Carbon dioxide (CO2) is the primary contributor to global warming (Lee et al. Reference Lee, Calvin, Dasgupta, Krinner, Mukherji, Thorne and Zommers2023) and the second most prevalent greenhouse gas globally, next to water vapour (United States Environmental Protection Agency 2023). In North America, the current average atmospheric CO2 concentration is 419.7 ppm, with projections suggesting it could reach 1000 ppm by 2100 if current CO2 production rates persist (Siegert et al. Reference Siegert, Haywood, Lunt, Van de Flierdt and Francis2020). As CO2 concentrations continue to rise, their potential effects extend beyond global warming, influencing various ecological systems, including the biology of forest insects (Skendžić et al. Reference Skendžić, Zovko, Živković, Lešić and Lemić2021). Most current research on the effects of CO2 on forest insects has focused on the indirect effects of elevated CO2 on host trees (DeLucia et al. Reference DeLucia, Casteel, Nabity and O’Neil2008) or on how CO2-driven climate change, such as warmer winters, influences insect range expansion. In Canada, rising ambient temperatures have indirectly influenced the survival rates of major forest insect species such as Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) (Bellemin-Noël et al. Reference Bellemin-Noël, Bourassa, Despland, De Grandpré and Pureswaran2021) and the mountain pine beetle, Dendroctonus ponderosae Hoptions (Coleoptera: Curculionidae) (Safranyik et al. Reference Safranyik, Carroll, Régnière, Langor, Riel and Shore2010), by inducing warmer winters, which have resulted in the expansion of their native ranges. Furthermore, some studies have shown that elevated CO2 concentrations can have either positive or negative direct impacts on the development of some insect species, including some lepidopterans (see reviews by Kocsis and Hufnagel Reference Kocsis and Hufnagel2011; Amiri-Jami et al. Reference Amiri-Jami, Sadeghi and Shoor2012; Li et al. Reference Li, Sun, Gou, Zhang, Zhang, Zhou and Liu2021; Macartney et al. Reference Macartney, Crean and Bonduriansky2022; Zaman et al. Reference Zaman, Shah, Shan, Ullah, Ishangulyyeva and Erbilgin2024). Together, these findings demonstrate an urgent need for more research on the direct physiological impact of elevated CO2 concentrations on major forest insect pest species such as C. fumiferana in North America.
Choristoneura fumiferana, native to the boreal forest of Eastern North America, experiences an outbreak approximately every 25–40 years (Boulanger et al. Reference Boulanger, Arseneault, Morin, Jardon, Bertrand and Dagneau2012). Multiple years of severe defoliations by C. fumiferana can lead to tree mortality, depending on tree species and age and climate conditions (Gauthier et al. Reference Gauthier, Vaillancourt, Leduc, Grandpré, Kneeshaw and Morin2009; Rossi et al. Reference Rossi, Plourde and Krause2018; Lavoie et al. Reference Lavoie, Girona and Morin2019; MacLean Reference MacLean2019). As with all budworm species, C. fumiferana is a univoltine species (Nealis Reference Nealis and Capinera2008). Larvae construct hibernacula in July and August and remain in the second-instar larval stage as they overwintering in the crevices on the branches and bud scales of balsam fir, Abies balsamea (Linnaeus) Miller, white spruce, Picea glauca (Moench) Voss (Pinaceae), and to a lesser extent, red spruce, Picea rubens Sargent (Pinaceae), and black spruce, Picea mariana (Miller) Britton et al. (Pinaceae), showing a preference for balsam fir and white spruce due to their earlier budburst compared to black and red spruce (Lavoie et al. Reference Lavoie, Girona and Morin2019). In late April and early May, before the buds expand, the second-instar larvae emerge from diapause and move towards the branch tips, mining old needles, flowers, and unopened buds until the buds begin to grow. Once the newly formed buds expand, the larvae bore into the new buds, moult into the third instar, and continue feeding until they reach the sixth-instar stage approximately eight weeks after emerging from diapause. By late June to early July, larvae stop feeding and pupate, with adult moths emerging about 10 days later. Female C. fumiferana lays light-green eggs arranged in a shingled pattern, typically in masses of 15–20 (Nealis Reference Nealis and Capinera2008), although well-nourished females can lay more than 150 eggs (Nealis Reference Nealis and Capinera2008; Subedi et al. Reference Subedi, Marchand, Bergeron, Morin and Girona2023). Depending on the climate condition, eggs typically hatch 7–14 days later.
The impact of increasing atmospheric CO2 concentrations on the biology of C. fumiferana remains unknown. Through in vitro experiments, we compared C. fumiferana development and realised fecundity between larvae exposed to elevated (1000 ppm) or ambient (469 ppm) CO2 concentrations. The selection of elevated CO2 concentration was based on predictions and observations reported in 810 peer-reviewed publications (reviewed by Xia et al. Reference Xia, Lam, Kiese, Chen, Luo and van Groenigen2021). This study will contribute to understanding how elevated CO2 concentrations directly influence C. fumiferana development.
We reared the second-instar larvae to the third-instar stage on the McMorran diet under laboratory conditions (23 ± 1 °C, 50% ± 5 relative humidity, 16:8-hour light:dark photoperiod). The larvae and diet were obtained from the Insect Production and Quarantine Laboratories (Product code: Glfc:IPQL:Cfum14, Great Lakes Forestry Centre, Natural Resources Canada, Canadian Forest Service, Sault Ste. Marie, Ontario, Canada; Roe et al. Reference Roe, Demidovich and Dedes2018). At the third-instar stage, we randomly assigned 50 larvae to either ambient or elevated CO2 treatments. Before placing each larva in its respective treatment, we measured the weight of the McMorran diet and the third-instar larvae and then placed the larvae individually into 60-mL Dixie cups. Each cup contained 20 two-millimetre holes on the top lid and bottom to ensure adequate aeration. We then placed the cups into two separate growth chambers (81.28 cm × 81.28 cm × 160.02 cm; Growth Tent Model OS-GTB; Opulent Systems; Los Angeles, California, United States of America). One chamber maintained 1000 ppm CO2 using a liquefied petroleum gas cylinder and CO2 generator (Two Burners Carbon Dioxide Generator LP; Titan Control, Vancouver, Washington, United States of America) and a controller (GZ USCO2 Controller; Grozone Control, Saint-Pascal, Québec, Canada), whereas the control chamber maintained ambient conditions, monitored daily (CO2 = 469 ± 169 ppm). Environmental parameters in both chambers were kept constant and recorded daily (23 ± 1 °C, 58 ± 5% relative humidity, 16:8-hour light:dark photoperiod).
The larvae were monitored daily until pupation. The third-instar to sixth-instar development time was determined by distinct morphological changes. Based on earlier publications, we identified when C. fumiferana larvae reached the sixth-instar stage by using distinct morphological characteristics (brown–black body, cream-coloured longitudinal stripes, white spots on each body segment). We removed C. fumiferana larvae from their respective cups and measured their weight. Simultaneously, we removed the remaining McMorran diet from each cup and recorded its weight after removing larval feces and any webbing produced during feeding. Both larval and diet weight were measured twice: once when the larvae were introduced to their cups and again when they reached the sixth-instar stage. Using this information, we calculated the amount of diet consumed (as the difference between the initial and final diet weight) and larval weight gain (as the difference in weight from third-instar stage to sixth-instar stage).
We then placed each C. fumiferana larva back in its respective cup and returned the remaining McMorran diet. Larvae were monitored daily to determine when pupation occurred. On the first day of pupation, we recorded their weight and sex, based on size, colour, and number of rings (males: small, brownish with four rings; females: large, greenish with three rings; Mattson et al. Reference Mattson, Simmons and Witter1988). We then randomly paired one male and one female for mating (n = 13 pairs for elevated CO2; n = 14 pairs for ambient CO2). After removing the pairs from their prior CO2 exposure treatments, we placed each pair in individual Dixie cups lined with white filter paper under ambient rearing conditions. Subsequently, we checked the cups daily and recorded when adult emergence occurred and the number eggs laid. The total number of eggs laid per female, or realised fecundity, was counted upon the female’s death. The eggs were quantified using a stereomicroscope with polarised lighting at 100× magnification and gamma enhanced, mounted with a camera connected to a large computer screen. Motic 2.0 software (Swift SM101C; Fisher Scientific, Ottawa, Ontario, Canada) was used for analysis.
We employed an independent Student’s t-test when the data met the normality and homogeneity of variance assumptions for mean larval weight gain, diet consumed, time of the first oviposition, and realised fecundity. We used the Kolmogorov–Smirnov test for normality and Levene’s test for homogeneity of variance. When these assumptions were not met, we used the Mann–Whitney U-test. We used a generalised linear model to analyse the development time for three developmental stages: third-instar larvae (L3) to sixth-instar larvae (L6), L3 to pupae, and L3 to adult emergence, with treatment as a factor and larval (L3–L6) or pupal weight (mg at the first day of pupation). We carried out all statistical analyses and created figures using Jamovi software, version 2.4 (https://www.jamovi.org/), with additional analysis and visualisation performed in R, version 4.1 (R Core Team 2022).
We analysed the following variables using statistical tests to compare the effects of elevated versus ambient CO2 concentrations on C. fumiferana biology: (1) larval weight gain (mg, the difference in weight between third-instar larvae and sixth-instar larvae, reflecting the growth of larvae during this period); (2) diet consumed (g, the change in weight of the diet provided to the larvae, calculated as the difference between the initial and final weight at the L3 and L6 stages, respectively); (3) development time (days, from L3 to L6, from L3 to pupation, and from L3 to adult emergence); (4) pupal weight (mg, on the first day pupation); (5) sex of pupae (based on size, colour, and number of rings); (6) adult emergence (days, from L3 to adult emergence); (7) time of first oviposition (days, from L3 to the first occurrence of oviposition); and (8) realised fecundity (the total number of eggs laid, estimated at the time of female death). The sample size was 43 for variables 1–6 and 13 pairs for elevated and 14 pairs for ambient treatments for variables 7 and 8.
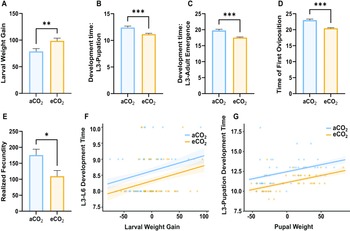
We found that larvae exposed to elevated CO2 treatment exhibited 20% higher mean weight gain from the third-instar larval stage to the sixth-instar larval stage compared to those exposed to the ambient condition (t-test: t 84 = –2.76, P = 0.0004; Fig. 1A), and no difference was observed between treatments in amount of diet consumed (U-test: U 43 = 913, P = 0.921; Fig. 2) or the development time from the third-instar larval stage to the sixth-instar larval stage (U-test: U 43 = 772, P = 0.126; Fig. 2). The time to pupate was 15% shorter for larvae exposed to the elevated CO2 concentration than for those in the ambient conditions (U-test: U = 520 and P < 0.001; Fig. 1B), with similar pupal weights observed between treatments (U-test: U 43 = 827, P = 0.40; Fig. 2). Furthermore, development time from the third-instar stage to adult emergence was 15% shorter in larvae exposed to elevated CO2 than in those maintained in ambient conditions (U-test: U = 294, P < 0.001; Fig. 1C). In addition, the day of the first oviposition occurred three days earlier for larvae exposed to the elevated CO2 than for the ambient-CO2 group (t-test: t 25 = 6.31, P < 0.001; Fig. 1D). Realised fecundity was lower in C. fumiferana that had been exposed to elevated CO2 compared to that in the ambient-CO2 group (t-test: t 25 = 2.59, P = 0.008; Fig. 1E). The generalised linear model analysis showed that increased larval weight gain was associated with slightly longer development times across both ambient and elevated CO2 for both L3–L6 and L3–pupal stages (β = 0.005, P < 0.05 and β = 0.018, P < 0.001). However, although heavier larvae generally take longer to develop, the elevated CO2 can somewhat mitigate this effect, promoting faster development (eCO2 – aCO2: β = –0.31, P < 0.05 and β = –1.35, P < 0.001, respectively).

Figure 1. The effects of ambient (469 ppm) and elevated levels of CO2 (1000 ppm) on the development and reproduction of Choristoneura fumiferana over 26 days: A, larval weight gain (mg, the difference in weight between L3 and L6); B, development time, L3 to pupation (days); C, development time, L3 to adult emergence (days); D, time of first oviposition (days, from L3 to the first day of oviposition); E, realised fecundity (the total number of eggs laid, estimated at the time of female death); F, L3 to L6 development time versus larval weight gain (mg); and G, L3 to pupation development time versus pupal weight (at the first day of pupation) analysed by generalised linear model. The bars denote the standard error of the mean. aCO2, ambient CO2; eCO2, elevated CO2.

Figure 2. Mean difference (± standard error) of the amount of A, diet consumed by Choristoneura fumiferana (g, the change in weight of the diet provided to the larvae, calculated as the difference between the initial and final weights at the L3 and L6 stages, respectively), B, L3 to L6 development time (days), and C, pupal weight (mg, on the first day of pupation). We conducted the Mann–Whitney U-test.
Choristoneura fumiferana exposed to elevated CO2 concentrations developed in 3–4 fewer days than did those maintained under ambient-CO2 conditions. This is the first reported effect of elevated CO2 concentrations on C. fumiferana biology. Our results align with findings in other lepidopterans, where elevated CO2 has been shown to influence their biology. For instance, in cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae), exposure to elevated CO2 concentrations up to 750 ppm reduced larval survival and growth but increased relative growth rate, fecundity, and pupal weight (Akbar et al. Reference Akbar, Pavani, Nagaraja and Sharma2016). Similarly, research on cowpea pod borer, Maruca vitrata (Lepidoptera: Crambidae), found that elevated CO2 increased feeding but also reduced pest fitness over time, suggesting a trade-off between short-term damage and long-term fitness costs (Megha et al. Reference Sreenivas, Sushila, Hadimani and Beladhadi2022). These findings support the idea that elevated CO2 can affect insect growth and population dynamics in complex ways (Choi et al. Reference Choi, Ahn, Kim, Ahn, Jung, Go and Kim2018). Although we did not investigate the mechanism behind the faster development of C. fumiferana under elevated CO2, the insect may experience altered nutrient allocation under elevated CO2, leading to faster development (Wu et al. Reference Wu, Chen and Ge2006; Li et al. Reference Li, Sun, Gou, Zhang, Zhang, Zhou and Liu2021).
Choristoneura fumiferana larvae (from L3 to L6) gained more weigh under elevated CO2 concentration, which is consistent with findings from earlier studies. Because we found no correlation between larval weight and food consumption, these results suggest that elevated CO2 may provide a more favourable environment for faster larval development, likely by enhancing metabolic rate. This is supported by research on other lepidopterans, where elevated CO2 affected metabolic rates and developmental timelines. For example, spongy moth, Lymantria dispar (Lepidoptera: Erebidae), and forest tent caterpillar, Malacosoma disstria (Lepidoptera: Lasiocampidae), showed variable responses to elevated CO2, including altered development times and feeding behaviour (Kocsis and Hufnagel Reference Kocsis and Hufnagel2011). Although the biochemical mechanisms behind the increased weight gain in C. fumiferana remain unknown, they may involve metabolic changes similar to those observed in other lepidopteran species.
Choristoneura fumiferana exposed to elevated CO2 showed reduced realised fecundity, a trend consistent with studies on other insects, where elevated CO2 negatively impacts reproductive success. In contrast, oviposition in tobacco hornworm moth, Manduca sexta (Lepidoptera: Sphingidae), was unaffected by elevated CO2 concentrations up to 1200 ppm (Guerenstein and Hildebrand Reference Guerenstein and Hildebrand2008). Similarly, Zhou et al. (Reference Zhou, Criddle and Mitcham2001) found that omnivorous leafrollers (Lepidoptera: Torticidae) experienced a decrease in metabolic heat rate as CO2 concentrations increased. Because metabolic heat rate reflects energy expenditure, this reduction suggests lower energy production, which may limit the energy available for vital functions such as reproduction. Other studies, such as those on H. armigera, also show that, although elevated CO2 reduced larval growth, it also increased fecundity, indicating a potential shift in energy allocation strategies under changing CO2 conditions (Akbar et al. Reference Akbar, Pavani, Nagaraja and Sharma2016). Together, these results highlight the species-specific effects of elevated CO2 concentrations on lepidopteran reproduction, and they underscore the need for further research to understand how elevated CO2 affects reproductive biology of C. fumiferana. Such insights will be crucial for predicting pest outbreaks in the context of climate change.
In conclusion, our results offer the first insights into the direct effects of elevated CO2 concentration on C. fumiferana development and reproductive biology. Accelerated larval development and increased weight gain could lead to faster population growth and more generations per year, but reduced realised fecundity may counterbalance these effects. However, in univoltine species like C. fumiferana, shorter development times may not always be beneficial to the insects because earlier hatching could cause larvae to deplete their energy reserves before winter diapause, potentially reducing survival before spring. Such a reduction in brood production may stem from disrupted nutrient availability and metabolic changes induced by elevated CO2, which affect reproductive functions. However, future generations may develop transgenerational hormetic traits, potentially becoming resistant to elevated CO2 (Zaman et al. Reference Zaman, Shah, Shan, Ullah, Ishangulyyeva and Erbilgin2024). The species-specific responses observed in other lepidopteran species suggest that the response of C. fumiferana to elevated CO2 concentration may vary depending on environmental and physiological factors. In addition, the impact of faster larval development on predation or parasitism rates remains uncertain but could influence survival and population growth. Given these uncertainties, along with many confounding abiotic factors (e.g., warming climate) and biotic factors (e.g., changes in plant chemistry), the long-term implications for C. fumiferana population dynamics remain unclear. Further research is needed to clarify how elevated CO2 affects developmental and reproductive pathways in C. fumiferana over multiple generations. Such insights are crucial for predicting future population trends and mitigating the impact of C. fumiferana on forest ecosystems.
Acknowledgements
The authors thank I-STEAM Pathways: Environmental Research Internships for Indigenous Students for supporting S.F.
Competing interests
The authors declare that they have no competing interests.