INTRODUCTION
Clostridium difficile is a Gram-positive, anaerobic bacterium that produces pathogenic toxins A and B. C. difficile infection (CDI) is an important cause of nosocomial diarrhoea [Reference McFarland1, Reference Kelly, Pothoulakis and LaMont2]. The incidence of CDI has increased steadily over the past decades, but recent data suggest that its incidence and severity are now increasing markedly in Western countries [Reference Pepin3–Reference O'Connor, Johnson and Gerding5]. The emergence of more virulent strains [Reference Warny6] combined with the widespread use of broad spectrum antibiotics have been identified as potential causes for the increasing CDI burden. Currently, CDI is a growing concern in many countries, as it prolongs duration of hospital stay [Reference Miller7], increases the financial burden on healthcare systems [Reference Kyne8], and causes a marked increase in morbidity and mortality [Reference Muto9]. Many clinicians believe that the incidence and severity of CDI in Korea has increased, along with other countries worldwide. Indeed, several recent studies have reported that hospital- and community-acquired CDI have significantly increased in Korea [Reference Byun10–Reference Lee12]. Interestingly, Shin et al. [Reference Shin13, Reference Shin14] reported that toxin A-negative and toxin B-positive [(tcdA(–)tcdB(+)] strains were prevalent in Korea from 2003, and this strain reached 27% among toxigenic stains in 2005, which was responsible for the high rate of pseudomembranous colitis (PMC). Recently, Kim et al. [Reference Kim15] reported that binary toxin-producing strain (7·1%) is not rare in Korea. Moreover, in 2008, the first severe CDI case due to a hypervirulent strain was reported in Korea [Reference Tae16], as in Canada [Reference Pepin3], the USA [Reference McDonald17], Europe [Reference Kuijper18], and Japan [Reference Kato19]. To date, CDI has received limited attention in the Korean healthcare system. Currently, there is no surveillance system for CDI in place. Therefore, there are limited epidemiological data and information regarding the clinical burden caused by CDI. This nationwide study was designed as a first step in establishing the current incidence of CDI in Korea, which was initiated by the IBD Study Group of the Korean Association for the Study of Intestinal Diseases (KASID). We aimed to determine the healthcare-associated CDI incidence in Korea from 2004 to 2008 and to raise awareness about CDI in the healthcare system.
METHODS
Incidence
This nationwide study included patients from 17 tertiary hospitals, located in seven different provinces throughout Korea. Eight of the hospitals were located in Seoul; three in Gyeonggi; two in Gyeongsang; and one each in Jeolla, Gangwon, Chungcheong, and Jeju. The number of adult admissions (aged >18 years) and the number of cases that met the criteria for CDI were tabulated annually from 2004 to 2008. Incidence rates of CDI were then calculated and expressed as the number of patients with CDI/1000 adult admissions.
Clinical features
A retrospective analysis was conducted to identify the clinical features of CDI patients who were diagnosed in 2008. Basic demographic data, including gender, age, comorbidities responsible for the patient's admission, and medical treatments including the use of antibiotics were collected and analysed for all patients. In addition, the initial treatment regimens prescribed for patients with CDI, and recurrence rates were evaluated. The Institutional Ethics Committee of each hospital approved the study protocol.
Definition of CDI
Diarrhoea was defined as a bowel habit change with >3 unformed stools per day for >2 days [Reference Kyne20]. The case definition of CDI included admitted patients with documented diarrhoea and any positive outcome (one or more) in the following diagnostic tests: (1) C. difficile toxin A and/or B by enzyme immunoassay (EIA); (2) positive stool culture for C. difficile; and (3) typical endoscopic findings, such as multiple white or yellow plaques suggestive of PMC. The stool samples were tested with VIDAS C. difficile toxin (A II or A/B; bioMérieux, France), or the Wampole C. difficile Tox A/B II method (TechLab, USA). Stool culture for C. difficile was performed by inoculating stool samples into anaerobically reduced cycloserine-cefoxitin-fructose agar (CCFA) at 37 °C under anaerobic conditions for 48–72 h in each institute. The identification of suspected C. difficile was conducted through analysis of Gram stain, spore stain, and/or a biochemical assay using an ANA identification test kit (bioMérieux).
Improvement of CDI was defined as resolution of diarrhoea by day 6 after treatment. Recurrence of CDI was defined as the development of CDI, after at least 8 weeks of symptom resolution. Complicated CDI was defined when toxic megacolon, colectomy, or death related to CDI resulted from septic shock or perforation occurred within 30 days of diagnosis of CDI.
Statistical analysis
Descriptive analyses were performed and values were expressed as mean ± standard deviation (s.d.). Categorical variables were expressed as proportions. Continuous data were compared using Student's t test. Statistical analysis was performed using the SPSS package for Windows version 13.0 (SPSS Inc., USA), and two-tailed P values <0·05 were considered to be statistically significant.
RESULTS
Incidence of CDI
The incidence of CDI was 1·7 cases/1000 adult admissions in 2004, 2·0 cases/1000 in 2005, 2·1 cases/1000 in 2006, 2·4 cases/1000 in 2007, and 2·7 cases/1000 in 2008 (Fig. 1). The incidence of CDI in 2008 was significantly greater than in 2004 (P = 0·028).

Fig. 1. Rates of Clostridium difficile infection (CDI) per 1000 adult admissions in Korea from 2004 to 2008. The incidence of CDI in 2008 was significantly greater than in 2004 (P = 0·028).
Clinical features
A total of 1367 patients was diagnosed with CDI in 2008 (669 males, 698 females). The mean age of these patients was 61·6 ± 15·3 years (range 19–96 years). CDI occurred throughout the year without any significant seasonal variation. The majority (63·4%) of patients were treated in medical units and the mean duration of hospital stay prior to diagnosis of CDI was 45·6 ± 75·8 days (range 3–1145 days). Most patients had severe underlying disease such as malignancy (Table 1). Only 74 (5·4%) had underlying gastrointestinal disease, 30 had inflammatory bowel disease, 21 had colon cancer, and 19 had ischaemic colitis. Most (1254, 91·7%) patients had received antibiotics prior to the diagnosis of CDI, and the mean duration of antibiotic usage prior to CDI was 16·0 ± 18·4 days (range 1–276 days). The most common antibiotics taken prior to CDI were cephalosporin (41·2%), fluoroquinolones (13·7%), and penicillins (9·8%) (Table 2). Eleven patients who had taken anti-tuberculosis medication such as rifampin developed CDI.
Table 1. Demographic and clinical characteristics of 1367 patients with CDI diagnosed in 2008
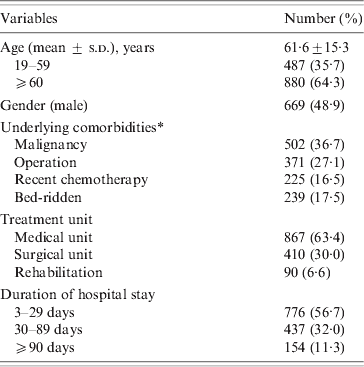
CDI, C. difficile infection.
* One or more conditions are possible.
Table 2. Antibiotic exposures of the CDI patients (2008)
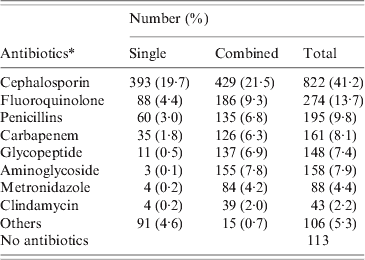
CDI, C. difficile infection.
* Antibiotics could be used in a single or combined with other agents.
Diagnostic tools
The most common tool used to diagnose CDI was an EIA for toxins A and/or B in the stool. Stool toxin assay, stool culture for C. difficile, and endoscopy were performed for 1174, 370 and 330 patients, respectively. Of these, stool toxin was positive in 1112 patients. Stool culture for C. difficile was positive in 254 patients and 278 patients were diagnosed as having PMC by endoscopy.
Treatment outcome
Of the 1367 patients with CDI, 846 (61·9%) were prescribed oral metronidazole as first-line treatment, and 796 (94·1%) patients improved with oral metronidazole. Other common treatments included intravenous metronidazole (113 patients, 8·3%) and oral vancomycin (34 patients, 2·5%). However, 235 (17·2%) patients improved without specific therapy after discontinuation of the offending antibiotic. Relapse occurred in 122 (8·9%) patients; 91 (74·6%) patients experienced one episode of relapse, and 31 (25·4%) patients had two or more episodes. The presumed causes of relapse were considered to be: ongoing use of offending antibiotics (43·9%), insufficient dose or duration of therapy (18·7%) or severe comorbidities (15·0%). Complicated CDI, such as colectomy was encountered in only 49 (3·6%) patients and seven (0·51%) patients died of causes related to CDI.
DISCUSSION
Our nationwide, hospital-based study revealed that the incidence of CDI in Korea increased significantly between 2004 and 2008. The incidence of 1·7 cases/1000 adult admissions in 2004 increased steadily to 2·7/1000 in 2008. To our knowledge, this study represents the first comprehensive study on a nationwide scale documenting the occurrence of nosocomial CDI in Korea. A recent prospective surveillance study in Canada found that the incidence of CDI was 4·6 cases/1000 adult admissions between 2002 and 2007 [Reference Gravel21]. Similarly, in the USA, the healthcare facility-associated CDI incidence was 3·5 cases/1000 adult admissions in 2006 [Reference Dubberke22]. Our calculated overall rates of CDI in Korea are low compared to rates in Canada and the USA. However, these incidence rates are similar to those obtained in a recent surveillance study covering eight European countries, which reported the incidence of CDI to be 1·1 cases/1000 admissions [Reference Barbut23]. In the light of the findings of this study, we conclude that Korea is no longer ‘safe’ from CDI, and that systematic surveillance is undoubtedly needed. We anticipate that future prospective surveillance studies will yield more precise data. The reason why CDI has increased in Korea is not clear. However, a recent single-centre study reported that requests for stool samples for CDI increased markedly from 53 cases in 2003 to 706 in 2008, and may reflect the increase in clinicians' perception of CDI [Reference Lee11]. Another study revealed that along with the increase of CDI, the use of intravenous fluoroquinolones increased about threefold from 2001 to 2007 [Reference Lee12]. We believe that several factors, such as an increase in antibiotic usage, improper control of CDI, and increased awareness, are responsible for the increase of CDI in Korea.
In order to assess the clinical characteristics of CDI in Korean patients, we retrospectively analysed the CDI patients (n = 1367) who were diagnosed in 2008 in 17 centres. Most (91·7%) patients with CDI had been previously exposed to antibiotics. We found rifampin, an anti-tuberculosis medication, to be a possible culprit in 11 patients. This finding raises potential concerns, as tuberculosis is still prevalent in Korea and many patients are prescribed anti-tuberculosis therapy. Previously Jung et al. reported six cases of PMC developing after anti-tuberculosis therapy [Reference Jung24]. In our study, stool toxin assays by EIA are the most commonly used method for diagnosing CDI and 1112 (81·3%) patients showed positive stool toxin assay results. Two hundred seventy-eight (20·3%) patients were found to have typical findings of PMC, suggesting that endoscopy is a very useful diagnostic tool when PMC is strongly suspected [Reference Lee25]. Stool culture for identification of C. difficile is not widely used, and we believe that clinicians' lack of knowledge about the importance of C. difficile culture may be responsible for this result. With respect to treatment of CDI, most patients improved with oral metronidazole treatment, which is quite different from Pepin et al.'s report that metronidazole treatment caused a poor outcome [Reference Pepin26]. Moreover, the attributable mortality rate in our study was 0·51%, suggesting that at present the clinical features of CDI in Korea are relatively milder than in Western countries, in which the mortality related to CDI is reported as 1–2·5% [Reference Schroeder27].
There were several limitations to our study. First, it was conducted retrospectively by reviewing CDI cases in 17 centres. There may be differences between the hospitals regarding suspicion about CDI, identifying CDI cases and data collection. There is still a lack of knowledge of CDI in Korea, therefore we assumed that all diarrhoeal stool samples were not tested for C. difficile toxin or culture. In addition, the standardization of the tests for CDI between different hospitals was incomplete. Identification of toxigenic isolates was not performed in our study and therefore we could not rule out the possibility of C. difficile colonization. Second, most patients were tested with only one test (stool toxin assay) to diagnose CDI. Although EIA for detection of toxins A and B in the stool is a relatively inexpensive, fast, and convenient method, EIA tests have variable sensitivities (63–73%) [Reference Peterson28, Reference Delmee29], suggesting that the diagnosis of CDI could be missed for many patients. Currently, there is no testing strategy that is optimally sensitive and specific for diagnosis of CDI [Reference Cohen30]. If a proper surveillance system is introduced to Korea, more accurate data will be gained about the incidence and clinical features of CDI. In addition, through the surveillance system, a standardized strategy for the diagnosis of CDI in the current situation in Korea will be established. Third, only tertiary hospitals participated in the study, so the study subjects tended to be more severely ill than patients in other hospitals. Therefore, it is unlikely that our results represent the present situation of CDI for all hospitalized adult patients in Korea. However, despite these limitations, our study provides important information concerning the clinical impact of CDI in Korea.
In conclusion, the incidence of CDI in Korea increased significantly between 2004 and 2008. This nationwide study has established the importance and clinical burden of CDI. Although the incidence and severity of CDI tend to be mild in Korea compared to Western countries, the current trend of increasing case numbers, and the presence of hypervirulent strains in Korea, should be a warning to Korean clinicians. Ongoing surveillance systems are needed to closely monitor the incidence of these infections, and especially infections caused by hypervirulent strains.
DECLARATION OF INTEREST
None.





