Introduction
Irritable bowel syndrome (IBS) is the most frequent functional bowel disorder. IBS is defined according to Rome IV criteria(Reference Lacy, Mearin and Chang1) as chronic abdominal pain associated with transit disorders that last for at least 3 months. It is divided into three predominant subtypes according to the gastrointestinal (GI) symptoms experienced by patients: (1) IBS with constipation; (2) IBS with diarrhoea; and (3) mixed IBS. The prevalence of IBS is about 5 to 20 % in Western European and North American populations according to Rome IV diagnostic criteria(Reference Whitehead, Palsson and Simren2). In 2000, the direct and indirect costs of IBS in the USA were estimated at 1·7 billion dollars(Reference Sandler, Everhart and Donowitz3). While in Europe the direct annual cost per patient ranged from 1421 up to 2487 euros and the indirect annual cost per patient ranged from 339 up to 11 248 euros(Reference Tack, Stanghellini and Mearin4).
Food intake has been identified as a trigger of IBS symptoms by patients(Reference Simren, Mansson and Langkilde5) and meal tests have been used to study symptom response in IBS(Reference Posserud, Strid and Storsrud6). Patients with increased digestive postprandial symptoms tend to have higher levels of depression and somatisation disorder(Reference Van Oudenhove, Tornblom and Storsrud7). The occurrence or the exacerbation of IBS symptoms as a result of food intake has been associated with more severe symptoms and reduced quality of life(Reference Bohn, Storsrud and Tornblom8,Reference Chey9) . The deleterious and symptomatic role of poorly absorbable and rapidly fermentable carbohydrates (fermentable oligo-, di-, mono-saccharides and polyols (FODMAP)) in IBS was first proposed by Gibson & Shepherd(Reference Gibson and Shepherd10). The mechanisms underlying the effects of unabsorbed carbohydrates on IBS symptoms may involve osmotic load, alteration of GI tract functions (for example, permeability and intestinal immunity) or modification of gut microbiota composition and functions (for example, fermentation and production of gas)(Reference Eswaran11). Among FODMAP, fructose is of particular interest. Consumption of high-fructose corn syrup (HFCS) and sucrose has increased dramatically during the recent decades, resulting in a current average fructose daily intake of 50 g per individual in the USA and most Western countries(Reference Beyer, Caviar and McCallum12). Fructose intake challenges the absorption capacity of the small intestine and leads to fructose malabsorption even in patients without hereditary fructose intolerance(Reference Jones, Butler and Brooks13). Unabsorbed fructose may be fermented by the intestinal microbiome and can lead to gas production such as hydrogen which is known to participate in IBS symptoms. Fructose has also been shown to trigger or worsen symptoms in IBS patients(Reference Hammer and Hammer14). All these mechanisms suggest that a low-fructose diet may be of potential interest in the management of IBS.
Objectives
The main objective of the present narrative review is to define the prevalence of fructose malabsorption in patients with IBS.
Secondary objectives are: (1) to provide an overview of the potential mechanisms underlying fructose-related IBS symptoms; (2) to determine the role of a low-fructose diet in the overall management of patients with IBS; and (3) to identify those patients who could benefit from a low-fructose diet.
Methods
All published studies related to the subject were retrieved from the PubMed database. For the main objective, we used the key words: fructose, fructose malabsorption, fructose breath test, with IBS. Only papers written in English were eligible. Papers about IBS in children, case reports, case–control-led studies and reviews were excluded (Fig. 1). Studies were selected by two independent reviewers.

Fig. 1. Systematic review search strategy.
FODMAP and irritable bowel syndrome
The link between food intake and GI dysfunctions in IBS has been confirmed in a prospective study(Reference Ragnarsson and Bodemar15). The majority of patients with IBS consider their symptoms to be related to specific food items; therefore they often change their diet by limiting the food they perceive as problematic(Reference Hayes, Corish and O’Mahony16). Nevertheless, daily nutrient intake in patients with IBS is similar to the diet of the general population and meets national nutrient recommendations(Reference Bohn, Storsrud and Simren17). Reporting by IBS patients of specific foods as IBS symptom triggers has led to focusing attention on some dietary factors such as FODMAP and, more specifically, fructose(Reference Boeckxstaens, Drug and Dumitrascu18).
FODMAP
Ultra-processed foods of the Western diet are of particular interest as their consumption has increased and they are associated with IBS(Reference Schnabel, Buscail and Sabate19,Reference Buscail, Sabate and Bouchoucha20) . One of the features of ultra-processed foods is the high amount of sugar they contain(Reference Poti, Mendez and Ng21). A recent study revealed a correlation between carbohydrate intake and IBS severity(Reference Solar, Santos and Yamashita22). Moreover, the majority of patients with IBS are intolerant to incompletely absorbed carbohydrates (70 %)(Reference Bohn, Storsrud and Tornblom8) and malabsorbed carbohydrates, i.e. carbohydrates that are not absorbed in the upper GI tract (fructose, fructans, sorbitol, etc.), have been linked to IBS symptoms(Reference Rumessen23). The poorly absorbed carbohydrates have been collectively grouped under the FODMAP concept. They are frequently associated with and even trigger GI symptoms(Reference Shepherd, Lomer and Gibson24,Reference Staudacher and Whelan25) even if some of them participate in the maintenance of the normal microbial community. Furthermore, diets low in FODMAP improved symptoms in patients with IBS in more than ten controlled randomised trials(Reference Shepherd, Lomer and Gibson24,Reference Staudacher and Whelan25) , reducing overall symptoms, abdominal pain, bloating and quality of life in comparison with a traditional diet, Western diet or diet recommendation for IBS(Reference Altobelli, Del Negro and Angeletti26,Reference Schumann, Klose and Lauche27) . Even short-term exposure to an enriched-FODMAP diet (diet enriched in fermentable oligo-, di-, monosaccharides and polyols) favoured gut symptoms in patients with IBS when compared with a low-FODMAP diet(Reference Ong, Mitchell and Barrett28). However, the unique role of fermentable oligosaccharides in IBS is more controversial. Indeed, some of them such as inulin, fructans or galacto-oligosaccharides have prebiotic actions and their elevated quantity in a high-FODMAP diet may increase the abundance of beneficial bacteria. Consumption of up to 7 g of trans-galacto-oligosaccharide per d as a prebiotic supplementation was associated with a beneficial increase in faecal Bifidobacterium abundance and a decrease in IBS symptoms and anxiety scores in IBS patients(Reference Silk, Davis and Vulevic29). However, larger doses of prebiotics, for example, 19 g of fructans in children or 40 g of inulin in adults, have a negative impact on IBS symptoms(Reference Chumpitazi, McMeans and Vaughan30,Reference Major, Pritchard and Murray31) . In contrast, dietary restriction of fermentable carbohydrates has shown efficacy in improving IBS symptoms(Reference Eswaran11,Reference Staudacher and Whelan25,Reference Bohn, Storsrud and Liljebo32-Reference McIntosh, Reed and Schneider35) . However, the mechanisms by which FODMAP exacerbate IBS symptoms remain unclear. FODMAP could contribute to GI symptoms by increasing the luminal water volume or by promoting bacterial fermentation and subsequent gas production, mainly hydrogen and methane. These two effects may explain GI symptoms in patients with visceral hypersensitivity. The involvement of microbial composition and metabolism(Reference Zhou, Gillilland and Wu36), the increase in faecal pH(Reference Halmos, Christophersen and Bird37) and changes in intestinal permeability(Reference Zhou, Gillilland and Wu36) are also suspected but need further investigation.
Fructose
Fructose is a monosaccharide belonging to FODMAP. Its intake worsens symptoms in patients with IBS(Reference Shepherd, Parker and Muir38). It is found in many fruits and in honey and is present as added sugars in many industrial foods containing sucrose or HFCS(Reference Muir, Rose and Rosella39). In these foods, fructose is either free or is part of the sucrose disaccharide while HFCS is composed of a mix of non-bonded glucose and fructose in approximately 1:1 ratio.
Fructose consumption pattern
For thousands of years, man consumed < 5 g of fructose per d from fruit and honey(Reference Douard and Ferraris40). Since the 1970s, fructose has been increasingly consumed in developed countries due to the increase in total sugar consumption and the advent of HFCS. In the USA, this raised the per capita fructose daily intake to a staggering 50–80 g(Reference Douard and Ferraris40). Despite scarcity of available data for other countries, the consumption of total sugar (including fructose) has increased in most continents over the past 20 years(Reference Douard and Ferraris40-Reference Tappy, Le and Tran44). In Europe, the Netherlands is one of the few countries for which recent data on fructose intake are available, revealing a fructose intake of 35–60 g/d in the population aged 7–69 years(Reference Sluik, Engelen and Feskens41). In the UK, the daily mean fructose intake has reached 15–18 g for individuals over 4 years old but it can reach 37–43 g/d for the top 2·5th percentile of the same age group(45). Normal fructose absorption should be considered alongside fructose intake. Indeed, one out of two adults cannot fully absorb a 35 g load of fructose(Reference Jones, Butler and Brooks13). Fructose absorption is also highly age dependent(Reference Douard, Cui and Soteropoulos46). Infants display the highest predisposition for fructose malabsorption(Reference Jones, Butler and Moore47), while they are the age group which consumes the most fructose. Thus, changes in food intake patterns have probably created a prevalent condition of fructose malabsorption leading to its overspill in distal GI tract regions(Reference Muir, Rose and Rosella39).
Intestinal fructose absorption and its regulation
Fructose is mainly absorbed in the proximal small intestine. Since only monosaccharides can be absorbed, sucrose is cleaved into glucose and fructose at the brush border by sucrase–isomaltase. Recently, functional variants in the sucrase–isomaltase gene which result in reduced or defective enzymic activity were identified in IBS patients(Reference Garcia-Etxebarria, Zheng and Bonfiglio48-Reference Kim, Calmet and Garrido52), supporting the potential link between sugar absorption defects and IBS. Fructose is mainly passively transported across the apical brush-border membrane of the small intestine via the GLUT5 transporter(Reference Burant and Saxena53-Reference Douard and Ferraris56) and subsequently exits enterocytes to enter the blood via a different transporter, GLUT2, present at the basolateral membrane. GLUT5 is the only fructose-specific transporter unable to transport glucose or galactose at physiological concentrations(Reference Kane, Seatter and Gould57,Reference Manolescu, Salas-Burgos and Fischbarg58) , whereas GLUT2 can transport the three monosaccharides (glucose, fructose and galactose)(Reference Uldry and Thorens59). GLUT2 can also be recruited transiently at the apical membrane of enterocytes in response to high luminal glucose concentrations (> 1 mm) in order to support glucose transport across this membrane(Reference Gouyon, Caillaud and Carriere60-Reference Barone, Fussell and Singh64).
Luminal fructose exerts a rapid, strong and specific up-regulation of GLUT5 mRNA expression above the basal level, leading to an increase in GLUT5 protein and activity levels(Reference Ferraris65,Reference Ferraris and Diamond66) . In enterocytes, the first step of the main pathway of fructose metabolism involves ketohexokinase (KHK or fructokinase), a specific enzyme of fructose metabolism converting fructose into fructose-1-P(Reference Diggle, Shires and Leitch67). Recently, the use of a KHK knockout (KO) (KHK–/–) mouse model demonstrated that the suppression of intracellular fructose metabolism prevents fructose-induced up-regulation of GLUT5 in the small intestine and leads to major fructose malabsorption(Reference Patel, Douard, Yu and Gao68-Reference Zhang, Grosfeld and Williams70). While aldolase B is a known marker of hereditary fructose intolerance(Reference Oppelt, Sennott and Tolan71), the role of other enzymes specific to the fructose metabolic pathway in fructose malabsorption in humans remains unknown.
Fructose breath test
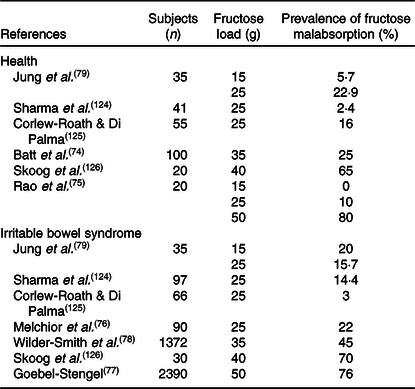
Breath tests estimate amounts of unabsorbed ingested carbohydrates by measuring hydrogen or methane generated by fermentation of the unabsorbed sugar by the intestinal bacteria. These tests can be used for the diagnosis of various carbohydrate malabsorption syndromes and small-intestinal bacterial overgrowth. Breath testing remains a useful, inexpensive, simple and safe diagnostic tool in gastroenterology and is performed with several substrates (for example, glucose, lactulose, fructose, sorbitol, sucrose and inulin) and at various doses. A positive breath test following fructose ingestion, defined as a rise ≥ 20 parts per million(Reference Rezaie, Buresi and Lembo72), indicates that bacteria are able to ferment fructose before its absorption. This may be due to all or any of the following scenarios – inefficient fructose absorptive mechanisms, rapidity of small bowel transit leaving insufficient time for absorption, or small-intestinal bacterial overgrowth. Small-intestinal bacterial overgrowth is a condition characterised by an abnormally high level of bacterial population in the small intestine where fructose is normally absorbed. This condition may produce false-positive sugar breath test results which could be ruled out with a glucose breath test(Reference Nucera, Gabrielli and Lupascu73). Fructose malabsorption in humans increases with fructose intake concentration and in healthy adults, there is a significant positive relationship between fructose dose and the breath test result(Reference Jones, Butler and Brooks13). In healthy volunteers, after 15, 25, 35 and 50 g fructose loads, breath tests were positive in 0, 10, 52 and 65 % of the individuals tested, respectively (Table 1)(Reference Batt, Fanning and Drake74,Reference Rao, Attaluri and Anderson75) . In healthy subjects the absorption of 15 to 25 g of fructose was not associated with malabsorption signs, suggesting that the intestinal absorptive capacity for fructose is about 25 g per food intake(Reference Rao, Attaluri and Anderson75). Conversely, increasing fructose doses (>50 g) were associated with more positive breath tests. Therefore doses > 50 g are not helpful to detect true malabsorbers(Reference Rao, Attaluri and Anderson75). Thus, a 25 g load seems to be the most specific dose to diagnose fructose malabsorption which has been accepted as the optimal dose by the North American Consensus(Reference Rezaie, Buresi and Lembo72).
Table 1. Studies assessing fructose malabsorption in health and in irritable bowel syndrome according to fructose load
Fructose malabsorption
According to the North American Consensus(Reference Rezaie, Buresi and Lembo72), the prevalence of fructose malabsorption in IBS patients is 22 %(Reference Melchior, Gourcerol and Dechelotte76), which is higher than in healthy individuals(Reference Rao, Attaluri and Anderson75). Indeed, after 15, 25, 35 and 50 g fructose loads, breath tests were positive in 20, 22, 45 and 64 % of IBS patients, respectively (Table 1)(Reference Melchior, Gourcerol and Dechelotte76-Reference Jung, Seo and Cho79). Surprisingly, fructose malabsorption prevalence is independent of the IBS subtype (diarrhoea, constipation or mixed)(Reference Melchior, Gourcerol and Dechelotte76). As mentioned above, current fructose intake per food intake probably exceeds the capacity of human absorption(Reference Beyer, Caviar and McCallum12). As an example, one can of Pepsi® or Coca-Cola® (33 cl) contains up to 24·6 g of fructose(Reference Ventura, Davis and Goran80). Fructose absorption is also dependent on the presence of glucose in the lumen(Reference Jones, Butler and Brooks13); simultaneous consumption of glucose and fructose increases fructose absorption(Reference Latulippe and Skoog81). However, the mechanisms underlying the limited capacity of the intestine to absorb fructose remains unclear. In a randomised controlled study, a 40 g fructose test meal was associated with increased fructose malabsorption in healthy individuals after injection of corticotrophin-releasing hormone (a peptide hormone involved in the stress response) in comparison with placebo(Reference Murray, Lam and Rehman82). In rat, GLUT2 translocation is inhibited by stress(Reference Shepherd, Helliwell and Mace83). Interestingly, IBS patients often report that the onset of IBS is associated with stress(Reference Ragnarsson and Bodemar15) and the stress scores of patients also correlated with the medical impact of IBS(Reference Boeckxstaens, Drug and Dumitrascu18). Therefore, one possible mechanism of fructose malabsorption in IBS could be the inhibition of GLUT2 translocation in response to stress. However, so far GLUT2 has not been identified as a major player in fructose malabsorption(Reference Wilder-Smith, Li and Ho84,Reference Kato, Iizuka and Takao85) or as an IBS marker. Carbohydrate responsive element-binding protein (ChREBP) is a transcription factor regulated by sugar intake. ChREBP-KO mice fed with a high-fructose diet developed fructose malabsorption with diarrhoea and caecum distension in association with decrease in expression of genes involved in fructose transport and metabolism(Reference Kato, Iizuka and Takao85,Reference Kim, Astapova and Flier86) . More specifically, ChREBP-KO mice were associated with insufficient induction of GLUT5 in response to fructose, which could potentially explain fructose malabsorption(Reference Oh, Sohn and Lee87). However, GLUT5 mRNA expression and protein level are not affected in patients with fructose intolerance(Reference Shepherd, Helliwell and Mace83). The mechanism underlying fructose malabsorption in humans is largely unknown and may depend on the transport capacity of GLUT5 or on fructose transporter regulation. However, the reason for a higher prevalence of fructose malabsorption in IBS patients requires further investigation.
Mechanisms underlying fructose-related symptoms in irritable bowel syndrome
The deleterious role of fructose in IBS was emphasised 10 years ago when uncontrolled studies using a low-fructose diet reported an improvement in IBS symptoms(Reference Heizer, Southern and McGovern88), while 25 and 50 g intake of fructose was found to promote IBS-like GI symptoms in healthy individuals(Reference Beyer, Caviar and McCallum12,Reference Murray, Wilkinson-Smith and Hoad89) . Fructose is also able to cause GI symptoms (abdominal pain, diarrhoea) in IBS patients but at a dose as low as 14 g/d(Reference Choi, Kraft and Zimmerman90,Reference Gibson, Newnham and Barrett91) .
Unabsorbed fructose may play a role in osmotic load. Fructose is osmotically active in the intestine when poorly absorbed and in healthy humans, unabsorbed fructose increases water volume in the small bowel(Reference Murray, Wilkinson-Smith and Hoad89). Similar results were found in IBS patients in which fructose-induced small bowel water content was associated with increasing symptoms(Reference Murray, Wilkinson-Smith and Hoad89).
Furthermore, induction of GI symptoms after a fructose load is linked to intestinal fermentation and gas production (hydrogen, carbon dioxide and methane)(Reference Wilder-Smith, Olesen and Materna92). In non-IBS and IBS individuals fructose intake increased colonic luminal volume, gas production and breath hydrogen levels, but only IBS patients experienced increased abdominal symptoms(Reference Major, Pritchard and Murray31). This suggests that colonic hypersensitivity to distension produces fructose-related symptoms only in patients with IBS(Reference Major, Pritchard and Murray31) even if the role of visceral hypersensitivity in carbohydrate-related symptoms is still debated(Reference Tuck, McNamara and Gibson93).
The role of barrier function and inflammation has also been proposed. In animal models, there is an association between fructose intake, increased intestinal permeability and inflammation(Reference Do, Lee and Oh94-Reference Volynets, Louis and Pretz97). However, in humans, fructose malabsorption in IBS patients does not seem to be linked to low-grade inflammation or to increased intestinal permeability(Reference Melchior, Aziz and Aubry98,Reference Kuzma, Cromer and Hagman99) .
The importance of the microbiota in IBS has been suggested by the transplantation of microbiota from IBS patients into mice and rats which leads the recipient animals to develop IBS-like symptoms(Reference De Palma, Lynch and Lu100,Reference Crouzet, Gaultier and Del’Homme101) . In a context of fructose malabsorption, unabsorbed fructose spills over into the distal small intestine and the colon, where it is fermented by anaerobic bacteria. Lactic acid and SCFA, predominantly propionate and butyrate, are some of the potential by-products of this fermentation(Reference Zhang, Grosfeld and Williams70,Reference Louis and Flint102,Reference Jang, Hui and Lu103) . Differences in the ability of intestinal microbiota to metabolise carbohydrates exist and are related to microbiome composition(Reference Chassard and Lacroix104). Fructose can be metabolised by several groups of bacteria(Reference Endo, Maeno and Tanizawa105,Reference Endo, Futagawa-Endo and Dicks106) and in humans it is mainly fermented by lactic acid bacteria (mainly Lactobacillus species), and also by Clostridium cluster IV genus Faecalibacterium (Reference Moens and De Vuyst107). IBS patients with functional variants of the sucrase–isomaltase gene displayed a specific faecal microbiota composition including higher Blautia abundance(Reference Thingholm, Ruhlemann and Wang108). In a rodent fructose malabsorption model (KHK–/– mice) fructose intake alters microbiota composition and metabolism, including a drastic increase in Coriobacteriaceae, Corynebacteriaceae and Lactobacillaceae as well as higher levels of propionate and lactate in the caecal content(Reference Zhang, Grosfeld and Williams70). In rodent models of IBS, butyrate was suggested to sensitise the colon, through acid-sensing ion channel 3 (ASIC3) and transient receptor potential vanilloide 1 (TRPV1)(Reference Jones, Otsuka and Wagstrom109-Reference Matricon, Muller and Accarie111), while lactate production favours luminal acidity which has been associated with an increase in visceral hypersensitivity(Reference Holzer112).
Low-fructose diet in irritable bowel syndrome
A low-fructose diet consists in reducing daily fructose intake; the most frequently allowed dose is under 6 g/d. The efficacy of a low-fructose diet has been suggested in several studies (Table 2). However, the dose for fructose restriction in low-fructose diet has not yet been established. In a study which included twenty-six patients with IBS, patients compliant with a low-fructose diet had improvement in their GI symptoms (abdominal pain and diarrhoea), with a moderate impact on their quality of life(Reference Choi, Kraft and Zimmerman90). A larger study, with 182 IBS patients, found that a low-fructose diet improved symptom scores (abdominal pain) but had a modest effect on stool frequency(Reference Berg, Fagerli and Martinussen113). In patients with IBS with fructose and sorbitol malabsorption, 81 % reported improvement after 1 month of a low-fructose and -sorbitol diet and 67 % at 12 months(Reference Fernandez-Banares, Rosinach and Esteve114). On the other hand, after 22 weeks of a low-fructose diet, 70 % of IBS patients challenged with fructose and fructans reported symptoms in a dose-dependent manner compared with only 14 % in the placebo group with glucose(Reference Shepherd, Parker and Muir38). To summarise, in open-label studies, the efficacy of a low-fructose diet achieves adequate symptom relief in 46 to 81 % of IBS patients (Table 2)(Reference Choi, Kraft and Zimmerman90,Reference Berg, Fagerli and Martinussen113,Reference Shepherd and Gibson115) . These large fluctuations could be explained by the different endpoints used in the studies (abdominal pain, transit, quality of life, etc.). Unfortunately, most of the studies provided a low level of evidence with retrospective analysis and were done without control or placebo groups. Moreover, it is still unclear which patients could benefit from a low-fructose diet. In several open studies, the fructose breath tests were not predictive of the efficacy of a low-fructose diet on IBS symptoms(Reference Berg, Fagerli and Martinussen113,Reference Helwig, Koch and Koppka116,Reference Melchior, Desprez and Houivet117) . Different doses of fructose for breath testing may have to be used to better select the candidates for this diet. Moreover, in IBS patients, certain factors appear to be involved in the efficacy of low-FODMAP or low-fructose diets. For instance, sucrase–isomaltase variants in IBS patients were associated with a lower efficacy to reduce IBS symptoms in response to a low-FODMAP diet(Reference Husein and Naim50,Reference Zheng, Eswaran and Photenhauer118) . In three randomised trials, the efficacy of a low-FODMAP diet in IBS patients was predicted by the amount of volatile organic compounds present in the faeces(Reference Rossi, Aggio and Staudacher119), by the initial faecal bacterial profiles of the patients(Reference Bennet, Bohn and Storsrud120) or the increased peak concentrations of breath methane during the fructose breath test preceding the low-FODMAP intervention(Reference Wilder-Smith, Olesen and Materna121). These data suggest a potential role of the gut microbiota composition and metabolism of the patients in their ability to respond to the dietary interventions.
Table 2. Studies assessing low-fructose diet in irritable bowel syndrome (IBS)

Irritable bowel syndrome patients’ management regarding fructose
One of the first steps regarding IBS patients’ management regarding fructose would be to avoid high fructose consumption. Indeed, high fructose consumption can lead to GI symptoms without IBS. Adolescents are among the higher fructose consumers(Reference Douard and Ferraris40), their consumption exceeds intestinal ability to absorb fructose. Lowering their fructose to a normal consumption often resolves the GI symptoms they experience.
A second step would be to identify the individuals in whom fructose malabsorption should be tested. Those included in priority the IBS patients refractory to first-line therapies and/or with a clear link between carbohydrate intake and GI symptoms. For instance, young male IBS patients could be at a higher risk for fructose malabsorption and be systematically tested(Reference Melchior, Gourcerol and Dechelotte76).
Diet could be recommended in all IBS patients in second-line therapy. The only validated restrictive diet is the low-FODMAP diet. Initially, a 4-week low-FODMAP diet could be introduced and, if efficient, FODMAP have to be reintroduced progressively to identify foods triggering symptoms, as a low-FODMAP diet could lead to nutritional deficiency such as fibre, Ca, Fe, Zn, folate, vitamins B and D and natural antioxidants(Reference Catassi, Lionetti and Gatti122). Following this, the patient can follow a less restrictive diet that only excludes their personal FODMAP triggers(Reference Whelan, Martin and Staudacher123). The restriction of individual FODMAP (such as lactose, fructose, etc.) could be of interest for long-term management.
Conclusion
Fructose plays an important role in IBS. Fructose malabsorption is frequent in patients with IBS but its mechanisms are not well understood. Exceeding the capacity of intestinal fructose absorption leads to an osmotic effect and fermentation by-products by the microbiome. The roles of visceral hypersensitivity and specific microbiota profiles in fructose-induced symptoms require better understanding. Further controlled studies are needed to identify predictive factors of the efficacy of a low-fructose diet on IBS symptoms.
Acknowledgements
The authors are grateful to Gregory Mosni (Physiology Department, Rouen University Hospital) for his technical help and to Nikki Sabourin-Gibbs (Rouen University Hospital) for her help in editing the manuscript.
There was no funding.
G. G., V. D., M. C. and C. M. contributed to the writing and editing of the draft and approved the submitted version of the manuscript. All authors agree to be personally accountable for their own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which they were not personally involved, are appropriately investigated, resolved and documented in the literature. C. M. is the guarantor of the review.
There are no conflicts of interest.





