Introduction
Exposure to a broad category of adversities during the fetal period or delivery, herein called obstetric complications (OCs), increases schizophrenia risk 1.5–5 fold (Cannon et al., Reference Cannon, Jones and Murray2002b; Geddes and Lawrie, Reference Geddes and Lawrie1995; Nosarti et al., Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012; Pugliese et al., Reference Pugliese, Bruni, Carbone, Calabro, Cerminara, Sampogna, Luciano, Steardo, Fiorillo, Garcia and De Fazio2019). Recent findings on the interaction of genetic and OCs risk factors have linked neural insult to schizophrenia (Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli, Hamilton, Mitjans, Maddalena, Begemann, Seidel, Yanamori, Jaffe, Berman, Egan, Straub, Colantuoni, Blasi, Hashimoto, Rujescu, Ehrenreich, Bertolino and Weinberger2018). The liability towards schizophrenia explained by genetic risk predicted the diagnosis in the presence of OCs, but not in their absence (Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli, Hamilton, Mitjans, Maddalena, Begemann, Seidel, Yanamori, Jaffe, Berman, Egan, Straub, Colantuoni, Blasi, Hashimoto, Rujescu, Ehrenreich, Bertolino and Weinberger2018). The influence of OCs in the risk for developing bipolar disorders is less clear (Nosarti et al., Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012; Pugliese et al., Reference Pugliese, Bruni, Carbone, Calabro, Cerminara, Sampogna, Luciano, Steardo, Fiorillo, Garcia and De Fazio2019; Scott et al., Reference Scott, McNeill, Cavanagh, Cannon and Murray2006).
Impaired cognition is common in psychiatric disorders (Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill, Gurvich, Pantelis, Malhotra, Rossell and Burdick2017), yet the degree of neurodevelopmental abnormalities is greater in schizophrenia than bipolar disorder (Demjaha et al., Reference Demjaha, MacCabe and Murray2012), i.e. excessive neuromotor delays and cognitive difficulties were found in children who later developed schizophrenia compared to those who later developed affective disorders (Cannon et al., Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002a). Neuromotor abnormalities relate consistently to intelligence quotient (IQ) abilities and remain central in adult schizophrenia (Vaskinn et al., Reference Vaskinn, Ueland, Melle, Agartz, Andreassen and Sundet2014). Even though schizophrenia and bipolar disorder are nosologically distinct, studies have revealed shared cognitive heterogeneity (Bora and Pantelis, Reference Bora and Pantelis2015; Hill et al., Reference Hill, Reilly, Keefe, Gold, Bishop, Gershon, Tamminga, Pearlson, Keshavan and Sweeney2013; Tamminga et al., Reference Tamminga, Pearlson, Keshavan, Sweeney, Clementz and Thaker2014; Van Rheenen et al., Reference Van Rheenen, Bryce, Tan, Neill, Gurvich, Louise and Rossell2016; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill, Gurvich, Pantelis, Malhotra, Rossell and Burdick2017).
Certain features of cognition appear to be unaffected by disease processes (e.g. schizophrenia, dementia, depression), and cognitive measures such as word-reading ability can act as ‘hold tests’ to estimate premorbid IQ (Nelson and Willison, Reference Nelson and Willison1991). Other aspects of cognition seem to deteriorate with the course of illness; tests of more fluid intelligence provide an indication of current cognitive abilities in patients. In fact, lower premorbid IQ was related to more severe cognitive impairment across schizophrenia and bipolar diagnoses with larger deficits observed in schizophrenia (Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill, Gurvich, Pantelis, Malhotra, Rossell and Burdick2017). Premorbid IQ was highly predictive of psychotic disorders in an umbrella review (Khandaker et al., Reference Khandaker, Barnett, White and Jones2011; Radua et al., Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee, Amir, Yenn Thoo, Oliver, Davies, Morgan, McGuire, Murray and Fusar-Poli2018; Woodberry et al., Reference Woodberry, Giuliano and Seidman2008), confirming the role of a cognitive antecedent in psychosis onset.
To date, little is known about the effects of having experienced OCs on cognition in severe mental illness. The most recent study of OCs’ association to cognitive functioning was a clinical cohort study of 157 patients in the year 2000 and showed no relationship within patients with affective psychosis and schizophrenia (Gilvarry et al., Reference Gilvarry, Takei, Russell, Rushe, Hemsley and Murray2000). They did, however, find differences in premorbid IQ between patients and their first-degree relatives, where the greatest IQ disparity was found between patients with OCs and their relatives. The larger deficit in patients with OCs could not be explained by shared genetic factors alone and is an indication that OCs might contribute to the lower premorbid IQ in these patients.
The evolving inference is that the intra-uterine environment partly mediates the genomic risk of schizophrenia, which has basic implications for pathogenesis (Birnbaum and Weinberger, Reference Birnbaum and Weinberger2017; Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli, Hamilton, Mitjans, Maddalena, Begemann, Seidel, Yanamori, Jaffe, Berman, Egan, Straub, Colantuoni, Blasi, Hashimoto, Rujescu, Ehrenreich, Bertolino and Weinberger2018). Investigating the pre- and perinatal period can be challenging, as data are usually collected retrospectively. Small sample sizes, lack of statistical power to measure small-interactive effects and inefficient reporting on the pre- and perinatal period have restricted knowledge on the contribution of OCs in mental illness (Cannon et al., Reference Cannon, Jones and Murray2002b; Radua et al., Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee, Amir, Yenn Thoo, Oliver, Davies, Morgan, McGuire, Murray and Fusar-Poli2018).
To overcome these limitations, we utilized the Medical Birth Registry of Norway (MBRN) to provide unique insight into pregnancy and birth information on 1492 patients and healthy controls in the Thematically Organized Psychosis (TOP) study cohort. The main goal of this study was to investigate putative effects of a history of OCs on adult cognition in schizophrenia and bipolar spectrum disorders. Neurodevelopmental implications of severe OCs might be marked cognitive impairments present before and after disease onset. A more severe disease progression indicated by a cognitive decline that reflects both early cognitive deficits and more severe course of pathology from the time of illness onset (Wells et al., Reference Wells, Swaminathan, Sundram, Weinberg, Bruggemann, Jacomb, Cropley, Lenroot, Pereira, Zalesky, Bousman, Pantelis, Weickert and Weickert2015; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill, Gurvich, Pantelis, Malhotra, Rossell and Burdick2017). Our study objectives in the clinical cohort of patients on the schizophrenia-bipolar spectrum and healthy controls aimed to: (1) investigate the prevalence of severe OCs across diagnoses through information obtained in the MBRN; (2) examine premorbid and current IQ of OCs-defined subgroups; (3) determine differential effects of OCs by diagnostic category; (4) assess the effects of the number of co-occurring OCs and (5) examine the degree to which OCs-defined subgroups could be differentiated on demographic and clinical measures.
Materials and methods
Participants
The TOP study is a thematic study research effort focused on the disease mechanisms of psychotic disorders and is the main study protocol at the Norwegian Centre for Mental Disorders Research (NORMENT, Oslo, Norway; www.med.uio.no/norment/english). Adult patients were recruited consecutively from psychiatric units (outpatient and inpatient) of public hospitals in the Oslo region, which have the complete catchment-based service area covering about one million inhabitants of Oslo's urban area. The hospitals are located in different parts of the city and are representative of the city's variation in sociodemographic characteristics. The healthy controls (HC) were randomly selected from the national population register and were residents in the same catchment area as the patients. After a complete description of the study, all participants gave written informed consent. The Regional Committee for Research Ethics and the Norwegian Data Inspectorate approved the study.
Exclusion criteria for both patients and HC were hospitalization for previous moderate or severe head injury, neurological disorder, medical conditions thought to interfere with brain function and age outside the range of 18–65 years. Additional exclusion criteria for HC were current or previous somatic illness and substance misuse disorders or dependency within the last 6 months. HC were also excluded if they or a first-degree relative had a lifetime history of severe psychiatric disorder.
All patients underwent thorough clinical investigation by trained psychologists and physicians. Clinical diagnoses were assessed using the Structured Clinical Interview for DSM-IV axis 1 disorder (SCID-I) module A-E (Spitzer et al., Reference Spitzer, Williams, Gibbon and First1988). Psychosocial function was assessed with the Global Assessment of Function scale, split version (GAF; Pedersen et al., Reference Pedersen, Hagtvet and Karterud2007). Current psychotic symptoms were rated by the use of the Positive and Negative Syndrome Scale (PANSS; Kay et al., Reference Kay, Fiszbein and Opler1987).
HC were interviewed by trained research assistants and examined with the Primary Care Evaluation of Mental Disorders (Prime-MD) to ensure no current or previous psychiatric disorders (Spitzer et al., Reference Spitzer, Williams, Kroenke, Linzer, deGruy, Hahn, Brody and Johnson1994).
From the onset of the study in October 2002 until June 2015, the total subject sample (n = 1492) consisted of patients with a DSM-IV diagnosis within the schizophrenia spectrum (SCZ): schizophrenia (DSM-IV 295.1, 295.3, 295.6, and 295.9; n = 346), schizophreniform disorder (DSM-IV 295.4; n = 43), schizoaffective disorder (DSM-IV 295.7; n = 70) or psychosis not otherwise specified (DSM-IV 298.9; n = 148); or within the bipolar spectrum (BIP): Bipolar I disorder (DSM-IV 296.0–7; n = 164), Bipolar II disorder (DSM-IV 296.89; n = 80) or bipolar disorder not otherwise specified (DSM-IV 296.80; n = 19); and HC (n = 622).
Obstetric complications
Birth data were collected from the national MBRN. In Norway, there is mandatory reporting on all births after gestational week 16 (online Supplementary material – Appendix 1: MBRN form from 1967–1998). MBRN data were scored for the presence and severity of OCs (McNeil and Sjöström, Reference McNeil and Sjöström1995) by two physicians (UKH and KE) who were blinded to patient/ control status (inter-rater reliability = 0.94).
The validated McNeil–Sjöström scale (McNeil et al., Reference McNeil, Cantor-Graae and Sjostrom1994; McNeil and Sjöström, Reference McNeil and Sjöström1995) includes several hundred events of potential harm to the fetus/offspring, each classified according to severity on an ordinal scale from 1 to 6. As in other reports (Nicodemus et al., Reference Nicodemus, Marenco, Batten, Vakkalanka, Egan, Straub and Weinberger2008; Haukvik et al., Reference Haukvik, McNeil, Lange, Melle, Dale, Andreassen and Agartz2014), an incidence of severe OCs was reported in those participants who had experienced one or more complications of a grade 5 or 6. Subjects with complications of grade 4 and below were classified as having an absence of severe OCs. A complication of grade 5 is defined as an event that is ‘potentially clearly greatly relevant/harmful’ to the central nervous system of the developing fetus/offspring, and a complication of grade 6 is defined as an event that causes ‘very great harm to or deviation in offspring’ (McNeil and Sjöström, Reference McNeil and Sjöström1995). Examples of grade 5 or 6 are as follows: severe preeclampsia, bleeding before 28 weeks, asphyxia, discolored placenta/amniotic fluid, emergency caesarean delivery, preterm birth ⩽35 weeks, low Apgar score (0–3 at 1 min or 0–7 at 5 min), bleeding during labor, low birth weight ⩽2000 g and eclampsia (see online Supplementary material – Note: Obstetric complications for more details).
IQ measures
Current IQ was evaluated using the Wechsler Abbreviated Scale of Intelligence (WASI) full scale IQ assessment (Wechsler, Reference Wechsler2007). The National Adult Reading Test (NART) was designed to give an indication of cognitive functioning preceding symptoms of disease and word-reading skills are significantly correlated with Wechsler-based IQ scores (Nelson and Willison, Reference Nelson and Willison1991). The reliability and validity of the Norwegian language version of the NART have been established (Sundet and Vaskinn, Reference Sundet and Vaskinn2008). The premorbid IQ estimate was calculated using the WASI full scale IQ formula (Sundet and Vaskinn, Reference Sundet and Vaskinn2008). Cognitive decline was calculated by subtracting premorbid IQ from current IQ scores (current IQ – premorbid IQ) and will be referred to as IQ difference scores.
Statistical analyses
Statistical analyses were performed within the Statistical Package of Social Sciences (SPSS), version 25 (IBM, USA; www.spss.com).
Demographic and clinical characteristics were compared between groups using chi-squared tests for categorical variables and analysis of variance (ANOVA) or covariance (ANCOVA) for continuous variables. Age, years of education and sex distributions were identified as potential confounders on cognitive variables, and we adjusted accordingly in between-group analyses. Subjects with missing data on any particular variable were omitted from analyses involving that variable, but were included in analyses for which all required variables were present.
Three separate multiple linear regressions were calculated for premorbid IQ, current IQ or IQ difference scores (as dependent variables), respectively. Age, education, sex, OCs, HC v. BIP, HC v. SCZ, OC*BIP and OC*SCZ were entered as independent variables. Premorbid IQ was estimated from NART total errors, and covariates (age, education and sex) were adjusted for in the regression models.
To assess differences in the number of OCs frequency between groups, we preformed chi-squared tests. Further, to examine if cognitive abilities were directly related to the number of co-occurring OCs that were rated 5 and 6 in each group, we performed two additional multiple regression analysis entering IQ variables as dependent variables and age, education level, sex, HC v. SCZ, HC v. BIP, One OC, Two or more (2+) OCs as independent variables. In the second regression, age, education level, sex, One OC, Two OCs and Three or more (3+) OCs were compared in only HC v. SCZ because the BIP group had too few cases of 3 or more OCs.
Results
Demographic and clinical variables
There were significant differences in sex distribution and education levels between the three participant groups (Table 1). Age did not differ between patient groups, but it did differ between patient groups and HC. Between the patient groups, age at illness onset, illness duration, PANSS scores and GAF scores were significantly different. In the BIP group, age of illness onset was earlier than in the SCZ group. The duration of illness was longer in the BIP group than in the SCZ group. A greater degree of clinical symptoms was found in the SCZ group compared to the BIP group and functioning was significantly reduced in the SCZ group, as well. Age at illness onset, illness duration, PANSS and GAF variables did not differ as a function of OCs within patient groups. Current IQ and IQ difference scores differed between groups. Negative IQ difference scores indicated a discrepancy between current IQ and premorbid IQ. Premorbid IQ estimates did not differ between the BIP group and HC, but differed between the SCZ group and HC. Across all three groups, there were no significant differences in birth weight or gestational age.
Table 1. Demographic and clinical characteristics
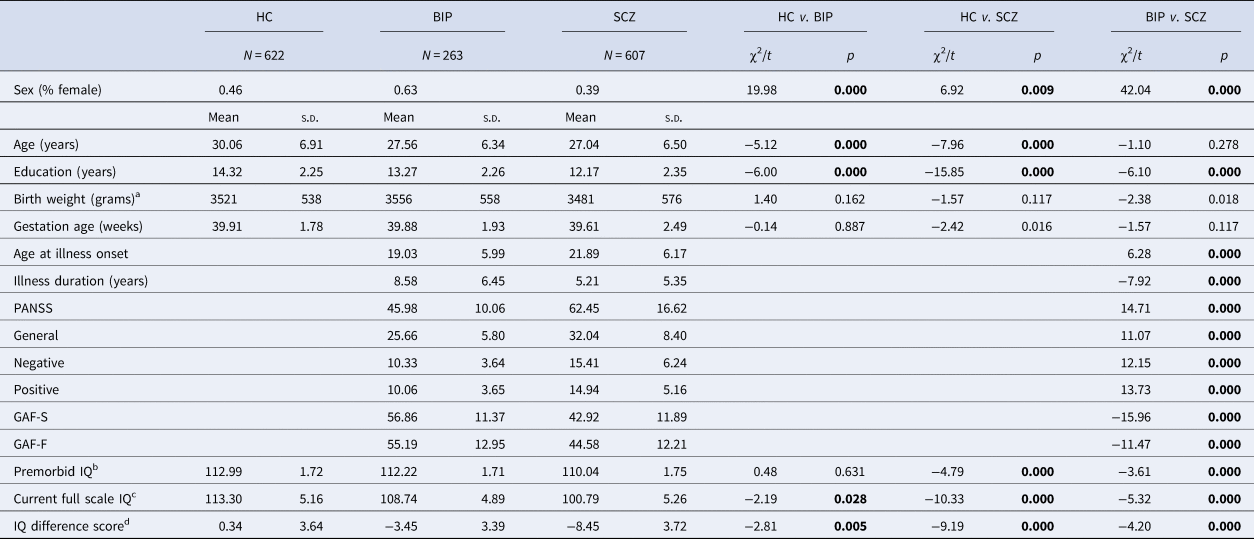
a Adjusted for sex; s.d. (standard deviation); HC (healthy controls); BIP (bipolar spectrum); SCZ (schizophrenia spectrum); PANSS (Positive and Negative Syndrome Scale); GAF-S (Global Assessment of Functioning- symptoms); GAF-F (Global Assessment of Functioning-functioning). Bold numbers represent significant values.
b NART premorbid IQ estimate adjusted for age, education and sex (HC: N = 582, BIP: N = 230 and SCZ: N = 411).
c WASI full scale IQ assessment adjusted for age, education and sex (HC: N = 580, BIP: N = 235 and SCZ: N = 428).
d IQ difference score (current IQ – premorbid IQ) adjusted for age, education and sex (HC: N = 579, BIP: N = 228 and SCZ: N = 396)
Obstetric complications
There were no significant differences in the frequency of OCs between groups (Table 2). However, two of the complications (preterm and low birth weight) were more frequent in the group with SCZ patients, compared to the HC. Bleeding during labor occurred more often in the HC in comparison to the SCZ group. The most frequent OCs across groups were asphyxia (⩾50%) and discolored placenta/amniotic fluid (⩾39%).
Table 2. Frequency of obstetric complications
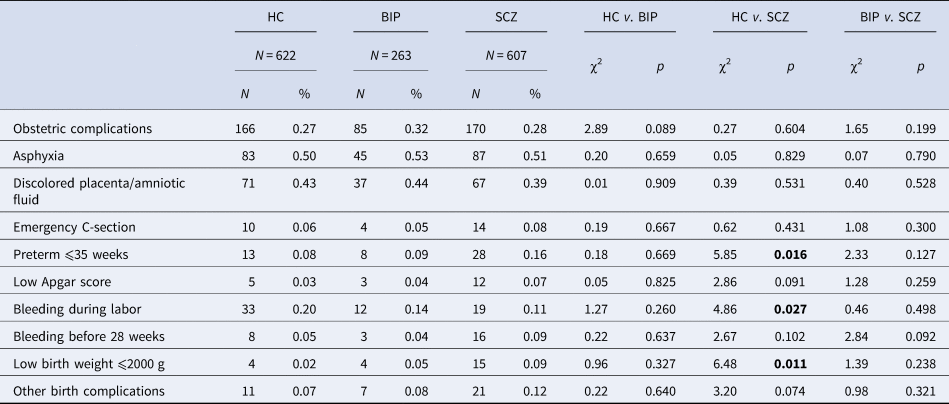
HC, healthy controls; BIP, bipolar spectrum; SCZ, schizophrenia spectrum. Bold numbers represent significant values.
IQ measures
Multiple linear regression analyses revealed that the independent variables predicted premorbid IQ, current IQ and IQ difference scores in the overall models (Table 3). Each model explained 24%, 31% and 23% of the variance in the change of IQ scores, respectively. We found an OCs by SCZ group interaction on premorbid and current IQ variables. An interaction effect indicated that within the SCZ group, patients with a history of OCs had lower premorbid and current IQ compared to SCZ patients without a history of OCs. This was not the case for the HC or BIP groups.
Table 3. Multiple regression analyses by premorbid IQ, current IQ and IQ difference scores
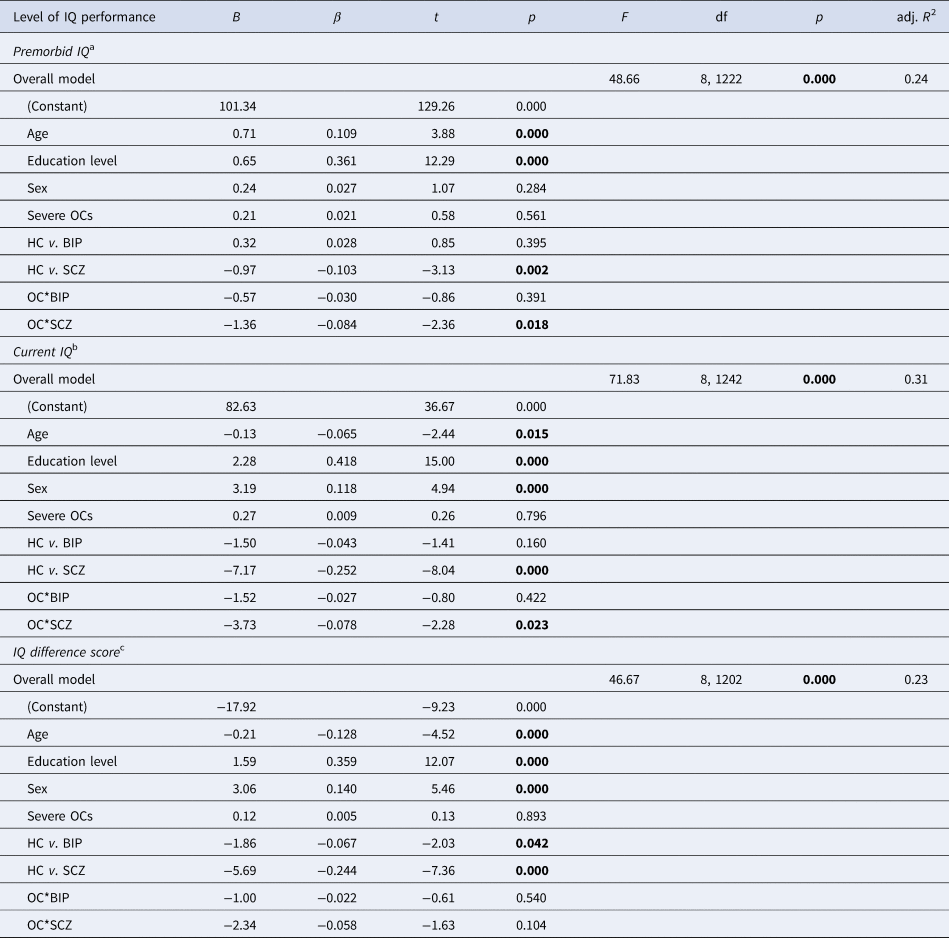
HC, healthy controls; BIP, bipolar spectrum; SCZ, schizophrenia spectrum; df, degrees of freedom. Bold numbers represent significant values.
a NART premorbid IQ estimate adjusted for age, education and sex (HC: N = 582, BIP: N = 230 and SCZ: N = 411).
b WASI full scale IQ assessment adjusted for age, education and sex (HC: N = 580, BIP: N = 235 and SCZ: N = 428).
c IQ difference score (current IQ – premorbid IQ) adjusted for age, education and sex (HC: N = 579, BIP: N = 228 and SCZ: N = 396).
Because OCs are not isolated events and commonly occur together (McNeil and Sjöström, Reference McNeil and Sjöström1995), we assessed the number of OCs that were rated 5 and 6 in each group using chi-squared analyses. We found no statistically significant differences (χ2 (2) = 1.63, p = 0.442) in the number of co-occurring OCs (1 and 2+) between the SCZ group (1 OC = 56%; 2 + OCs = 44%), the BIP group (1 OC = 61%; 2 + OCs = 39%) and HC (1 OC = 63%; 2 + OCs = 37%). In the BIP group, there were only 3 cases with 3 + OCs, so the BIP group was not included in these analyses. We found no statistically significant differences (χ2 (2) = 4.87, p = 0.088) in the number of co-occurring OCs (1, 2 and 3+) between the SCZ group (1 OC = 56%; 2 OCs = 31%; 3 + OCs = 13%) and HC (1 OC = 63%; 2 OCs = 31%; 3 + OCs = 6%).
Furthermore, we found premorbid IQ scores were significantly lower with more than 2 severe OCs in both adult patients with SCZ and HC (online Supplemental materials: Table S1B and Fig. 1a). This was not the case for BIP group, as they did not differ from the HC on premorbid IQ measures. Current IQ measures were significantly lower if more than one severe OC was experienced, and this effect was observed in all groups (online Supplemental materials: Table S1A and Fig. 1b). IQ difference scores (current IQ – premorbid IQ) showed a significant decrease in IQ scores in those that had experienced more than one severe OC, also seen in all groups (online Supplemental materials: Table S1A and Fig. 1c). In those with 2 + OCs, asphyxia-related OCs were identified in 88% (50 out of 57) of the healthy controls, 81% (21 out of 26) of patients in the bipolar group and 71% (29 out of the 41) of patients in the schizophrenia group. We identified 15 schizophrenia patients with current IQ below 70 in our sample, and 33% (5 out of 15) had severe OCs in their histories.
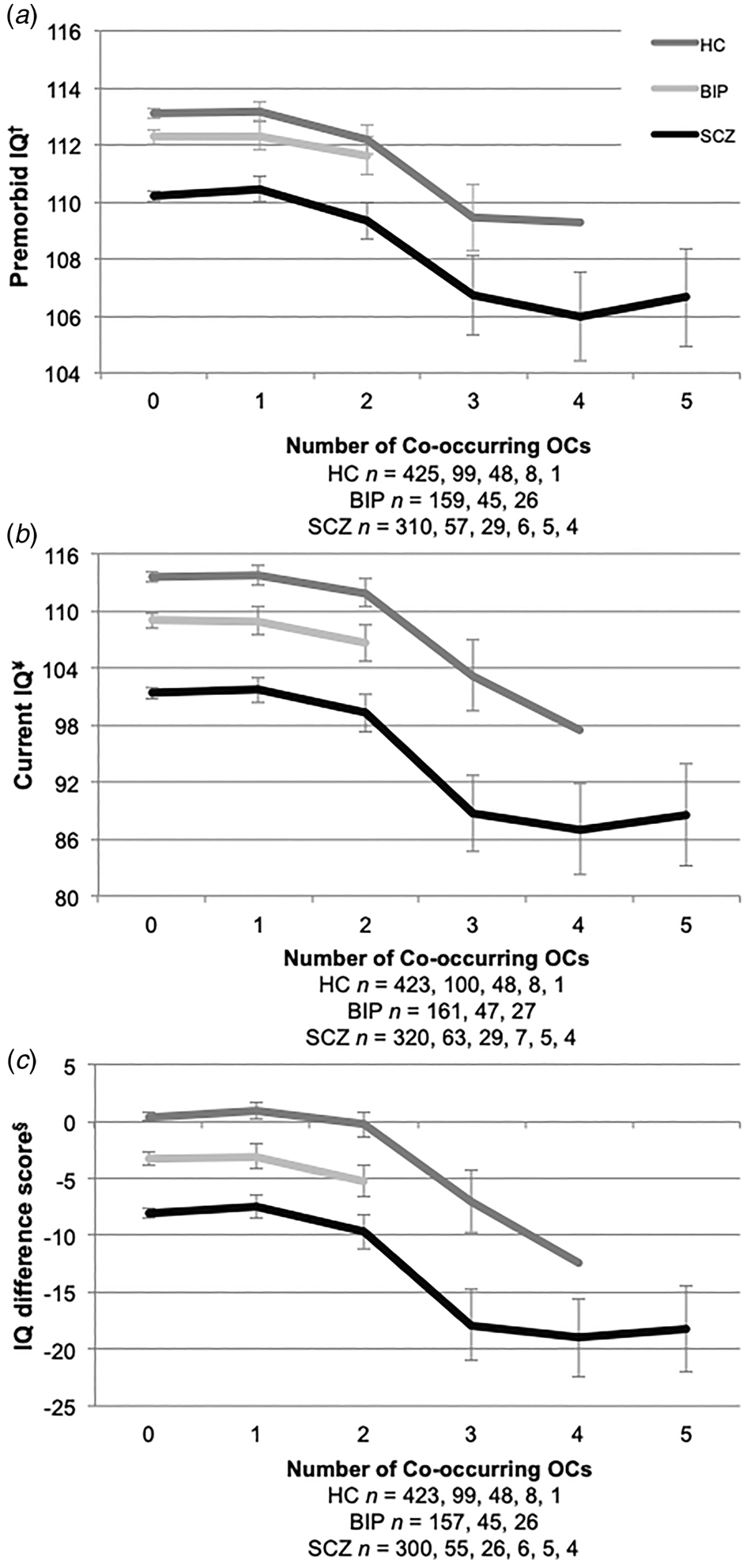
Fig. 1. IQ across the number of co-occurring severe OCs. HC, healthy controls; BIP, bipolar spectrum; SCZ, schizophrenia spectrum. (a) †NART premorbid IQ estimate; (b) ¥WASI full scale assessment; and (c) §IQ difference score (current IQ – premorbid IQ). The numbers below the x-axis refer to the number of co-occurring OCs in participant histories. Error bars represent ±2 standard errors.
Discussion
We found severe OCs to be equally common across groups, and the number of OCs co-occurring was also similar. An interaction effect indicated that within the schizophrenia group, patients with a history of OCs had lower premorbid and current IQ compared to schizophrenia patients without a history of OCs. To have experienced more than two co-occurring OCs was specifically associated with lower premorbid IQ in both patients within the schizophrenia group and healthy controls, but not in the bipolar group. However, more than one co-occurring OC was related to lower current IQ and greater decreases in IQ difference scores in all participant groups.
Despite scientific reports that have stressed the role of OCs as a risk factor for the development of schizophrenia (Cannon et al., Reference Cannon, Jones and Murray2002b; Geddes and Lawrie, Reference Geddes and Lawrie1995; Nosarti et al., Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012; Pugliese et al., Reference Pugliese, Bruni, Carbone, Calabro, Cerminara, Sampogna, Luciano, Steardo, Fiorillo, Garcia and De Fazio2019), the precise nature of their relationship to cognitive functioning in psychiatric disorders is unknown. We report that severe OCs occurred in 28% of patients within the schizophrenia group, but we also observed similar numbers in both the bipolar patient group and healthy control group, which is comparable to what has been reported in other studies (Haukvik et al., Reference Haukvik, Lawyer, Bjerkan, Hartberg, Jonsson, McNeil and Agartz2009; Nicodemus et al., Reference Nicodemus, Marenco, Batten, Vakkalanka, Egan, Straub and Weinberger2008; Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli, Hamilton, Mitjans, Maddalena, Begemann, Seidel, Yanamori, Jaffe, Berman, Egan, Straub, Colantuoni, Blasi, Hashimoto, Rujescu, Ehrenreich, Bertolino and Weinberger2018). These findings support the idea that having a history of OCs might be an additive/interactive risk for the development of a psychiatric disorder (Walter and Holford, Reference Walter and Holford1978). Since OCs were equally occurring in all groups, psychiatric disorders must be mainly determined by other factors and not directly caused by OCs. Severe OCs might be an additional factor, wherein their presence further increases the probability of developing a condition with a more severe cognitive course. In this study, we found that the associations between OCs and IQ were stronger in patients within the schizophrenia group, compared to the bipolar group, but were also observed in the healthy controls.
IQ is the most discriminating neuropsychological variable between patients with schizophrenia and affective disorders (Goldberg et al., Reference Goldberg, Gold, Greenberg, Griffin, Schulz, Pickar, Kleinman and Weinberger1993). We found that patients with schizophrenia and OCs had lower premorbid and current IQ than patients who had not experienced OCs. Severe OCs in this patient group can be presumed to have early neurodevelopmental consequences on cognition, as reduced IQ was present before illness onset. Common genetic variants associated with schizophrenia are enriched for associations with lower intelligence (Smeland et al., Reference Smeland, Bahrami, Frei, Shadrin, O'Connell, Savage, Watanabe, Krull, Bettella, Steen, Ueland, Posthuma, Djurovic, Dale and Andreassen2019) and intracranial volume (Smeland et al., Reference Smeland, Wang, Frei, Li, Hibar, Franke, Bettella, Witoelar, Djurovic, Chen, Thompson, Dale and Andreassen2018), a proposed biomarker of brain development (Woodward and Heckers, Reference Woodward and Heckers2015). Lower IQ performance might indicate brain alterations and differing developmental trajectories within schizophrenia. Even though we did not identify differences on clinical measures between OCs-related subgroups, more studies are need to clarify the relationship between OCs, genetic risk, brain changes and clinical characteristics.
We found that having more than one severe OC, which is clearly harmful to the developing central nervous system of the fetus/offspring (McNeil and Sjöström, Reference McNeil and Sjöström1995), was specifically associated to lower current IQ and greater IQ difference scores (current IQ – premorbid IQ) in both patient groups and healthy controls, which indicates a decline in cognition. The significant discrepancies between current and premorbid IQ in both patient groups might imply that common pathogenic processes operate once illness has begun. Additionally, processes may have started earlier in schizophrenia, as IQ did not differ between the bipolar group and healthy controls, premorbidly, which gives an indication that there might be better resilience in bipolar disorder. Yet, lower current IQ and greater IQ difference scores in those who had experienced more than one OC might suggest a more vulnerable neurodevelopmental subgroup of both patient groups and healthy controls who are less resilient to age-related processes or pathological processes beginning later in life.
We found that 50% of all severe OCs were related to asphyxia at birth (i.e. deprivation of oxygen). Of the participants with more than one OC, we identified asphyxia-related OCs in 88% of healthy controls, 81% of patients in the bipolar group and in 71% of patients in the schizophrenia group. We observed a pattern of lower IQ as the number of OCs increased that might reflect the magnitude of neurodevelopmental insult of OCs on cognitive brain development. Asphyxia might specifically impact the developing brain. It appears that both a greater number of harmful OCs and asphyxia experienced at birth influence adult IQ, more so in vulnerable individuals who later develop a severe mental illness, but a series of OCs do not necessarily lead to a disorder. A direction for future research might be to improve knowledge of neural injury due to OCs and implement neuroprotective interventions for better outcome in vulnerable newborns.
Current pharmacological treatment for schizophrenia mainly improves positive symptoms, but has no documented improvement on cognitive impairments, which together with negative symptoms are the most disabling features of schizophrenia long-term (Fusar-Poli et al., Reference Fusar-Poli, Papanastasiou, Stahl, Rocchetti, Carpenter, Shergill and McGuire2015). We identified 15 schizophrenia patients with current IQ below 70 in our sample. Excluding this group from analyses did not alter group proportions, means or results, and doing so, would not reflect the number of patients with compromised intellectual function in the schizophrenia population (Wells et al., Reference Wells, Swaminathan, Sundram, Weinberg, Bruggemann, Jacomb, Cropley, Lenroot, Pereira, Zalesky, Bousman, Pantelis, Weickert and Weickert2015). Severe OCs were found in 33% of these patients, which might have been an additive risk factor for schizophrenia. We realize that there are other non-purely genetic risk factors, defined as socio-demographic, parental, later factors and antecedents (Radua et al., Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee, Amir, Yenn Thoo, Oliver, Davies, Morgan, McGuire, Murray and Fusar-Poli2018), that may increase the risk or decrease any protective effects and influence the likelihood of developing a psychiatric disorder. Urbanization, migration, childhood social withdrawal, childhood trauma, Toxoplasma gondii Immunoglobulin G, and non-right handedness might interact or be additive risks (Radua et al., Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee, Amir, Yenn Thoo, Oliver, Davies, Morgan, McGuire, Murray and Fusar-Poli2018) to a genetic predisposition that operates by impairing the resilience of the fetal and neonatal brain (Murray et al., Reference Murray, Bhavsar, Tripoli and Howes2017). The neurodevelopmental significance of having experienced severe OCs is that cognitive deficits are major aspects of treatment-resistant schizophrenia. Therefore, we need to explore other avenues of treatment and optimize perinatal care in vulnerable individuals, for instance in mothers with a family history of severe psychosis.
Strengths of this study were the use of the Medical Birth Registry of Norway data, which allows for a more accurate and prospective assessment of OCs in severe mental illness. Our study provides a priori knowledge about the pre- and perinatal environment (indicated with OCs) in patients who later develop psychiatric disorders. We observed normal-ranged IQ means (i.e. ≈100) across groups, yet we were still able to identify adverse effects of OCs on IQ measures. Importantly, it reveals clinical evidence on early life factors and their possible impact on cognition in adult patients, which is currently missing in the field (Radua et al., Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee, Amir, Yenn Thoo, Oliver, Davies, Morgan, McGuire, Murray and Fusar-Poli2018). Including OCs as a component of risk may advance our ability to make prognoses in psychiatric disorders, which might soon compliment genetic vulnerability or other predictors.
The use of the NART as an index of prior intellectual ability (rather than current) is well established with high retrospective validity. In fact, a 66-year follow up study in healthy individuals found that NART performance at age 77 and IQ at age 11 had a highly significant correlation (r = 0.73, p < 0.001) (Crawford et al., Reference Crawford, Deary, Starr and Whalley2001). The validation for the Norwegian version of the NART for patients with schizophrenia and bipolar disorders also revealed highly significant correlations between estimated and actual IQ (r = 0.64, p < 0.001; r = 0.49, p < 0.001, respectively) (Sundet and Vaskinn, Reference Sundet and Vaskinn2008). The use of the NART as an estimate of premorbid intellectual ability in clinical populations is supported by this series of results and is in line with the findings of the present study.
This study has some limitations. Exclusion criteria in the TOP study precluded recruitment of healthy controls if they had a first-degree relative with a lifetime history of severe psychiatric disorder. Further, despite a random selection from the population, it seems that the controls were performing above average on cognitive tests. A recent study indicates that large proportions of the genomic risk underlying schizophrenia also influence intelligence (Smeland et al., Reference Smeland, Bahrami, Frei, Shadrin, O'Connell, Savage, Watanabe, Krull, Bettella, Steen, Ueland, Posthuma, Djurovic, Dale and Andreassen2019). It is possible that even more pronounced effect of OCs on IQ would have been evident had more genetically varied controls been included in the analyses.
Although the NART is a validated measure of premorbid IQ (Sundet and Vaskinn, Reference Sundet and Vaskinn2008), an actual IQ assessment administered before the onset of symptoms may more accurately describe participants, especially in cases of extreme scores (Mathias et al., Reference Mathias, Bowden and Barrett-Woodbridge2007).
Even though we did not find an association between OCs and premorbid IQ in the bipolar group, this group had half the sample size of the other two groups in our study. Since each type of OC event is infrequent in a population, the smaller sample size in this group might lack the statistical power to measure small and interactive effects. Cognitive decline, indicated by IQ difference scores, in the bipolar patients with OCs was significantly lower when the number of co-occurring OCs was taken into account.
In conclusion, the relevance of this study is that a history of severe OCs seems to be associated with cognitive functioning in adult participants. These findings add to new reports indicating that genetic risk for schizophrenia is found to start in the womb, interacting with placental integrity (Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli, Hamilton, Mitjans, Maddalena, Begemann, Seidel, Yanamori, Jaffe, Berman, Egan, Straub, Colantuoni, Blasi, Hashimoto, Rujescu, Ehrenreich, Bertolino and Weinberger2018). Even though the placenta might be a key environmental factor for psychopathology, placenta status is also of major importance for IQ (Jacobs et al., Reference Jacobs, Van Gestel, Derom, Thiery, Vernon, Derom and Vlietinck2001; Patterson, Reference Patterson2007). Low IQ might be a marker for schizophrenia development and an experience of OCs appears to increase the risk. A better understanding of early pre- or perinatal factors such as OCs and genetic risk in the context of brain development could advance the role of perinatal care for reducing the burden of severe mental illness. Also, it may improve strategies for primary prevention of schizophrenia.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719002046
Acknowledgements
The authors thank the participants for their contribution in this study. We express gratitude to the clinicians and research assistants at NORMENT for their time and effort invested in data collection and to all referring hospital units.
Financial support
This work was supported by the Research Council of Norway (grant number 223273) and the South-Eastern Norway Regional Health Authority (grant number 2017–093).
Conflict of interest
The authors report no financial relationships with commercial interest, other than Dr Andreassen, who received speaker's honoraria from Lundbeck.







