Introduction
Rare earth elements (REE) represent a unique set of elements having coherent behaviour and very similar properties which are responsible for their similar geochemistry. The International Union of Pure and Applied Chemistry (IUPAC) definition of the REE group includes lanthanides (Ln), yttrium (Y) and scandium (Sc). However, due to the substantially smaller ionic radius of Sc3+ with respect to the rest of the group, Sc frequently enters different structural sites, and therefore Sc is commonly not included into the REE in natural geological environments. Due to the lanthanide contraction phenomenon, the REE are further divided into larger LREE (light Ln, La–Sm), medium MREE (Sm–Dy) and smaller HREE (heavy Ln, Tb–Lu and Y).
Minerals belonging to the xenotime group are anhydrous orthophosphates, orthoarsenates and orthovanadates with tetragonal symmetry and space group I41/amd (#141). Among phosphates, xenotime-(Y) is the most common species and contains predominantly Y3+ and usually low contents of other HREE as well as negligible amounts of LREE based on their preference of the xenotime-type crystal structure (cf. Ni et al., Reference Ni, Hughes and Mariano1995). On the other hand, xenotime-(Yb) and Yb-, Dy- and Gd-rich xenotime-(Y) are rare minerals occurring in some granitic pegmatites and metamorphic-hydrothermal lithologies (Demartin et al., Reference Demartin, Pilati, Diella, Donzelli, Gentile and Gramaccioli1991; Förster and Rhede, Reference Förster and Rhede1995; Franz et al., Reference Franz, Andrehs and Rhede1996; Förster, Reference Förster1998; Buck et al., Reference Buck, Cooper, Černý, Grice and Hawthorne1999; Masau et al., Reference Masau, Černý and Chapman2000; Repina, Reference Repina2011; Repina et al., Reference Repina, Khiller and Makagonov2014; Franz et al., Reference Franz, Morteani and Rhede2015; Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Majzlan, Pollok, Mikuš, Milovská, Molnárová, Škoda, Kopáčik, Kurylo and Bačík2023b, Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c).
In contrast to numerous minerals with REE as their essential constituents (particularly Ce and Y, more rarely La, Nd, Sm and Yb), there are currently only two Gd-dominant minerals approved by the International Mineralogical Association, Commission on New Minerals, Nomenclature and Classification (IMA-CNMNC, Pasero, Reference Pasero2024): lepersonnite-(Gd) CaGd2(UO2)24(SiO4)4(CO3)8(OH)24⋅48H2O, a rare REE-uranyl carbonate from the Shinkolobwe U deposit in the DR Congo (Deliens and Piret, Reference Deliens and Piret1982) and monazite-(Gd) (Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Majzlan, Pollok, Mikuš, Milovská, Molnárová, Škoda, Kopáčik, Kurylo and Bačík2023b) which is cogenetic with the xenotime-(Gd) described here.
In this work, we describe a new Gd-dominant mineral xenotime-(Gd), discovered in a hydrothermal quartz vein with REE–U–Au mineralisation at the Zimná Voda occurrence near Prakovce, Slovakia. Xenotime-(Gd) is a new Gd-dominant member of the xenotime group and it is related to xenotime-(Y) and xenotime-(Yb) by substitution of Gd for other REEs, having the REE composition distinctly shifted towards the MREE enrichment. The new mineral and its name have been approved by the IMA-CNMNC (IMA 2023-091; Ondrejka et al., Reference Ondrejka, Bačík, Majzlan, Uher, Ferenc, Števko, Čaplovičová, Milovská, Mikuš, Rößler, Matthes and Molnárová2024) and the Levinson modifier for rare earth minerals (Levinson, Reference Levinson1966; Bayliss and Levinson, Reference Bayliss and Levinson1988). The symbol Xtm-Gd was given to the new mineral. The holotype specimen of xenotime-(Gd) (FIB lamella of the thin section ZV-2A4) is deposited in the collection of the Slovak National Museum, Natural History Museum, Vajanského nábrežie 2, P.O. BOX 13, 810 06 Bratislava, Slovak Republic under the catalogue number M-20412. The crystallographic information file (cif) is deposited in American Mineralogist Crystal Structure Database (AMCSD; Downs and Hall-Wallace, Reference Downs and Hall-Wallace2003) under the code 0021444 and is also available as Supplementary material (see below).
Occurrence
The Zimná Voda REE–U–Au occurrence was discovered in 1975, during exploration for uranium ores (Novotný and Čížek, Reference Novotný and Čížek1979). The sample containing xenotime-(Gd) was collected by the authors in September 2017 during reconnaissance of the Zimná Voda REE–U–Au quartz vein, Prakovce, Gelnica Co., eastern Slovakia. The site is located near the main ridge of the Slovenské Rudohorie Mts., ~5.6 km to the S of the Prakovce village, 600 m to the NW of the Tri Studne elevation point (969 m a.s.l.) and 400 m NW of Trohánka bivouac shelter, at an altitude of ~950 m a.s.l., ca. 23 km WNW of Košice town at 48.7666°N, 20.9137°E.
The hydrothermal REE–U–Au vein mineralisation at the Zimná Voda occurrence is hosted in the Lower Palaeozoic metamorphic rocks of the Bystrý Potok Formation, a part of the Gelnica Group in the Gemeric tectonic unit of the Western Carpathians, which is part of the Alpine-Carpathian Mountain belt (Bajaník et al., Reference Bajaník, Hanzel, Mello, Pristaš, Reichwalder, Snopko, Vozár and Vozárová1983; Ivanička et al., Reference Ivanička, Snopko, Snopková and Vozárová1989). Two quartz veins (Western and Eastern) containing REE, U and Au mineralisation were found in the area. Xenotime-(Gd) was found in the Western vein. The vein is hosted in fine-grained micaceous phyllites interbedded with fine-grained quartzites. It has an E–W strike, total length of ~90 m with an average dip of 65° to the S, and conforms to the schistosity of the host rocks. The thickness of the vein ranges from 3 to 30 cm. Along the contact, the rocks are intensively argillitised, and locally silicified, and impregnated by pyrite. Supergene alteration of pyritised rocks caused their limonitisation.
The metamorphic rocks were intruded by Hummel granites which outcrop 600 m to the SW of the investigated occurrence. These igneous rocks are leucocratic peraluminous rare-metal granites with S-type affinity and relatively high degree of magmatic fractionation, evidenced by higher P concentrations in K-feldspar and elevated contents of rare lithophile elements (Li, Rb, Cs, B, Sn, W, Nb and Ta) and F. They originated and were emplaced during the post-Variscan, Permian (260–270 Ma) extension stage (e.g. Villaseñor et al., Reference Villaseñor, Catlos, Broska, Kohút, Hraško, Aguilera, Etzel, Kyle and Stockli2021, and references therein).
In addition to xenotime-(Gd), the following minerals were identified in the Western vein: arsenopyrite, native bismuth, bismuthinite, brannerite, chlorite, cobaltite, fluorapatite, galena, gersdorffite, glaucodot, native gold, hingganite-(Y), kobellite, pyrite, quartz, molybdenite, monazite-(Ce), monazite-(Gd), monazite-(Nd), monazite-(Sm), muscovite, rutile, stibnite, tetrahedrite-(Fe), tintinaite, tourmaline-group minerals, uraninite and xenotime-(Y); supergene minerals are represented by arseniosiderite, goethite and other undifferentiated iron oxyhydroxides, pharmacosiderite–bariopharmacosiderite, philipsbornite–segnitite, scorodite, zeunerite, other uranyl arsenates–phosphates (most abundant are kahlerite, nováčekite, threadgoldite, rarely arsenuranospathite, autunite, chistyakovaite related mineral phases and phosphuranylite); Ondrejka et al. (Reference Ondrejka, Ferenc, Majzlan, Števko, Kopáčik, Voleková, Milovská, Göttlicher, Steininger, Mikuš, Uher, Biroň, Sejkora and Molnárová2023a, Reference Ondrejka, Uher, Ferenc, Majzlan, Pollok, Mikuš, Milovská, Molnárová, Škoda, Kopáčik, Kurylo and Bačík2023b, Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c). The mineralisation probably originated by fluid-driven hydrothermal mobilization of REE, U and Au from the surrounding metamorphic rocks induced by the intrusion of the granitic rocks (Rojkovič et al., Reference Rojkovič, Háber and Novotný1997; Reference Rojkovič, Konečný, Novotný, Puškelová and Streško1999). The perigranitic origin is supported by the age correlation of uraninite (246–265 Ma), the geochemical signatures and the spatial proximity of the mineralised veins with Permian rare-metal granites of the Gemeric Unit (cf. Kohút and Stein, Reference Kohút and Stein2005; Ferenc et al., Reference Ferenc, Števko, Mikuš, Milovská, Kopáčik and Hoppanová2021; Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c). Further details and regional geology can be found in Ondrejka et al. (Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c).
Experimental methods
The chemical composition of xenotime-(Gd) was studied by a JEOL JXA-8530F electron probe microanalyser (EPMA) in the wavelength-dispersive spectrometry (WDS) mode at the Earth Science Institute, Slovak Academy of Sciences in Banská Bystrica, Slovakia. An accelerating voltage of 15 kV and a probe current of 20 nA were used. The typical spot beam diameter varied from 2 to 6 μm; a more focused ≤1–3 μm beam was used only occasionally to avoid any intermediate composition in strongly heterogeneous micro-scale areas. The determination was calibrated using natural and synthetic calibrants (Table 1), and raw counts were converted to wt.% of oxides using the ZAF matrix correction. Corrections of line interferences were provided using the method by Åmli and Griffin (Reference Åmli and Griffin1975). The detection limit for all elements is typically between 0.01 and 0.15 wt.%. Element contents in the mineral formula are expressed in atoms per formula unit (apfu). The xenotime-(Gd) formula was normalised to 4 oxygen atoms.
Table 1. Conditions used for the electron microprobe analyses.

Depolarised Raman measurements were conducted on an unoriented section of xenotime-(Gd) using a Labram HR800 spectrometer (Horiba Jobin-Yvon), which was linked to an Olympus-BX41 optical microscope (Earth Science Institute, Slovak Academy of Sciences in Banská Bystrica, Slovakia). The samples were exposed to 532 nm frequency-doubled Nd-YAG and 633 nm He–Ne lasers. The system resolution was ~2 cm−1.
Slicing and polishing of a lamella for electron diffraction analysis were carried out using a scanning electron microscope (SEM) coupled with a gallium-focused ion beam (FIB) source (Bauhaus University Weimar, Germany). The SEM-FIB (Helios G4 UX, ThermoFisherScientific) is equipped with a high-performance FIB source (Phoenix) that allows the polishing of TEM lamella at very low acceleration voltage or beam current. This feature is essential for obtaining undisturbed thin lamella suitable for high-resolution TEM imaging. Thin sections of samples, as used for optical light microscopic investigation and other analyses, were sputtered with an ≈ 8 nm gold layer to ensure the electric conductivity of the full sample and to reduce sample abrasion during ion beam imaging. Sites for extraction of the lamellae were selected according to previous microscopic and spectroscopic characterisation of the samples. Areas of interest were covered with an approximately 15×3×3 μm layer of platinum to further protect the sample surface against ion beam damage.
Transmission electron microscopy (TEM), high-resolution TEM (HRTEM), and scanning transmission electron microscopy (STEM) characterisations were performed using double corrected TEM/STEM JEOL JEM-ARM200CF (JEOL Ltd., Tokyo, Japan) with cold-FEG cathode operated at 200 kV located in the Centre for Nanodiagnostics of Materials, Faculty of Materials Science and Technology, Slovak University of Technology in Bratislava, Slovakia.
Images from HRTEM were recorded using a Gatan Orius SC1000 CCD Camera (Gatan Inc., Pleasanton, CA, USA) with a resolution of 2048 × 2048 pixels using the DigitalMicrograph software package (version 3.60.4435.0, Gatan Inc., Pleasanton, CA, USA). The measurement of the reflection positions and angles in Fast Fourier Transform (FFT) patterns acquired from relevant HRTEM images was done using the DIFPACK module incorporated in DigitalMicrograph software. By clicking on the spots on the FFT pattern DIFPACK locates the spots at the brightest pixel value precisely.
A high-angle annular dark field (HAADF) STEM detector with inner-collection semi-angle of 90 mrad, and a probe convergence semi-angle of 22 mrad were utilised to obtain atomic resolution HAADF STEM images. Crystal structure simulations were carried out using CrystalMaker® for Windows version 2.3.0 (Begbroke, UK).
Appearance and physical properties
Xenotime-(Gd) forms rare crystal domains or zones (≤20 μm in size, usually ≤10 μm), in Gd-rich xenotime-(Y) crystals (≤100 μm in size, xenotime II, Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c) in close association with Gd-poor xenotime-(Y) (xenotime I, ibidem), monazite-group minerals, particularly monazite-(Gd) and monazite-(Sm), uraninite, supergene uranyl arsenates and phosphates, dispersed in quartz–muscovite gangue (Fig. 1a). The holotype specimen (FIB lamella) is positioned in the Gd-richest domain of the xenotime crystal (Fig. 1b–d). Among two xenotime-group species recognised in the Western vein at Zimná Voda occurrence, xenotime-(Y) is the most common and occurs in all of 6 samples studied (ZV-1 to ZV-6). Xenotime-(Gd) was found only in the ZV-2 sample and unlike monazite-(Gd) with occurrence in multiple thin sections, xenotime-(Gd) was found only in the thin section ZV-2A4. The lustre, hardness, cleavage and parting of xenotime-(Gd) could not be determined, nor could density be measured due to an insufficient quantity of mineral for physical measurement. We assume, however, that these properties are similar to those observed in other xenotime-group minerals. The density of 5.26 g/cm3 was calculated on the basis of the average empirical formula and calculated unit-cell volume (see below). The density of the ideal xenotime-structured formula GdPO4 is 5.66 g cm–3 (Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013) to 5.77 g cm–3 (Ni et al., Reference Ni, Hughes and Mariano1995) calculated from the unit-cell volume of synthetic GdPO4 due to the significant presence of lighter elements, mostly Y. In comparison, the density of natural xenotime-(Y) varies between 4.4 and 5.1 g cm–3 (Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2024). Optical properties could not be determined because of the small sample size, but they are probably similar to other xenotime-group minerals. The value of n = 1.87 calculated from the Gladstone-Dale relationship (Mandarino, Reference Mandarino1979, Reference Mandarino1981) using an empirical formula and calculated density of the holotype specimen is slightly higher than the refractive index of natural xenotime-(Y), which is up to 1.83 (Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2024) due to enrichment in heavier REE.

Figure 1. Back-scattered electron images (BSEI) of (a) a large aggregate of monazite-group minerals (MGM) with xenotime-(Gd) (Xtm-Gd), xenotime-(Y) (Xtm-Y), uraninite (Urn) and uranyl arsenates-phosphates (U-As-P) in quartz-muscovite gangue (Qz + Ms). The red dashed area indicates the Gd-richest (Gd-apfu-dominant) composition Gd2O3>29 wt.%; Y2O3<16 wt.%; black dashed area indicates Gd-rich (Gd-apfu-dominant) Gd2O3=28–29 wt.%. The position of the holotype FIB lamella (#M-20412) is documented herein. (b) The spot positions of electron microprobe analyses in Table 2. (c) The FIB lamella (holotype specimen) positioned in the Gd-richest domain of the xenotime crystal. (d) The extraction of the FIB lamella from the sample. Mineral symbols from Warr (Reference Warr2021). Note: Images (b), (c) and (d) are rotated 90 degrees to the view in (a).
Chemical composition
Chemical analyses were carried-out on crystals in the ZV-2A4 thin section and are positioned within and in close vicinity of the FIB lamella holotype specimen (Fig. 1b). The representative chemical composition is shown in Table 2. The xenotime-(Gd) μm-scale domains are chemically relatively homogeneous, with no distinct variations in the Y/Gd mass ratio (0.47–0.50). However, those Gd-dominant domains are always a part of a larger and heterogeneous Gd-rich to Gd-medium-rich xenotime-(Y) with Y/Gd = 0.56–2.57. The analysed domains have a composition with Gd>Y>Dy > Sm > Tb (28.5–29.3 wt.% Gd2O3, 0.36–0.38 apfu Gd; 15.2–16.1 wt.% Y2O3, 0.31–0.33 apfu Y; 10.1–10.8 wt.% Dy2O3, 0.13 apfu Dy; 5.3–6.2 wt.% Sm2O3, 0.07–0.08 apfu Sm; 3.7–4.0 wt.% Tb2O3, 0.05 apfu Tb). The average chemical composition of xenotime-(Gd) calculated from six point electron-microprobe analyses is as follows (wt.%): %): P2O5 30.1, As2O5 0.5, SiO2 0.2, UO2 0.3, Y2O3 15.7, (La, Ce, Pr, Nd)2O3 0.5, Sm2O3 5.7, Eu2O3 1.4, Gd2O3 29.2, Tb2O3 3.9, Dy2O3 10.4, Ho2O3 0.4, (Er, Tm, Yb, Lu)2O3 2.1, (Ca, Fe, Pb, Mn, Ba)O 0.1, total 100.5. The corresponding empirical formula calculated on the basis of 4 oxygen atoms is: (Gd0.37Y0.32Dy0.13Sm0.08Tb0.05Eu0.02Er0.01Tm0.01Nd0.01…)Σ1.01(P0.98As0.01Si0.01)O4. The empirical formula of xenotime-(Gd) in its Gd-richest spot is: (Gd0.38Y0.31Dy0.13Sm0.08Tb0.05Eu0.02Er0.01Nd0.01Ho0.01…)Σ1.01(P0.98As0.01Si0.01)O4 which leads to the end-member formula GdPO4 requiring (in wt.%): Gd 62.35, P 12.28, and O 25.37, or Gd2O3 71.86, P2O5 28.14, total 100.00 wt.%.
Table 2. Representative and average EPMA data and mineral formula of xenotime-(Gd). Spot numbers correspond to positions on Fig. 1.

*Note: ΣA = sum of cations at the A site (Th+U+Al+REE+Fe+Pb+Mn+Ca+Ba+Na); ΣB = sum of cations at the B site (S+P+As+Si).
In general, the element distribution shows enrichment towards the MREE, depletion of HREE+Y and negligible LREE abundances. The chondrite-normalised patterns exhibit conspicuous maxima at Gd and Tb (MREE hump; for further details, see Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c). The concentration of Th was usually below the detection limit of the EPMA; U attains only 0.4 wt.% UO2 (0.004 apfu U). Arsenic is always present (≤0.7 wt.% As2O5, 0.014 apfu As) and attests to limited PAs–1 (chernovite) substitution. Satisfactory analytical totals (99 − 101 wt.%), excellent stoichiometry calculated on an anhydrous basis and Raman spectra (see below) indicate that a potential role of a tetrahedral array of (OH)– groups is negligible, if any. Other trace elements (Ca, Sr, Fe, Pb, Si, F and S) have negligible concentrations or are below detection limits (Table 2).
Crystallography
Xenotime-(Gd) is isostructural with other xenotime-group minerals; its tetragonal (space group I41/amd) structure consists of isolated PO4 tetrahedra separated by intervening (REE)O8 polyhedra. A smaller REE-containing polyhedron in the xenotime structure with the 8-fold coordination compared to a monoclinic monazite structure with the 9-fold coordination accommodates the smaller cations; therefore, the xenotime structure prefers HREE over MREE and LREE (e.g. Miyawaki and Nakai, Reference Miyawaki, Nakai, Gschneidner and Eyring1993; Ni et al., Reference Ni, Hughes and Mariano1995). Consequently, xenotime crystals with MREE enrichment are rare.
To determine the crystal structure of the mineral, HRTEM and STEM characterisation on focused ion beam (FIB) lamella was used. This procedure enables measurement of the same phases that were previously analysed by EPMA. A single-crystal X-ray diffraction study of xenotime-(Gd) was not carried out due to the small size of the crystals. Powder X-ray diffraction data could also not be obtained, but because the xenotime structure of synthetic GdPO4 is known (tetragonal, space group I41/amd, a = 6.9648(4) Å and c = 6.1050(5) Å; Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013; a = 6.9612(7) Å and c = 6.1026(10) Å (Clavier et al., Reference Clavier, Mesbah, Szenknect and Dacheux2018), a pattern was calculated using the cif (Table 3). The xenotime structure can be described as [001]-oriented chains formed of alternating phosphate tetrahedra and REE polyhedra (Vegard, Reference Vegard1927). Each PO4 tetrahedron is isolated from other PO4 tetrahedra and shares two of its edges with the REEO8 polyhedra. In synthetic GdPO4, the smaller P5+ cations are tetrahedrally coordinated with a P−O bond length of 1.554(4) Å and two different OPO angles [101.6(3)° and 113.6(2)°]. The PO4 tetrahedra are connected to Gd3+ cations with coordination number 8 and two unique Gd−O bond lengths [2.343(4) and 2.397(4) Å] (Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013). Therefore, a HRTEM with FFT techniques was used to determine the unit-cell parameters of the crystal examined.
Table 3. Calculated powder X-ray diffraction pattern (λ = 1.5406 Å) for xenotime-(Gd) compared to synthetic xenotime-(Gd) (Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013).*
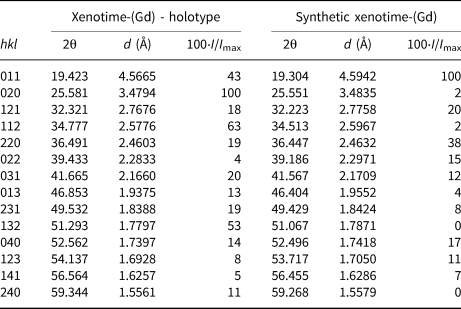
* The data were calculated with the program Diamond, v 4.0, for the composition and unit-cell parameters of the natural sample investigated in this work.
A thin lamella of a xenotime-(Gd) single crystal was tilted in the electron microscope to obtain a low-index zone axis parallel to the primary electron beam. At this setting, HRTEM resulted in the recording of a defect-free single crystalline structure (Fig. 2a). The corresponding FFT pattern was acquired from the entire recorded area and the interplanar distances obtained by measuring the reflection positions and the angles between individual reflections were determined (Fig. 2b). Using this method, a set of 25 reflections were measured using FFT and with refinement gave the following unit-cell parameters: a = 6.9589(5) Å, c = 6.0518(6) Å, V = 293.07(3) Å3 and Z = 4.

Figure 2. (a) High-resolution TEM image of the crystal lattice viewed along the [11$\bar{1}$![]() ] direction. (b) Relevant FFT pattern showing the [11$\bar{1}$
] direction. (b) Relevant FFT pattern showing the [11$\bar{1}$![]() ] zone axis.
] zone axis.
The measured angles and interplanar distances were compared with relevant data obtained using a standard stereographic projection of the tetragonal crystal of xenotime-(Gd) (Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013). Based on this, and also using the rules for indexing point electron diffractions, Miller indices were assigned to all reflections in the FFT pattern (Fig. 2b).
The experimentally obtained d-spacings of 0.5001 nm and 0.457 nm are in good agreement with that of 0.49015 nm and 0.45664 nm, respectively, reported for interplanar distances of ($\bar{1}10$![]() ) and ($\bar{1}$
) and ($\bar{1}$![]() 0$\bar{1}$
0$\bar{1}$![]() ) planes of tetragonal xenotime-(Gd) (Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013). Moreover, the measured angle of 62.90° between these reflections corresponds well with the 62.23° valid for the angle between ($\bar{1}10$
) planes of tetragonal xenotime-(Gd) (Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013). Moreover, the measured angle of 62.90° between these reflections corresponds well with the 62.23° valid for the angle between ($\bar{1}10$![]() ) and ($\bar{1}0\bar{1}$
) and ($\bar{1}0\bar{1}$![]() ) planes according to standard stereographic projection of xenotime-(Gd). By evaluation of the FFT pattern, it was determined that the zone axis of the examined xenotime-(Gd) single crystal is [$11\bar{1}$
) planes according to standard stereographic projection of xenotime-(Gd). By evaluation of the FFT pattern, it was determined that the zone axis of the examined xenotime-(Gd) single crystal is [$11\bar{1}$![]() ].
].
A Xenotime-(Gd) single crystal oriented in the [$11\bar{1}$![]() ] direction was also characterised using atomic-scale resolution imaging. Atomic-resolution HAADF STEM (Z contrast) and BF STEM images display the distribution of atomic columns in the tetragonal crystal structure of xenotime-(Gd) oriented along the [$11\bar{1}$
] direction was also characterised using atomic-scale resolution imaging. Atomic-resolution HAADF STEM (Z contrast) and BF STEM images display the distribution of atomic columns in the tetragonal crystal structure of xenotime-(Gd) oriented along the [$11\bar{1}$![]() ] zone axis (Fig. 3). Bright spots in HAADF STEM image (Fig. 3a) represent Gd atomic columns, which is concordant with a superimposed tetragonal crystal structure representation of xenotime oriented in the [$11\bar{1}$
] zone axis (Fig. 3). Bright spots in HAADF STEM image (Fig. 3a) represent Gd atomic columns, which is concordant with a superimposed tetragonal crystal structure representation of xenotime oriented in the [$11\bar{1}$![]() ] direction. Gadolinium atomic columns appear dark in the simultaneously recorded atomic resolution BF STEM image (Fig. 3b).
] direction. Gadolinium atomic columns appear dark in the simultaneously recorded atomic resolution BF STEM image (Fig. 3b).

Figure 3. (a) Atomic-resolution HAADF STEM image of a xenotime-(Gd) single crystal observed along the [11$\bar{1}$![]() ] direction. The superimposed atomic structural model (bottom right) viewed along the [11$\bar{1}$
] direction. The superimposed atomic structural model (bottom right) viewed along the [11$\bar{1}$![]() ] direction identifies the Gd atomic columns (red) in a xenotime-(Gd) single crystal. (b) A simultaneously recorded atomic-resolution BF STEM image of a xenotime-(Gd) single crystal observed from the [11$\bar{1}$
] direction identifies the Gd atomic columns (red) in a xenotime-(Gd) single crystal. (b) A simultaneously recorded atomic-resolution BF STEM image of a xenotime-(Gd) single crystal observed from the [11$\bar{1}$![]() ] direction. Dark spots represent Gd atomic columns.
] direction. Dark spots represent Gd atomic columns.
It can be assumed that the unit-cell parameters of the observed mineral correspond to the weighted average of the corresponding end-members unit-cell parameters. The unit-cell parameters of synthetic REEPO4 with a xenotime structure display a near-ideal linear trend of increasing unit-cell parameters with increasing ionic radii of REE cations. Moreover, the content of other possible substituents (U, Th, Ca, Si and As), which could alter the linear trend, is very low in the xenotime-(Gd) sample and, therefore, the influence of these elements is negligible for the cell-parameter calculation. The following are the calculated mean unit-cell parameters for studied xenotime-(Gd) from the weighted sum of end-members: a = 6.9319(4) Å, c = 6.0697(5) Å, V = 291.66(1) Å3 and Z = 4.
The refined and calculated unit-cell parameters are in very good agreement. Moreover, the empirical unit-cell parameters obtained from the FFT are slightly lower than the unit-cell parameters of synthetic analogues of xenotime-(Gd) of Rodriguez-Liviano et al. (Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013) and Clavier et al. (Reference Clavier, Mesbah, Szenknect and Dacheux2018), which reflects the presence of other REE with smaller ionic radii (Table 4).
Table 4. Unit-cell parameters for natural xenotime-(Y) and xenotime-(Gd) compared to the published data for synthetic xenotime compounds.

Raman spectroscopy
The Raman spectra of xenotime-(Gd) and Gd-poor xenotime-(Y) have been documented previously in Ondrejka et al. (Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c). These spectra are influenced significantly by the photoluminescence (PL) of REE3+, masking Raman signals in the low-frequency range (100–750 cm–1) (see Fig. 4). Despite the intense PL, specific bands can be discernible in xenotime-(Y) at 650, 580 and 484 cm–1, and in xenotime-(Gd) at 640, 576–577 and 483 cm–1. The Raman modes become clearly distinguishable between 800 and 1200 cm–1 in both spectra excited by 532 nm and 633 nm lasers. Xenotime-(Y) exhibits distinctive bands centred at 999, 1024 and 1057 cm–1, while the spectra of xenotime-(Gd) display lines at 994–996, 1018 and 1052 cm–1, along with an additional, less intense band at 965 cm–1, and minor broad features at approximately 870, 900 and 1150 cm–1.
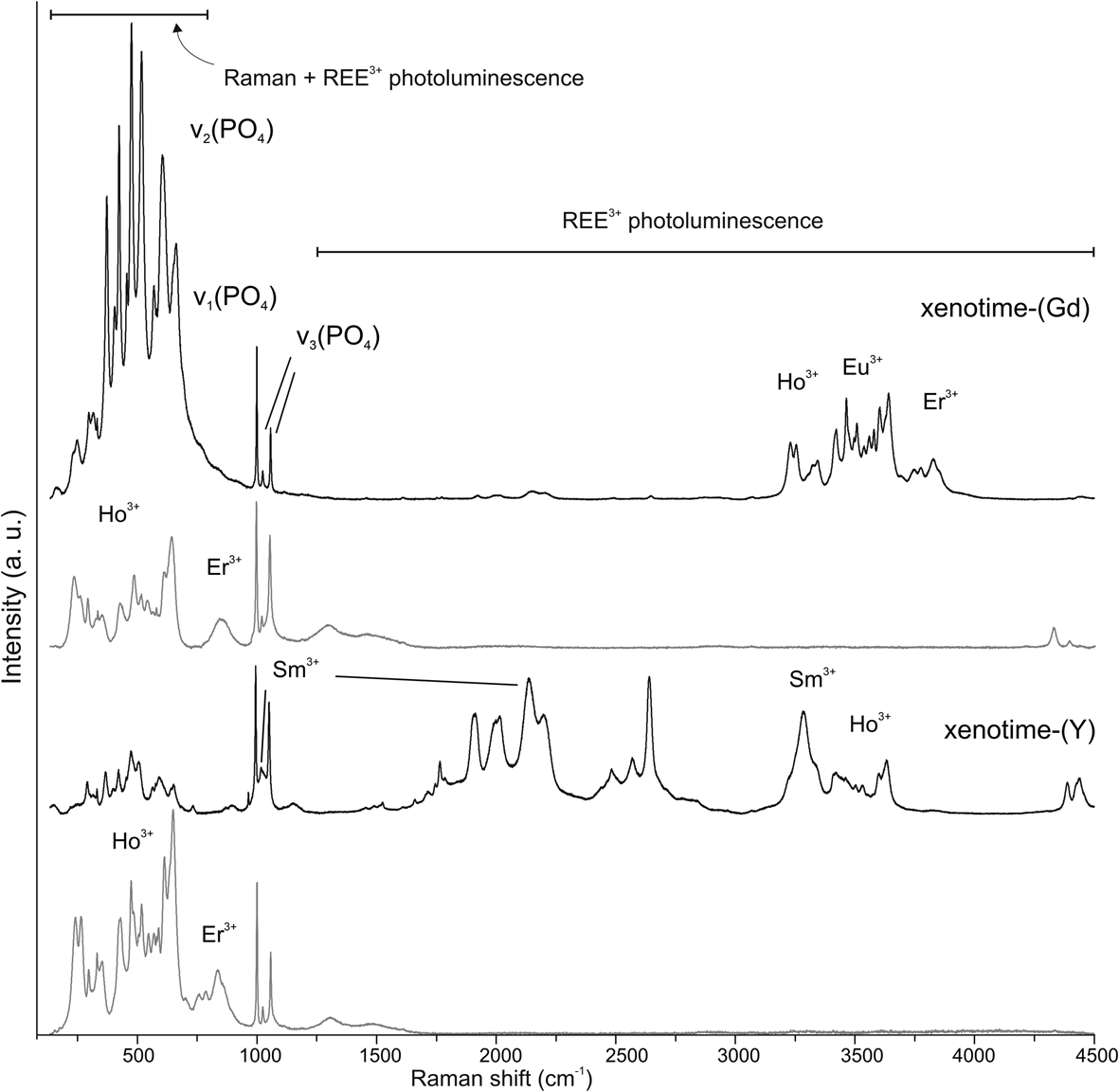
Figure 4. Depolarised Raman spectra captured for both natural xenotime-(Y) and xenotime-(Gd) (Prakovce, Zimná Voda ZV-2 sample) using two distinct laser excitations: 532 nm (black line, upper) and 633 nm (grey line, lower). The spectra are annotated with labelled sections indicating regions of photoluminescence (PL) and Raman bands. The intensity scale bar is provided in arbitrary units (a. u.).
The positions of the Raman bands for xenotime-(Gd) align with previously reported data on xenotime-type orthophosphates (Begun et al., Reference Begun, Beall, Boatner and Gregor1981; Lenz et al., Reference Lenz, Nasdala, Talla, Hauzenberger, Seitz and Kolitsche2015; Švecová et al., Reference Švecová, Čopjaková, Losos, Škoda, Nasdala and Cícha2016; Yahiaoui et al., Reference Yahiaoui, Hassairi and Dammak2017; Lösch et al., Reference Lösch, Hirsch, Holthausen, Peters, Xiao, Neumeier, Schmidt and Huittinen2019; Clavier et al., Reference Clavier, Mesbah, Szenknect and Dacheux2018). Generally, lattice vibration modes manifest below 300 cm–1 (Begun et al., Reference Begun, Beall, Boatner and Gregor1981; Lösch et al., Reference Lösch, Hirsch, Holthausen, Peters, Xiao, Neumeier, Schmidt and Huittinen2019) or up to 400 cm–1 (Clavier et al., Reference Clavier, Mesbah, Szenknect and Dacheux2018) encompassing translations and rotation modes of the entire (PO4)3– unit. According to this literature, bands in the spectral range of 950–1100 cm–1 relate to the P–O symmetric and antisymmetric stretching modes of (PO4)3– (Table 5). The frequencies observed for xenotime-(Gd) are lower than those for xenotime-(Y), aligning with the shifting positions of ν 1 and ν 3 observed in experimental studies on REE substitutions in xenotime-type orthophosphates (Begun et al., Reference Begun, Beall, Boatner and Gregor1981; Clavier et al., Reference Clavier, Mesbah, Szenknect and Dacheux2018). Peaks at 965 cm–1, 900 and 1150 cm–1 tentatively associate with Si and As substitution in an anion position in natural xenotime, necessitating further detailed examination. The strong PL is caused by the presence of Er3+ and Ho3+ (under 633 nm excitation), and by Er3+, Eu3+, Ho3+ and Sm3+ (under 532 nm excitation) (Fig. 4). Notably, Sm3+ probably contributes to the broadening of the 1017 cm–1 band, and the faint, broad band around 1150 cm–1 is possibly attributed to this luminescent centre (Lenz et al., Reference Lenz, Nasdala, Talla, Hauzenberger, Seitz and Kolitsche2015). Despite the Raman signal being overshadowed by PL, we deduce the absence of structural H2O or (OH)– in xenotime-(Gd) because of the absence of bands within the stretching modes range of these groups after 633 nm excitation.
Table 5. Raman bands for xenotime-(Gd).
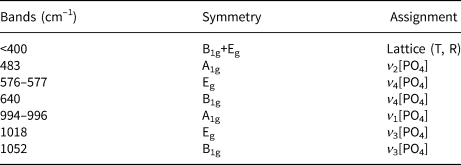
Notes: ν1 and ν3 – symmetric and antisymmetric stretching; ν2 and ν4 – symmetric and antisymmetric bending. Lattice modes: T – translation, R – rotation; REE photoluminescence bands not included.
Discussion
Xenotime-(Gd) is a new lanthanide orthophosphate mineral of the xenotime group with a natural unique Gd-dominance over the other REE cations. It represents only the third Gd-dominant mineral, described in Nature, and approved by IMA-CNMNC (Pasero, Reference Pasero2024), after lepersonnite-(Gd), CaGd2(UO2)24(SiO4)4(CO3)8(OH)24⋅48H2O, from the Shinkolobwe uranium deposit, DR Congo (Deliens and Piret, Reference Deliens and Piret1982) and cogenetic dimorphous monazite-(Gd) from the Zimná Voda occurrence near Prakovce, Slovakia (Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Majzlan, Pollok, Mikuš, Milovská, Molnárová, Škoda, Kopáčik, Kurylo and Bačík2023b). However, xenotime-(Gd) contains a significantly higher content of Gd: ≤29.3 wt.% Gd2O3, in comparison to lepersonnite-(Gd) (only 2.1 wt.% Gd2O3; Deliens and Piret, Reference Deliens and Piret1982) and monazite-(Gd) ≤23.4 wt.% Gd2O3 (Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Majzlan, Pollok, Mikuš, Milovská, Molnárová, Škoda, Kopáčik, Kurylo and Bačík2023b). Consequently, xenotime-(Gd) contains the highest Gd concentration among approved minerals. We emphasise the use of appropriate and precise analytical methods in these data, as there are other published data that document the possible presence of other Gd-dominant ‘minerals’, unfortunately without suitable analytical method or measurements. The presence of Gd-dominant ‘monazite' with 42.5 wt.% Gd2O3 and an empirical formula (Gd0.55Y0.25Dy0.1Sm0.05Nd0.05Th0Ca0)(PO4) is noted from alkali-feldspar syenite pegmatite at the Myan Gyi mine, near Mogok, Myanmar (Kartashov, web data at mineralienatlas.de; mindat.org); however, with no further information. The impressive REE accumulations in lignite coals from the Russian Far East Pavlovka deposit contain fine-grained authigenic unknown Gd- and Dy-dominant minerals (Seredin, Reference Seredin1996). Several nano- to micro-sized particles of Gd–Ti–Zr oxides with dominant Gd occupancy and 9−57 wt.% Gd2O3 were identified in the lunar regolith from Mare Crisium (Bogatikov et al., Reference Bogatikov, Mokhov, Kartashov, Magazina, Koporulina, Ashikhmina and Gorshkov2004; Mokhov et al., Reference Mokhov, Kartashov, Gornostaeva, Bogatikov and Ashikhmina2011). In addition, an unnamed Gd-dominant mineral (Gd > Ce > La) close to the Gd2Ti4O11 formula was noted from fumaroles in the active Kudriavy Volcano, Kuril Islands, Russia (Bogatikov et al., Reference Bogatikov, Mokhov, Kartashov, Magazina, Koporulina, Ashikhmina and Gorshkov2004). However, all the above-mentioned findings of possible Gd-dominant minerals need to be taken and interpreted with caution, because the data were obtained using semiquantitative EDX analysis without appropriate analytical details available. Furthermore, other analytical data (e.g, XRD, micro-Raman) are lacking.
Beside xenotime-(Gd), xenotime-(Y) from the Zimná Voda REE–U–Au occurrence is also enriched in Gd (11.6 to 27.9 wt.% Gd2O3, 0.13 to 0.35 apfu Gd). Moreover, inclusions of xenotime-(Gd), monazite-(Gd) and monazite-(Sm) (≤50 μm in size) with up to 29.8 wt.% Gd2O3 (≤0.38 apfu Gd) have been found recently in spessartine garnet in the Otov rare-element granitic metapegmatite (beryl–columbite-phosphate subtype), Czech Republic (Vrtiška, Ondrejka and Uher, unpubl. data). Rare occurrences of Gd, Dy-rich and Yb-dominant compositions in the xenotime-group minerals have also been described from some magmatic and metamorphic-hydrothermal systems (Demartin et al., Reference Demartin, Pilati, Diella, Donzelli, Gentile and Gramaccioli1991; Förster and Rhede, Reference Förster and Rhede1995; Förster, Reference Förster1998; Buck et al., Reference Buck, Cooper, Černý, Grice and Hawthorne1999; Masau et al., Reference Masau, Černý and Chapman2000; Franz et al., Reference Franz, Morteani and Rhede2015). In addition to Prakovce-Zimná Voda, the highest published Gd2O3 content in xenotime-(Y) attains 25 wt.% (0.31 apfu Gd), from the quartz vein of Au–REE mineralisation in the Subpolar Urals, Russia (Repina, Reference Repina2010, Reference Repina2011).
Such unusual enrichment of Gd in xenotime-(Gd) as well as in some other REE minerals [e.g. monazite-(Gd), hingganite-(Y)] and the progressive development of a MREE enhanced signature could be a result of local selective complexing of REE during granite to pegmatite sequence solidification in aqueous F–Cl–Li–CO2-bearing fluids (Masau et al., Reference Masau, Černý, Cooper, Chapman and Grice2002; Twardak et al., Reference Twardak, Pieczka, Kotowski and Nejbert2023). Moreover, hydrothermal xenotime-(Gd) and associated Gd-rich minerals precipitated in response to the alteration of MREE-selective, but nominally REE-free uraninite, brannerite and fluorapatite precursors by low-temperature hydrothermal fluids. Further details and description of Gd-MREE-enriched minerals in the Prakovce-Zimná Voda occurrence can be found in Ondrejka et al. (Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c).
The existence of GdPO4 and mixed GdxLn1–xPO4 dimorphism has been experimentally replicated and the kinetic-thermodynamic (meta)stability of the monazite and zircon structure types under various P-T conditions has been described in the literature (e.g. Mullica et al., Reference Mullica, Sappenfield and Boatner1990; Celebi and Kolis, Reference Celebi and Kolis2002; Kolitsch and Holtstam, Reference Kolitsch and Holtstam2004; Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013; Hay et al., Reference Hay, Mogilevsky and Boakye2013; Hefferman et al., Reference Hefferman, Ross, Spencer and Boatner2016; Meng et al., Reference Meng, Ding, Zhao, Li, C and Yang2016; Li et al., Reference Li, Ding, Meng, Ch, Wu and Yang2018; Muñoz and Rodríguez-Hernández, Reference Muñoz and Rodríguez-Hernández2018; and references therein). There is a general consensus that the structural boundary between monazite and xenotime usually lies between Gd and Tb (e.g. Ni et al., Reference Ni, Hughes and Mariano1995; Gratz and Heinrich, Reference Gratz and Heinrich1998; Hay et al., Reference Hay, Mogilevsky and Boakye2013; Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013). The investigation of natural GdPO4 orthophosphates from Prakovce, Zimná Voda (Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Majzlan, Pollok, Mikuš, Milovská, Molnárová, Škoda, Kopáčik, Kurylo and Bačík2023b, Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c) confirms the possibility of (1) substantial Gd incorporation into REE-selective structures and (2) the stabilisation of Gd-dominant orthophosphate with a zircon/xenotime-type structure by substitution of smaller HREE+Y cations in the A site (Mullica et al., Reference Mullica, Grossie and Boatner1986, Reference Mullica, Sappenfield and Boatner1990; Gratz and Heinrich, Reference Gratz and Heinrich1998; Clavier et al., Reference Clavier, Podor and Dacheux2011; Rodriguez-Liviano et al., Reference Rodriguez-Liviano, Becerro, Alcántara, Grazú, de la Fuente and Ocaña2013; Ondrejka et al., Reference Ondrejka, Uher, Ferenc, Milovská, Mikuš, Molnárová, Škoda, Kopáčik and Bačík2023c). The stabilisation of the monazite- versus xenotime-type structure of the natural REEPO4 species depends on the average REE3+ ionic radius (Kolitsch and Holtstam, Reference Kolitsch and Holtstam2004). In addition, the xenotime-(Gd) – monazite-(Gd) pair represents the first naturally occurring dimorphism among the known REE phosphates.
Acknowledgements
This work was supported by the Slovak Research and Development Agency under the contract APVV-22-0092. We thank Owen P. Missen, two anonymous reviewers, Mihoko Hoshino (Associate Editor) and Stuart Mills (Principal Editor) for their constructive suggestions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2024.62.
Competing interests
The authors declare none.
















