Encapsulation of micro-objects with a polymeric membrane is desired in many applications such as pharmaceutics and microelectronics. This is currently achieved through three-dimensional (3D) printing, lithography, or microfluidics. These are demanding techniques. For example, microfluidics requires oil–water interfaces that may not be compatible with the chemistry of a drug. To address this issue, the research teams of Paolo Netti of the University of Naples Frederico II and Istituto Italiano di Tecnologia in Naples, Pietro Ferraro of the Institute of Applied Sciences and Intelligent Systems in Pozzuoli, Italy, and their colleagues are proposing an unusual and very efficient alternative, using polymer solutions. The “quick liquid packaging” method they developed was published in Science Advances (doi:10.1126/sciadv.aat5189). The approach stands out for its simplicity. It relies on surface tension and the need for two non-miscible solutions to minimize their interfacial energy.
In the process, a polymer solution such as poly(lactic-co-glycolic acid) (PLGA) is dissolved at a given concentration in a solvent, for instance dimethyl carbonate (DMC). PLGA is a common biocompatible polymer, whereas DMC is an organic solvent widely used for biomedical applications. DMC also easily dissolves PLGA, and evaporates slowly. When a hydrophilic object, such as a droplet of water, is placed in the DMC-PLGA solution, a PLGA film forms around it, adopting its form. DMC then diffuses into the water, leaving behind a stiff and highly packed 3D PLGA membrane.
Using the same method to produce self-standing PLGA films, the researchers recorded a thickness as small as 1.65 µm, hydrophilicity, transparency, and an elastic modulus of 75 MPa. This is in the range of polyethylene used in most electrical or water pipes. The PLGA film is also permeable to oxygen and other liquid exchanges. These properties were demonstrated by encapsulating a microdroplet containing the worm Caenorhabditis elegans. When encapsulated, the worms decreased their activity, but remained alive for the duration of the experiment, demonstrating the biocompatibility of the process. Biocompatibility was further confirmed by the cultivation of human stem cells directly onto the PLGA membrane.
Many other objects could also be packaged, such as polystyrene microparticles, pillar arrays, or even optical microfibers. “Packaging of optical fibers could provide an attractive solution for sensing applications revealed by a change in refractive index,” says Sara Coppola, the first author of the article. “For example, fiber optic biochemical sensors based on light absorption [could be developed] that use the polymer layer as a sensitive surface to change its refractive index in presence of the analyte,” she added.
Beyond photonics, future applications include the encapsulation of live cells. “We demonstrated the fabrication process on different liquids such as cell culture medium [etc]. We look forward to proposing the water packaging technique as a framework for microfluidic organ-on-chip technology,” says Ferraro.
Nicolas Martin, junior chair leader of IdEx Bordeaux and CNRS researcher, France, says that “it is remarkable to see how the PLGA membrane closely reproduces the morphology of the air–water interface, and that the process can be applied to both liquid and hydrogel surfaces. The ease, versatility, robustness and biocompatibility of the reported methodology are also extremely appealing, and open exciting perspectives for applications ranging from facile surface coating to encapsulation of fragile biological material.”
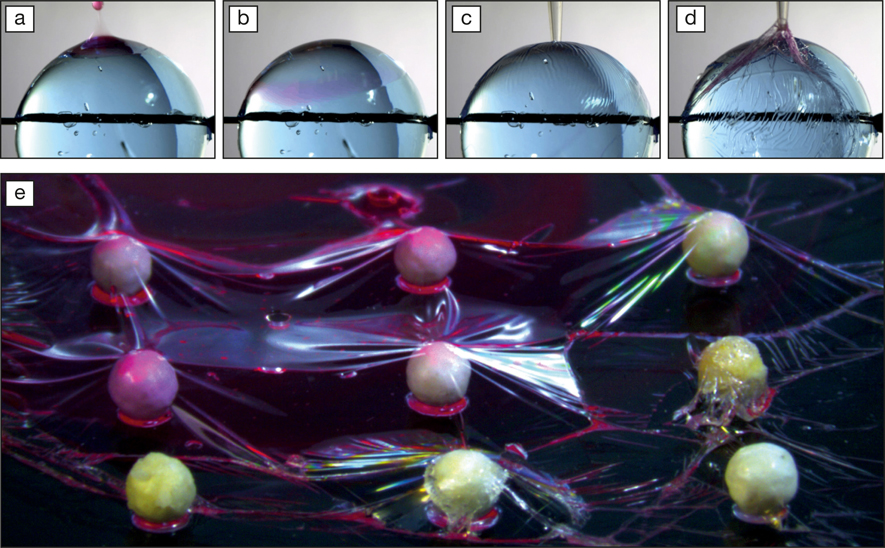
(a–d) Sequence of the formation of the poly(lactic-co-glycolic acid) (PLGA) film around a hydrogel sphere of diameter ∼200 µm with (a) deposition of the PLGA-dimethyl carbonate (DMC) solution (stained in pink), (b) its spreading, (c) the drying of the PLGA by DMC evaporation, forming a membrane, and (d) retrieving the membrane. (e) PLGA film formed around polystyrene spheres ∼50 µm in diameter, submerged in water. Credit: Sara Coppola.


