Frequency of different degrees of vitamin D deficiency according to criteria 2Footnote * in 9–12-year-old primary-school children, Tehran, autumn and winter 2007–2008

Frequencies were compared using the χ 2 test.
* Deficiency, 25-hydroxyvitamin D (25(OH)D) < 27·5 nmol/l; insufficiency, 27·5 ≤ 25(OH)D < 50·0 nmol/l; adequate, 25(OH)D ≥ 50·0 nmol/l.
Serum levels of 25(OH)D inversely correlated with iPTH (r = −0·16, 95 % CI −0·17, −0·06; P < 0·001), BAP (r = −0·150, 95 % CI −0·22, −0·11; P < 0·001) and Mg (r = −0·129, 95 % CI −0·17, −0·07; P < 0·001) and also with weight (r = −0·091, 95 % CI −0·13, −0·04; P = 0·003), height (r = −0·084, 95 % CI −0·13, −0·034; P = 0·005) and BMI (r = −0·074, 95 % CI −0·13, −0·01; P = 0·014). Interestingly, OST showed a positive correlation with height (r = 0·130, 95 % CI 0·08, 0·16; P < 0·001) but a negative correlation with BMI (r = −0·072, 95 % CI −0·131, −0·01; P = 0·018). For those variables without normal distribution, Spearman's correlations were also evaluated. In this case, serum levels of 25(OH)D inversely correlated with iPTH (rs = −0·154, 95 % CI −0·22, −0·10; P < 0·001), BAP (rs = −0·151, 95 % CI −0·22, −0·10; P < 0·001), Mg (rs = −0·142, 95 % CI −0·22, −0·11; P < 0·001), weight (rs = −0·116, 95 % CI −0·17, −0·06; P < 0·001) and BMI (rs = −0·092, 95 % CI −0·14, −0·04; P = 0·002) but directly correlated with duration of sun exposure (rs = 0·115, 95 % CI 0·11, 0·22; P < 0·001).
Discussion
A remarkably high prevalence of poor vitamin D status among 9–12-year-old schoolchildren during cold seasons in Tehran convincingly proved that vitamin D deficiency is a major health problem in this age group living in Tehran. Severity of this deficiency was higher in girls than in boys. Our data are comparable to those reported from Isfahan, central Iran (95·2 % in girls v. 49·0 % in boys)(Reference Moussavi, Heidarpour and Aminorroaya20). However, there are some differences between these two studies as in the Isfahan study: (i) participants were between 14 and 18 years of age; (ii) sampling was performed during all four seasons; (iii) adequacy and insufficiency of vitamin D were defined as serum levels of 25(OH)D above 80 nmol/l and below 50 nmol/l, respectively; and (iv) RIA was used to measure serum 25(OH)D(Reference Moussavi, Heidarpour and Aminorroaya20).
One of the factors affecting vitamin D status is body weight, notably BMI. The negative influence of fat mass (FM) on vitamin D status has already been reported(Reference Alemzadeh, Kichler and Babar26). It has been suggested that FM may act as a ‘metabolic well’ to reduce the bioavailability of vitamin D and its transformation to 25(OH)D(Reference Wortsman, Matsuoka and Chen27). This theory is well supported not only by our finding of an inverse correlation between serum 25(OH)D levels and BMI, but also by the epidemiological observation that vitamin D insufficiency is more prevalent among overweight and obese people(Reference Hypponen and Power28).
Duration of direct sun exposure was clearly not sufficient to protect the children, and especially girls, from vitamin D insufficiency. Higher prevalence of vitamin D deficiency among girls has been repeatedly reported by others(Reference Moussavi, Heidarpour and Aminorroaya20, Reference Bener, Al-Ali and Hoffmann29, Reference Sahu, Bhatia and Aggarwal30). Considering the very limited food sources of vitamin D in the Iranian diet(Reference Kalantari and Ghafarpour21), one can relate the higher vitamin D deficiency in girls to their lower outdoor activities as well as to their dress code.
According to Islamic regulations, girls after pubescence must be veiled. It is noteworthy that even long sun exposure in this situation may not be effective to produce adequate vitamin D in the body. The amount of UV energy for dermal vitamin D synthesis is about 18–20 mJ/cm(Reference Hashemipour, Larijani and Adibi2, Reference Matsuoka, Wortsman and Haddad31). Exposure of limited surfaces of the body, mainly face and hands, does not seem sufficient for vitamin D adequacy(Reference Matsuoka, Wortsman and Hollis32). The dermal response of individuals to UV may be different. In a study on ninety-three adults (thirty men and sixty-three women) with a mean age of 24·0 (sd 0·7) years and mean BMI of 23·6 (sd 0·4) kg/m2, even after 28·9 (sd 1·5) h/week of sun exposure 51 % of the participants had some degree of vitamin D insufficiency. In that study, serum levels of 25(OH)D below 75 nmol/l were considered as insufficient(Reference Binkley, Novotny and Krueger33).
Tehran is located at a latitude of 35·34°, where dermal synthesis of vitamin D in cold seasons is almost negligible(Reference Holick, Chen and Lu34). During this time, the body inevitably relies on vitamin D body stores. The extremely high prevalence of vitamin D deficiency in our study participants reflected well their inefficient vitamin D storage during warm seasons. In accordance with this finding, in a study on 168 girls aged 4–8 years, race and season were the strongest predictors of vitamin D status(Reference Stein, Laing and Hall35). Prevalence of vitamin D insufficiency in Lebanese children was reported as 52 % in 1999, with a peak in winter (65 %) and a fall in summer (40 %). In those children, socio-economic status and BMI were the most important predictors of serum vitamin D(Reference El-Hajj Fuleihan, Nabulsi and Choucair36).
Human diets usually fail to provide sufficient amounts of vitamin D(Reference Vieth, Bischoff-Ferrari and Boucher37). Even in North America, with the higher intakes of dairy products(Reference Kranz, Lin and Wagstaff38) compared with Iran(Reference Kalantari and Ghafarpour21) and fortification of milk with vitamin D(Reference Calvo, Whiting and Barton39), prevalence of vitamin D insufficiency is more than expected(Reference Hanley and Davison40, Reference Calvo and Whiting41). This could be, at least in part, because of insufficient intakes of dairy products. It has recently been reported that: first, dairy intake in the 4–18-year-old population of the USA is insufficient; and second, most children consume high-fat dairy products, which, compared with low-fat products, have a lower amount of Ca in a certain volume(Reference Kranz, Lin and Wagstaff38). Ca intake may decrease even more during transition from childhood to adolescence(Reference Larson, Neumark-Sztainer and Harnack42). The importance of Ca intake, with regard to vitamin D status, lies in the fact that sufficient Ca intake can improve serum levels of 25(OH)D by decreasing PTH secretion, thus decreasing hydroxylation of 25(OH)D to 1,25(OH)2D(Reference Wilson, Horst and Schedl43). The Ca intake in our study participants, however, was not enough to protect them from vitamin D insufficiency. This observation is quite explainable as there is no fortification and/or supplementation programme currently ongoing in Iran.
Significantly higher iPTH in girls than in boys is in accordance with girls’ lower vitamin D status. Regarding the lower age of puberty in girls, there is a compensatory increase in PTH secretion to produce enough 1,25(OH)2D needed for the accelerated growth. Higher levels of OST and BAP, together with iPTH, in girls compared with boys clearly indicate their higher bone turnover(Reference Levine44).
The high prevalence of vitamin D insufficiency and deficiency during cold seasons in primary-school children in Tehran should be regarded as a public health emergency by all stakeholders. Vitamin D deficiency-induced immune dysfunction can bring about such auto-immune disorders as T1DM in children and MS in adults(Reference Mark and Carson45). There are some reports of a higher occurrence of T1DM(Reference Moltchanova, Schreier and Lammi46) and MS(Reference McDowell, Amr and Langenberg47) during the cold seasons and it was related to the lower vitamin D status(Reference Holick48–Reference Orton, Morris and Herrera50). Viral infections, which are usually more common during cold seasons, have been proposed as possible aetiologies of T1DM(Reference Richer and Horwitz51–Reference Aarnisalo, Veijola and Vainionpää53). Vitamin D may exert its protective effects by inducing the expression of an antimicrobial peptide, cathelicidin(Reference White54, Reference Misawa, Baba and Ito55). Therefore, improvement of vitamin D intake in schoolchildren can help their bone health and growth and act, at least to some extent, as a ‘vaccination’ against auto-immune disorders and some other prevalent diseases such as CVD later in life(Reference Holick56).
It is noteworthy that the present study was conducted during cold seasons and does not necessarily show the vitamin D status of the children for the entire year. In a recent study conducted in 7–18-year-old children in Tehran, the prevalence of vitamin D insufficiency was reported as 53·6 % in girls and as 11·3 % in boys(Reference Rabbani, Alavian and Motlagh57). In a separate study on 313 children aged 8–18 years in Tehran, 26 % had vitamin D insufficiency(Reference Razzaghy-Azar and Shakiba58). These two studies were conducted in four seasons and both used RIA to measure serum 25(OH)D. Therefore, the time(Reference Bolland, Chiu and Davidson59) and the method of assessment(Reference Neyestani, Gharavi and Kalayi60) can both influence our judgement on the prevalence.
We achieved a very high response rate in all schools and there was no obvious difference between children with and without blood samples.
In conclusion, our data showed that the problem of vitamin D deficiency in children still persists 35 years after the earliest reports of clinical rickets in Iran(Reference Salimpour61). Vitamin D deficiency was more prevalent in girls than in boys. These findings warrant immediate appropriate interventions for nutritional support of 9–12-year-old (and most likely all) children.
Acknowledgement
The present study was funded by the National Nutrition and Food Technology Research Institute (NNFTRI). The authors do not have any duality of conflict of interest. The whole project was designed and conducted by T.R.N.; M.H., as a co-supervisor, actively participated in designing and conducting the project; dietary assessment, including the validation study, was carried out under the supervision of N.O.; sampling methodology and statistical analyses were performed under directions of M.R.E.; all laboratory analyses and fieldwork were conducted by T.R.N., A.G., A.K., N.S., N.K., H.H., T.Z. and B.N. The authors acknowledge the Deputy of Health of the Iranian Ministry of Education who allowed them to perform the present research in the primary schools of Tehran. They also appreciate all the school directors, principals and health instructors for their sincere cooperation. They especially thank all the children who participated in the present study and the parents who permitted their children to do so. All the laboratory bench works were carried out at the Laboratory of Nutrition Research at NNFTRI.






