Long-term care facilities (LTCFs) have been disproportionately affected by coronavirus disease 2019 (COVID-19). The high incidence and case fatality rate of LTCF residents highlights the vulnerability of frail individuals with numerous comorbidities in a congregate setting with a long duration of stay.Reference Ouslander1,Reference D’Adamo, Yoshikawa and Ouslander2 Across Canada and Europe, most COVID-19–related deaths have occurred in LTCFs.Reference Ouslander1-Reference Adlhoch, Kinross and Melidou3 In British Columbia, 59% of COVID-19–related deaths were in LTCFs, compared to 75% in Canada overall and 30%–60% across Europe.Reference Adlhoch, Kinross and Melidou3,Reference Sinha and McCleave4 In the United States, a single COVID-19 outbreak in an LTCF facility in Washington State resulted in 62% of the LTCF residents becoming infected, of whom 56.8% were subsequently hospitalized and 27.2% died.Reference McMichael, Clark and Pogosjans5
Many large COVID-19 outbreaks have been attributed to a failure in proactive surveillance and early recognition of potentially infected patients, as well as a failure to rapidly implement appropriate infection control measures.Reference Adlhoch, Kinross and Melidou3,Reference McMichael, Clark and Pogosjans5 A national Canadian military report of 5 LTCFs experiencing COVID-19 outbreaks highlighted serious concerns regarding infection control practices, frontline working conditions, limited supplies, and poor policies and procedures.Reference Mialkowski6 Additionally, increased crowding, use of communal spaces, low staffing ratios, and documented index infection in staff members all increase the risk of a COVID-19 outbreak in LTCFs.Reference Fisman, Bogoch, Lapointe-Shaw, McCready and Tuite7 Given the significant mortality among residents, proactive infection prevention measures, as well as effective outbreak management by public health, are necessary to reduce and/or prevent subsequent COVID-19 cases when they are detected in the facility.
The first Canadian LTCF COVID-19 outbreak and resident death occurred in British Columbia, within the Vancouver Costal Health (VCH) region.Reference Hager and Woo8 As a result, mitigating the transmission of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in LTCFs quickly became a top priority. A rapid, coordinated, and multistakeholder outbreak control response was developed to specifically support LTCFs. A multifaceted intervention containing a bundle of outbreak control measures was developed and immediately implemented following the initiation of each facility’s outbreak response. This was accomplished through collaboration between LTCF leadership and regional residential care, infection prevention and control (IPAC), and public health programs.
The objectives of this study were (1) to provide a descriptive overview of LTCF COVID-19 outbreaks, (2) to evaluate the effectiveness of the intervention (a bundle of outbreak control measures) in terms of reducing subsequent transmission among residents and staff, and (3) to inform the ongoing public health approach to managing COVID-19 outbreaks in LTCFs.
Methods
Setting
In British Columbia, acute, community, residential care as well as public health are delivered by 5 geographically defined regional health authorities (RHA), one of which is VCH. A unique and important feature of public health in British Columbia involves the licensing and regulation of LTCFs.9 Moreover, RHAs can also be responsible for directly operating or financially supporting many LTCFs within their region.
VCH is responsible for providing care to ∼1.25 million people (25% of the BC population). There are 75 LTCFs located within the VCH region (19% of all facilities in the province), of which 21% and 57% are respectively owned or financially supported by VCH. As of May 2020, 35% (76 per 100,000 population) of all COVID-19 cases in the province were located in the VCH region. The study period of our analysis spanned February 28, 2020, through May 24, 2020.
Study population
All LTCFs with a documented exposure to a laboratory-confirmed case of COVID-1910 among staff members or residents that resulted in <2 subsequent cases in the facility were excluded because there would not be enough data to carry out a segmented regression analysis. Asymptomatic cases (n = 19.6%) were excluded from the analysis because their incidence could not be clearly reliably attributed to the early outbreak period versus the postintervention period. Eligible facilities varied in size, ranging from 108 to 259 staff and from 107 to 210 residents (Appendix 1 online).
Data collection
All COVID-19 cases residing within VCH were contacted by public health staff for case management and contact tracing through a standardized data collection form.11 Staff collected case information including demographics, symptom onset date, exposure details, association with high-risk settings, and high-risk contacts through patient and family interviews and medical chart review. Data were centrally compiled to form a master case list and an individual facility line list. Cross validation of data for each case was carried out between the master case list and individual facility line lists. Conflicting or missing values were reconciled and corrected through a review of these cases. Total resident and staff numbers within each LTCF during the outbreak period were obtained from licensing records (ie, staff and resident census lists).
Study intervention
A bundle of outbreak control measures were imposed by public health upon outbreak declaration and are summarized in Table 1.
Table 1. Description of the Multisectoral Intervention Implemented in Long-Term Care Facilities
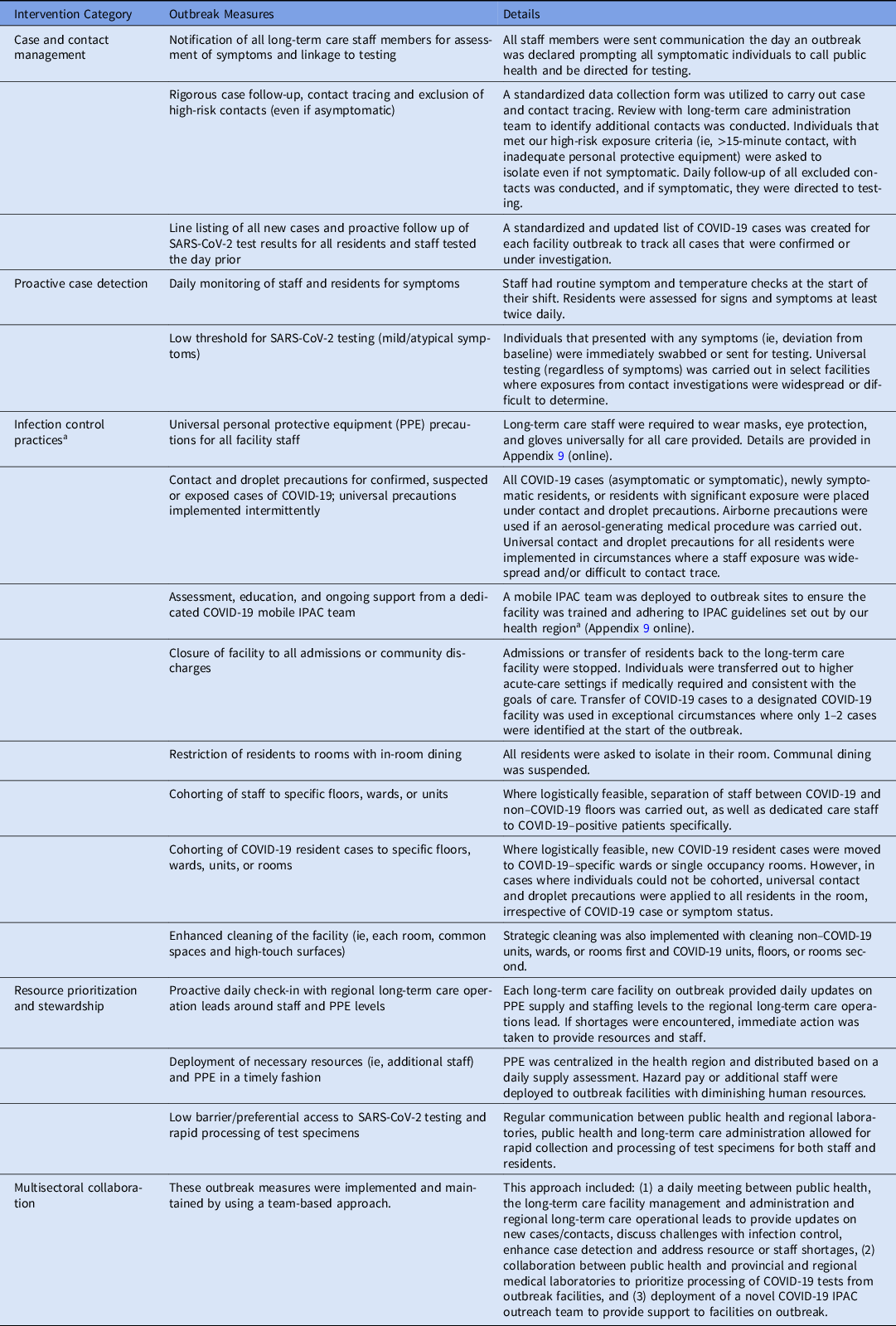
Note. PPE, personal protective equipment.
a Additional details around infection control and outbreak control measures can be found in greater detail in the British Columbia Infection Prevention and Control Requirements for COVID-19 in Long-Term Care and Seniors’ Assisted Living.40
Primary outcome
Our primary outcome of interest was the COVID-19 incidence rate within each facility, which was calculated for staff and residents using case counts over 2 days, divided by the total population in the facility at-risk (removing individuals who became cases in previous time periods). Symptom onset dates (instead of case report dates) were used as a marker for incidence because of the inherent delays between exposure and case identification.
Potential confounders
Staffing levels for IPAC were similar across facilities as it was delivered by an outreach team that would deploy immediately following declaration of a facility outbreak. A daily meeting between regional LTCF operation leads, public health representatives, and the LTCF administration ensured consistent resource allocation, maintenance of staffing levels, and adherence to consistent IPAC recommendations during each LTCF outbreak. Lastly, our model accounted for background community infection rates (Appendix 2 online).
Study design
The study was a quasi-experimental before-and-after study based on a segmented time-trend regression analysis of interrupted time-series data. Segmented regression analysis of time-series data is a widely used method to evaluate the effect of population-level interventions or policy changes implemented at a discrete point in time.Reference Bernal, Cummins and Gasparrini12 For these reasons, we used this method to evaluate the impact of this intervention on preventing further transmission and spread of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) within LTCFs experiencing an outbreak.
Our expectation was that the effect of these measures on the rate of new cases would be fully apparent, 14 days after implementation since individuals could incubate up to 14 days from their exposure to SARS-CoV-2 before showing COVID-19 symptoms.Reference Lauer, Grantz and Bi13
Statistical analyses
COVID-19 case demographics (age and sex) and case status by case type (staff vs resident) within LTCFs were summarized. Attack rates and case fatality rates for each facility were calculated using public health and licensing data. These statistical analyses were carried out using Stata version 15 software.14
A mixed-effect segmented Poisson regression was fit to our facility-specific COVID-19 case data against time to assess the association between the intervention and the COVID-19 incidence rate. The model was built using a standard approach for segmented regression of time series dataReference Penfold and Fang15 and the study followed the Outbreak Reports and Intervention Studies of Nosocomial Infection (ORION) reporting guidelines.Reference Stone, Cooper and Kibbler16 R version 3.6.2 software17 was used to perform generalized linear mixed-effects regression and generate figures with the ggplot2 package.
For each facility, the outbreak period was segmented into an early outbreak period (from the first case until 14 days following implementation of measures) and the postintervention period (after 14 days from the implementation of measures). We estimated 4 standard components: (1) the early outbreak trend in COVID-19 rate, (2) the postintervention trend in COVID-19 rate, (3) the magnitude of change in trend from early outbreak to postintervention, and (4) the change in the average COVID-19 rate from early outbreak to postintervention (ie, level change). Random intercept models (using facility as a random effect) were used to account for variation by facility in COVID-19 rates and for the nonindependence of cases within a facility arising from the infectious spread of SARS-CoV-2. Relative effects in the form of rate ratios (RRs) were calculated through exponentiation of the relevant model coefficients. A second model was constructed to evaluate case type (staff vs resident) as an effect modifier. Two-sided tests at 5% significance levels were used to determine statistically significant differences.
A counterfactual trend during the postintervention period was generated by setting all model coefficients, except early outbreak trend, to zero and predicting the COVID-19 rate as if the intervention were not effective or were not implemented. Full details regarding the model specification and residuals examination can be found in Appendix 2 (online).
Ethics approval
Research ethics board review was not required because this study was part of routine public health operations for quality improvement and program evaluation. Data were deidentified and aggregated, and results were suppressed where counts were <5 individuals.
Results
Descriptive analyses
Between February 28, 2020, and May 30, 2020, 18 of 75 (24%) of all LTCFs in the VCH region had at least 1 documented exposure from a COVID-19 case. Among those, 10 of 18 (56%) had a single staff case of COVID-19 with no documented transmission to another staff member or resident. One facility experienced only 1 subsequent case. Among these 18 LTCFs, 7 experienced 2 or more subsequent cases and were included in the analysis.
In total, 275 COVID-19 cases (165 staff and 110 residents) were reported to public health from these 18 study facilities. Appendix 3.1 (online) shows case counts by symptom onset or episode dates for long-term care staff. Appendices 3.2 and 3.3 (online) summarize the characteristics of symptomatic and asymptomatic COVID-19 cases by facility. For all of the LTCFs, except facility C, most cases occurred among residents. The facility attack rates ranged from <4% to 25%. The case fatality rate for infected residents among individual facilities ranged from 22% to 50%.
Appendices 3.4 and 3.5 (online) outline characteristics of symptomatic and asymptomatic COVID-19 cases by case type for the study facilities, respectively. The case fatality rate was 34% among residents, and no deaths were recorded among staff. Figure 1 illustrates the size and duration of COVID-19 outbreaks by facility as well as the varied characteristics of each outbreak and non-LTCF cases in VCH.

Fig. 1. Size and duration of COVID-19 outbreaks in study long-term care facilities by symptom onset date. Dots indicate cases and the dot size is proportional to the number of cases. Prior to April 8, 2020, testing was restricted to individuals that were either hospitalized, likely to be hospitalized, health care workers, residents of long-term care facilities or part of an investigation of a cluster/or outbreak (as decided by public health). Therefore, nonfacility cases were likely underestimated during that period.
Regression analyses
The results of the regression model are described in Table 2 and Appendix 4 (online). The segmented regression analyses are presented in Fig. 2 based on a model with the effect modification terms (model 2).
Table 2. Results of Segmented Regression Analysis to Evaluate the Impact of a Multisectoral COVID-19 Intervention

Note. RR, Rate Ratios; CI, confidence interval; *P < .05; ** P < .01; ***P < .001; ****P < .0001.
a Model 1 adjusts for baseline trend, change in rate, change in trend and case type (resident vs staff), and allows a random baseline COVID-19 rate among facilities.
b Model 2 adjusts for the same covariates as model 1 as well as interactions between case type and baseline trend, change in rate, and change in trend. It also allows for a random baseline COVID-19 rate among facilities.
c Ratio of relative rate between staff and residents.
d Average 2-day (daily) change in the rate of COVID-19 during the early outbreak period (prior to public health measures, plus 14 days).
e Difference in the average COVID-19 rate between the early outbreak period and the postintervention period (ie, level shift).
f Change in slope from the early outbreak period to the postintervention period.
g Average daily change in the rate of COVID-19 during the postintervention period (starting 14 days after the intervention).

Fig. 2. Segmented regression result for all study facilities. Time is based on symptom onset date. Rates were calculated for every 2-day period. A counterfactual (dotted line) was constructed to visually represent that predicted rate of COVID-19 if public health measures were not implemented or were not effective. The results from model 2 are shown.
After adjusting for case type, there was a significant upward trend in the COVID-19 incidence rate during the early outbreak period (RR, 1.07; 95% CI, 1.03–1.11; P < .001). Following 14 days from implementation of the intervention bundle, a significant reversal in trend was identified (RR, 0.68; 95% CI, 0.62–0.75; P < .001). In particular, the postintervention trend demonstrated a 27% decrease in the COVID-19 incidence rate every 2 days (RR, 0.73; 95% CI, 0.67–0.80; P < .001). We detected a decrease (level change) in the overall average incidence rate following the early outbreak period (RR, 0.83; 95% CI, 0.52–1.36) that was not statistically significant (P > .05).
Effect modification by case type
The upward COVID-19 incidence trend during the early outbreak period did not differ significantly between staff and resident (P > .05). Neither the change in trend during the early outbreak period versus postintervention period (RR, 1.07; 95% CI, 0.88–1.31), nor the downward postintervention trend (RR, 1.07; 95% CI, 0.88–1.30), varied significantly between staff and residents.
However, the level change from the early outbreak to postintervention period was significantly different between residents and staff. Specifically, staff had a 70% greater reduction in their average rate of COVID-19 compared to residents following the early outbreak period (RR, 0.30; 95% CI, 0.10–0.88; P < .05).
Discussion
Summary of findings
The results of our analysis provide an overview of the epidemiology of COVID-19 within LTCFs experiencing outbreaks in the VCH region. Most cases occurred among residents of these facilities, whereas only 1 facility had more COVID-19 cases among staff than residents. Our regression analysis demonstrated that the combination of outbreak control measures (Table 1) delivered through a collaborative approach were associated with a decrease in COVID-19 incidence rates 14 days from implementation in each LTCF. This change from an upward to downward trend in COVID-19 was consistently detected among both staff and residents and across facilities, regardless of the background rates of community transmission. In addition, the impact of the intervention varied between staff and residents, with a significantly greater decrease (level change) in the average rate of COVID-19 among staff compared to residents after the early outbreak period.
Explanation of findings
The pronounced effect of the intervention among staff cases may be attributable to the lower exposure risk experienced by staff because they spend less time in the facility and they use personal protective equipment daily. Also, many of the outbreak control measures are largely focused on rapidly identifying and removing symptomatic staff from the work environment, thereby decreasing the frequency of new COVID-19 introductions into the facility. The gradual but persistent decline of new resident cases after the intervention can be explained by the increased exposure time in the facility as well as challenges with resident isolation (ie, wandering due to cognitive impairment). This pronounced effect among staff is particularly important given that documented infections among staff has been demonstrated to be a strong risk factor of long-term care resident mortality.Reference Fisman, Lapointe-Shaw, Bogoch, McCready and Tuite18
Comparison of related studies in the literature
To the best of our knowledge, this is the first study to evaluate outbreak control measures to mitigate the transmission of COVID-19 in LTCF using a quasi-experimental design. Cheng et alReference Cheng, Wong and Chen19 evaluated a regional infection control response to COVID-19 using descriptive epidemiological methods. Various studies using interrupted time series analysis and segmented regression analysis have evaluated the impact of broader interventions such as social distancing,Reference Vokó and Pitter20 travel restrictions,Reference Tian, Liu and Li21 and lockdown policiesReference Figueiredo, Daponte Codina and Figueiredo22 on COVID-19 incidence and mortality.
In addition, various outbreak summary reports, commentaries, and media articles have highlighted the challenges with managing COVID-19 outbreaks in LTCFs in other regions of Canada, United States, and Europe. Key barriers included poor communication and collaboration between key actors, limited access to personal protective equipment (PPE), inadequate early identification of symptomatic staff and resident cases, and challenges in infection control education and adherence.Reference Adlhoch, Kinross and Melidou3,Reference McMichael, Clark and Pogosjans5,Reference Mialkowski6,Reference McMichael, Currie and Clark23-Reference Szczerbińska26 In contrast, our intervention was administered through a collaborative team-based approach that fostered excellent communication between public health and LTCF operators. This approach also facilitated the implementation of public health directives and troubleshooting ongoing concerns with the facility. Working directly within a regional health authority structure, PPE levels were monitored daily and were prioritized to LTCFs facing shortages. Access to accurate resident and staff census lists (through public health licensing officials) allowed early notification, assessment, and exclusion of all symptomatic or significantly exposed staff. Prioritization and low-barrier access to SARS-CoV-2 testing allowed for timely case identification and public health action. Furthermore, our intervention included outbreak measures that have been implemented to curb transmission across the United States, such as cohortingReference Eckardt, Guran and Hennemyre27 and routine symptom monitoring of staff and residents,Reference Mills, Kaye and Mody28 universal mask policies,Reference Walker, Fleece and Griffin29 appropriate PPE use/ensuring no PPE shortages.Reference Telford, Bystrom, Fox, Wiggins-Benn, McCloud, Holland and Shah30 As a result, our analysis provides additional support for the effectiveness of outbreak measures not implemented in large LTCF COVID-19 outbreaks in other jurisdictions and are comparable to recommended approaches in the United States and Canada.Reference D’Adamo, Yoshikawa and Ouslander2,Reference Mills, Kaye and Mody28,Reference Chen, Ryskina and Jung31-Reference Ouslander and Grabowski33
However, important difference exists in our approach compared to what has been reported and recommended in the United States. First, the rapid creation and deployment of a government-funded COVID-19 IPAC outreach team was critical in providing effective standardizedReference Mills, Kaye and Mody28 education to staff, carrying out infection control audits, and diminishing a substantial burden on the LTCF IPAC educators and administrators. Also, we did not conduct weekly, biweekly or bimonthly testing of LTCF staff without symptoms, which is currently recommended32,Reference Ouslander and Grabowski33 with reported effectiveness.Reference Escobar, Lanzi and Saberi34 However, we enacted broad and stringent infection control precautions, which likely reduced the benefit of serial testing.Reference Lanièce Delaunay, Saeed and Nguyen35 Lastly, during our study period universal facility-wide testing was not carried out following the first identified case but rather was determined by contact investigations. However, after the conclusion of our study period, the health region has adopted facility-wide testing to align with current evidence.Reference Dora, Winnett and Jatt36,Reference Hatfield, Reddy and Forsberg37
Strengths and limitations
Time-based segmented regression analyses are one of the strongest quasi-experimental designs to evaluate the impact of population-level interventions targeting nosocomial infection rates.Reference Shardell, Harris, El-Kamary, Furuno, Miller and Perencevich38 A mixed-effect model also adds rigor to account for dependency (correlation) of observations within each facility. A major strength of a multigroup analysis is the ability to assess for comparability between groups on our observed covariates. Using multiple facilities also increased the number of time points, adding additional power to detect significant effects.Reference Bernal, Cummins and Gasparrini12 A time-based approach also allows for the control of overall secular trends in rates, which can provide an estimate of the true impact of the intervention. Our model demonstrated a consistent effect across facilities while accounting for varying COVID-19 incidence rates among facilities and across time. Lastly, LTCFs that experienced significant COVID-19 outbreaks (>2 cases) occurred unsystematically in our region, providing essentially a random sample of LTCFs for analysis.
However, with the study of any model, there are limitations. First, we assumed that the bundle of measures was imposed upon outbreak declaration; however, the actual implementation of each measure may have occurred over a few days, underestimating the true effect of the intervention. Second, although the model evaluates the bundle of measures, it cannot determine the contribution of individual measure to the overall effect nor whether the intervention improved across time as it became more cohesive and comprehensive. Third, our findings should only be generalized to LTCFs experiencing COVID-19 outbreaks (with >2 cases). Our intervention may not be easily implemented or generalizable in jurisdictions that do not utilize a regional health authority structure to deliver health services. Fourth, asymptomatic cases could not be reliably included potentially biasing our results; however, it is unlikely that these cases would significantly drive our final model due to their small size. A final limitation is the lack of a control group (ie, an LTCF where the intervention was not implemented) given that this would have been unethical. Nonetheless, the early outbreak intervention period serves as control for the postintervention period, which still accounts for threats to internal validity and constitutes a methodologically acceptable study design for evaluating the impact of population-level intervention.Reference Ansari, Gray and Nathwani39
In conclusion, our comprehensive, timely intervention leveraged regional partnerships to reduce the incidence of COVID-19 in LTCFs, underscoring the value and importance of collaborative approaches for effective infection control. The findings of this study can help to inform and prepare key policy makers such as public health, infection control practitioners, healthcare professionals, and LTCF operators for future COVID-19 outbreaks. We hope our intervention and its team-based approach can be adapted and utilized by other jurisdictions to effectively decrease SARS-CoV-2 transmission and protect the vulnerable populations in LTCFs.
Acknowledgments
We would like to acknowledge the hard work of the front line public health and healthcare staff involved in the COVID-19 outbreak response in long term care facilities.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.1407







