Human diets contain both cholesterol and oxidised cholesterol (OxC). Cholesterol is susceptible to oxidation, forming a series of cholesterol oxidation products (COP) under various food processing conditions. The amount of COP can reach up to 10 % total cholesterol (TC) in foodsReference Kumar and Shingal1, particularly in Western countries where total fat intake is high and fried foods are popularReference Escharte, Zulet and Astiasaran2. More than thirty COP have been identified and reportedReference Smith3, Reference Osada, Komada, Cui, Yamada and Sugano4. The major COP include 7β-hydroxycholesterol, 7α-hydroxycholesterol, 5α-hydroxycholesterol, 7-ketocholesterol and α-epoxidesReference Tai, Chen and Chen5.
COP have been extensively studied for their undesirable effects including cytotoxicityReference Al Kanhal, Ahmad, Al Othman, Al Orf and Al Murshed6, mutagenesisReference Homma, Kondo, Kaneko, Kitamura, Nyou, Yanagisawa, Yamamoto and Katizoe7 and carcinogenesisReference Wrensch, Petrakis, Grenke, Miike, Ernster, King, Hauck, Craig and Goodson8. In addition, COP have been shown to be absorbed in a similar way to cholesterolReference Tomoyori, Carvajal, Nakayama, Kishi, Sato, Ikeda and Imaizumi9, Reference Emanuel, Hassel, Addis, Bergmann and Zavoral10, cause disturbance of lipid metabolismReference Seillan11 and alter the cell membrane functionReference Massey12, Reference van Reyk, Brown, Hult'en, Dean and Jessup13. Most importantly, COP could accelerate fatty streak lesion formation and promote atherosclerosisReference Staprans, Pan, Joseph and Feingold14, Reference Staprans, Pan, Joseph, Grunfeld and Feingold15. Human daily cholesterol intake is about 300–500 mg while that of COP could be up to 30–50 mg, provided that 10 % cholesterol in the diet is oxidised. It is well known that high cholesterol consumption elevates plasma TC and LDL-cholesterol levels and increases the risk of CHD. However, the effect of COP in the diet on blood cholesterol is unfortunately ignored and there is very limited information concerning relative hypercholesterolaemic and atherosclerotic activity of COP and cholesterol itselfReference Al Kanhal, Ahmad, Al Othman, Al Orf and Al Murshed6. The present study was therefore carried out to investigate the effect of OxC on blood cholesterol level, atherosclerotic plaque formation and endothelium function compared with that of non-oxidised cholesterol using hamsters as an animal model.
Experimental methods
Preparation of oxidised cholesterol
Pure cholesterol was purchased from Sigma Chemical Company. GLC analysis found that COP were less than 0·1 % of TC. An OxC mixture was prepared as previously describedReference Al Kanhal, Ahmad, Al Othman, Al Orf and Al Murshed6. In brief, pure cholesterol (100 g) was placed into a round-bottomed flask and heated in a 160°C oil bath in air for 72 h. The OxC mixture was dark-brown with an obnoxious smell. A proportion of the OxC mixture (20 mg) was converted to trimethylsilyl (TMS)-ether derivatives with a Sigma TMS-reagent (Sigma-Sil-A; Sigma, St Louis, MO, USA) and dissolved in the hexane. The TMS-ether derivatives were analysed in a fused silica capillary column (SACTM-5, 30 m × 0·25 mm; Supelco, Inc., Bellefonte, PA, USA) in a Shimadzu GC-14B equipped with a flame ionisation detector. GLC analysis demonstrated that the OxC mixture contained 78·0 % cholesterol, 8·3 % 7β-hydroxycholesterol, 4·9 % 7-ketocholesterol, 4·6 % 7α-hydroxycholesterol, 2·8 % α-epoxide and 1·8 % 5α-hydroxycholesterol (Fig. 1).
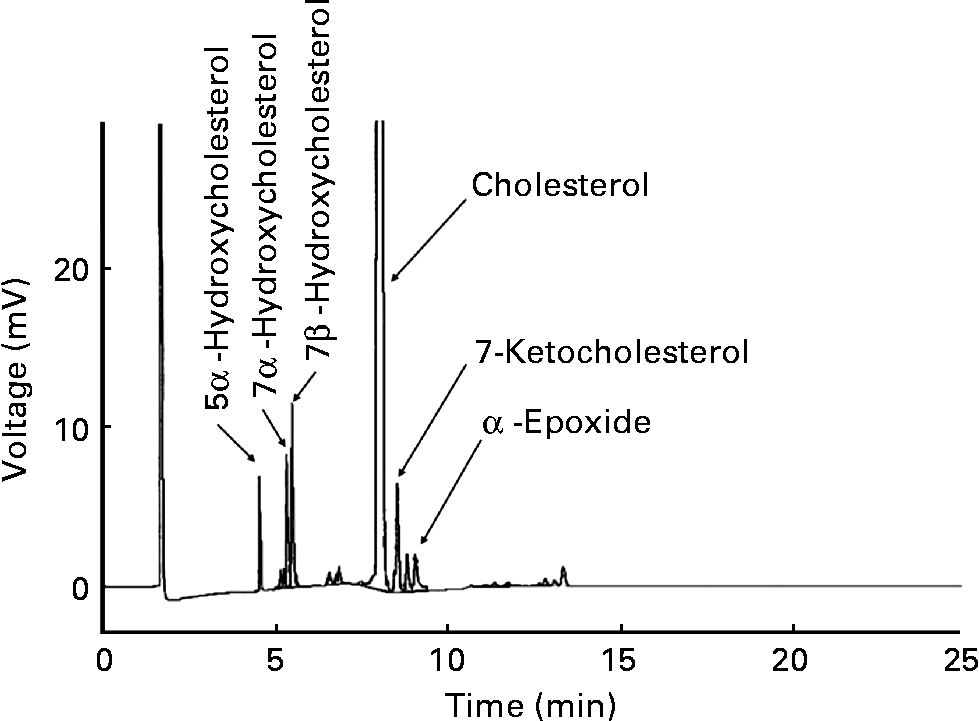
Fig. 1 Gas–liquid chromatogram of the oxidised cholesterol mixture.
Diets
The hypercholesterolaemic diet described by Sanders & Sandaradura was modified and used in the present studyReference Sanders and Sandaradura16. In brief, the control diet was prepared by mixing all powdered ingredients (g/kg): maize starch, 569; casein, 200; lard, 100; sucrose, 50; mineral mix, 40; vitamin mix, 20; dl-methionine, 1. The four experimental diets were prepared by adding 0·05 % non-oxidised cholesterol (C-0·05), 0·1 % non-oxidised cholesterol (C-0·1), 0·05 % OxC mixture (OxC-0·05) and 0·10 % OxC mixture (OxC-0·1), respectively. All five powdered diets were then mixed with a gelatin solution in a ratio of 200 g diet per litre solution. Once the gelatin had set, the diets were cut into approximately 20 g cubic portions and stored frozen ( − 20 °C). It should be pointed out that the actual amount of cholesterol in the diets was greater because lard contains 0·9 mg cholesterol/g, leading to an additional 0·01 % cholesterol in the diets. To be more specific, TC and OxC accounted for 0·01, 0·06, 0·06, 0·11 and 0·11 % in the control, C-0·05, OxC-0·05, C-0·1 and OxC-0·1 diets, respectively. All five diets contained 10 g lard/100 g of the following fatty acid composition (in % of total fatty acids): myristic acid, 2·5; palmitic acid, 24·.9; palmitoleic acid, 2·9; stearic acid, 19·0; oleic acid, 37·0; linoleic acid, 7·1; α-linolenic acid, 0·4.
Animals
Fifty Golden Syrian hamsters (Mesocricetus auratus; weight 100–120 g; age 3 months) were randomly divided into five groups (n 10) and fed one of the five diets. All hamsters were housed in an animal room at 23 °C with a 12 h light–12 h dark cycle. To minimise the oxidation of cholesterol, the fresh diets were given to the hamsters daily, and uneaten food was discarded. Food intake was measured daily and body weight was recorded twice per week. The faeces from each hamster were pooled for a period of the 2nd, 4th and 6th weeks. At the end of 6 weeks, all the hamsters were killed after overnight fasting. The blood was collected via the abdominal aorta. After clotting, the blood was centrifuged at 1500 g for 10 min and serum was collected. The liver was removed, washed with saline and stored at − 80 °C. The thoracic aorta was also removed and saved for the organ bath experiment and the analysis of atherosclerotic plaque.
Determination of serum lipoproteins
Several enzymic kits were purchased from Sigma (St Louis, MO, USA) to measure serum TAG (catalogue no. 336-20), TC (catalogue no. 352-20) and HDL-cholesterol (HDL-C; catalogue no. 352-4).
Measurement of liver and aorta cholesterol
In each case, the fresh aorta (100 mg) or liver sample (300 mg) was cleaned of adventitial tissue and washed in saline solution. Total lipids were extracted with the addition of 0·2 mg stigmastanol (1·0 mg for the liver sample) as an internal standard using chloroform–methanol (2:1, v/v). The lipid extracts were then saponified with 6 ml of 1 m-NaOH in 90 % ethanol at 90 °C for 1 h, and the non-saponified substances including cholesterol were converted to their TMS-ether derivatives by a commercial TMS reagent (Sigma-Sil-A; Sigma). The analysis of cholesterol TMS-ether derivatives was performed in a fused silica capillary column (SACTM-5, 30 m × 0·25 mm internal diameter; Supelco, Inc., Bellefonte, PA, USA) using a Shimadzu GC-14 B GLC equipped with a flame ionisation detector as previously describedReference Staprans, Pan, Joseph, Grunfeld and Feingold15.
Determination of faecal neutral and acidic sterols
Individual faecal neutral and acidic sterols were quantified as previously describedReference Chan, Fong, Cheung, Huang, Ho and Chen17. To simplify the analysis, only faecal samples collected in week 6 were analysed because the composition of faeces was relatively constant during this period. In brief, stigmasterol (0·3 mg) as an internal standard for neutral sterols was added to a faecal sample (300 mg). The sample was saponified using 9 ml 1 m-NaOH in 90 % ethanol containing 0·3 mg hyodeoxycholic acid as an internal standard for acidic sterols (Sigma, St Louis, MO, USA). The total neutral sterols were extracted using 8 ml cyclohexane and were then converted to their corresponding TMS-ether derivatives for GLC analysis.
After the cyclohexane extraction, 1 ml 10 m-NaOH was added to the remaining aqueous layer and heated at 120 °C for 3 h. After cooling down, 1 ml of distilled water and 3 ml 3 m-HCl were added and followed by extraction using 7 ml diethyl ether twice. The diethyl ether layers were then pooled followed by adding 2 ml methanol, 2 ml dimethoxypropane and 40 μl concentrated HCl (12 mol/l). After standing overnight at room temperature, the solvents were dried down and the acidic sterols were similarly converted to their TMS-ether derivatives at 60 °C for GLC analysis.
Aorta relaxation test
Aorta relaxation was used as an indication of change in arterial functionality. In brief, the thoracic aorta from non-oxidised and oxidised cholesterol groups was dissected out and surrounding connective tissues and fat were removed under a dissection microscope as previously describedReference Leung, Leng, Yao, Ko, Chen, Vanhoutte and Huang18. One part of the aorta was cut into 3 mm long aortic ring segments and each segment was suspended between two stainless-steel hooks in a 10 ml organ bath chamber filled with normal Krebs–Henseleit solution (119 mm-NaCl, 4·7 mm-KCl, 2·5 mm-CaCl2, 1 mm-MgCl2, 25 mm-NaHCO3, 1·5 mm-KH2PO4, 11·1 mm-d-glucose). The chamber was bubbled with a mixture of 95 % O2 and 5 % CO2 and kept at 37 °C throughout the entire experiment. For the two stainless-steel hooks, one of them was mounted at the bottom of the bath while another one was connected to a Grass FT03 force-displacement transducer (Grass Instruments, Quincy, MA, USA) and the isometric contraction was measured by transducer and recorded by the MacLab computer system (AD Instruments, Hastings, UK). Basal tension (1 g) was applied to all aortic rings and rings were allowed to equilibrate for 30 min. During this time, the bath solution was replaced with pre-warmed, oxygenated Krebs–Henseleit solution three to four times. After the equilibration period, the rings were first contracted with 0·3 μm-phenylephrine to test vessel contractibility and then relaxed by 0·3 μm-acetylcholine to assess integrity of the endothelial layer. Then, the rings were rinsed several times in pre-warmed and oxygenated Krebs–Henseleit solution until the basal tension was restored and allowed to equilibrate for 60 min. Then, the concentration–response curve to acetylcholine was constructed by adding 1 μm-phenylephrine and waiting until the rings' maximal contraction, followed by the addition of 3 nm- to 10 μm-acetylcholine to produce a concentration–response relaxation curve to acetylcholine.
Measurement of aorta atherosclerotic plaque
The percentage area of atherosclerotic plaque on the endothelial layer was determined as previously describedReference Zhang, James, Huang, Ho, Sahota and Chen19. In brief, the remaining part of the thoracic aorta was cut open vertically. The aorta was then stained with 0·5 g Sudan III in 10 ml ethanol for 3 h. The endothelial layer of aorta was then washed with distilled water for three times and scanned with a table scanner (Epson 1220 Perfection; Epson Co., Tokyo, Japan). The area of atherosclerotic plaque was measured with the aid of a computer image analysing program (Sigma Scan Pro 5.0; SPSS, Inc., Chicago, IL, USA).
Statistics
Data are expressed as mean values and standard deviations. The group means were statistically analysed using one-way ANOVA and post hoc LSD test on SigmaStat Advisory Statistical Software (SigmaStat version 14.0; SPSS, Inc.). Significance was defined as a P value less than 0·05.
Results
Body weight and food intake
The body-weight gain was similar among the five groups (data not shown). Oxidised cholesterol did not affect the weight gain compared with the control hamsters. Food intake ranged 10·9–11·2 g/d per hamster among the five groups. No significant difference in food intake was seen between the non-oxidised and oxidised cholesterol groups. COP intake by the OxC-0·05 and OxC-0·1 groups was 1·2 and 2·4 mg/d per hamster, respectively.
Serum total cholesterol, triacylglycerols and low-density lipoprotein cholesterol
Serum TC increased in response to the amount of cholesterol in the diet. As shown in Table 1, both dietary non-oxidised and oxidised cholesterol elevated serum TC in a dose-dependent manner. Compared with non-oxidised cholesterol, oxidised cholesterol was more hypercholesterolaemic (Table 1). To be more specific, serum TC level in the control, C-0·05, C-0·1, OxC-0·05 and OxC-0·1 groups was 2·85, 3·52, 4·30, 4·32 and 4·82 mmol/l, respectively. Serum TC in the OxC-0·05 and OxC-0·1 groups was 22 and 12 % higher, respectively, compared with the corresponding C-0·05 and C-0·1 hamsters (P < 0·05). Like TC, serum non-HDL-C exhibited similar change in response to dietary non-oxidised and oxidised cholesterol. Oxidised cholesterol had a greater raising effect on non-HDL-C level than non-oxidised cholesterol in the diet. For HDL-C level, no difference was observed between the C-0·05 and OxC-0·05 groups, but the OxC-0·1 group had an HDL-C level significantly higher than the C-0·1 hamsters (Table 1). Both non-oxidised and oxidised cholesterol groups had a greater non-HDL-C:HDL-C ratio than the control. However, no significant difference was observed between the non-oxidised and oxidised cholesterol groups. Serum TAG increased with the amount of dietary non-oxidised and oxidised cholesterol in a dose-dependent manner. However, it was demonstrated that the oxidised cholesterol groups had higher serum TAG levels than the corresponding hamsters maintained on the non-oxidised cholesterol diet (Table 1).
Table 1 Changes in serum total cholesterol (TC), triacylglycerols, HDL-cholesterol (HDL-C) and non-HDL-cholesterol (non-HDL-C) in hamsters fed the control (CON) and the experimental diets containing 0·05 % non-oxidised cholesterol (C-0·05), 0·10 % non-oxidised cholesterol (C-0·1), 0·05 % oxidised cholesterol (OxC-0·05) and 0·10 % oxidised cholesterol (OxC-0·1) for 6 weeks
(Mean values and standard deviations)
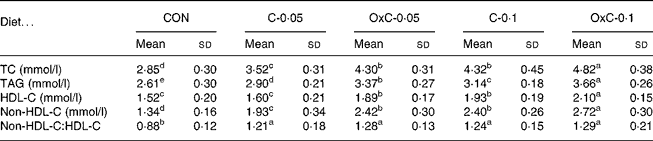
a,b,c,d,e Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
Liver cholesterol and cholesterol oxidation products
The amount of cholesterol in the liver increased with both non-oxidised and oxidised cholesterol in the diets in a dose-dependent manner (Table 2). There was no difference in hepatic cholesterol between the C-0·05 and OxC-0·05 groups. In contrast, hepatic cholesterol in the C-0·1 group was greater than that in OxC-0·1 hamsters. The results demonstrated that dietary COP could deposit in the liver, with 7α- hydroxycholesterol and 7β-hydroxycholesterol accumulating the most (Table 2). COP in the liver of the OxC-0·05 and OxC-0·1 groups were three to five times greater than that of the corresponding non-oxidised cholesterol groups.
Table 2 Changes in liver total cholesterol (TC) and cholesterol oxidation products (COP) in hamsters fed the control (CON) and the experimental diets containing 0·05 % non-oxidised cholesterol (C-0·05), 0·10 % non-oxidised cholesterol (C-0·1), 0·05 % oxidised cholesterol (OxC-0·05) and 0·10 % oxidised cholesterol (OxC-0·1) for 6 weeks
(Mean values and standard deviations)
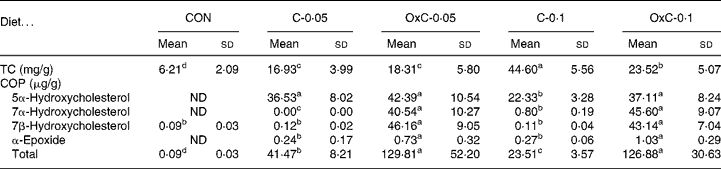
ND, not detectable.
a,b,c,d Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
Aortic cholesterol and atherosclerotic plaque
Both the non-oxidised and oxidised cholesterol diets increased deposition of cholesterol in the aorta compared with the control diet (Fig. 2). The two groups fed the OxC-0·05 and OxC-0·1 diets demonstrated greater deposition of cholesterol in the aorta compared with the corresponding hamsters fed diets containing 0·05 % and 0·1 % non-oxidised cholesterol (P < 0·05). Similarly, both groups fed diets containing oxidised cholesterol had a greater area of atherosclerotic plaque than the corresponding groups fed diets containing non-oxidised cholesterol. However, only the OxC-0·1 group had an area of atherosclerotic plaque significantly larger than the C-0·1 hamsters (P < 0·05).
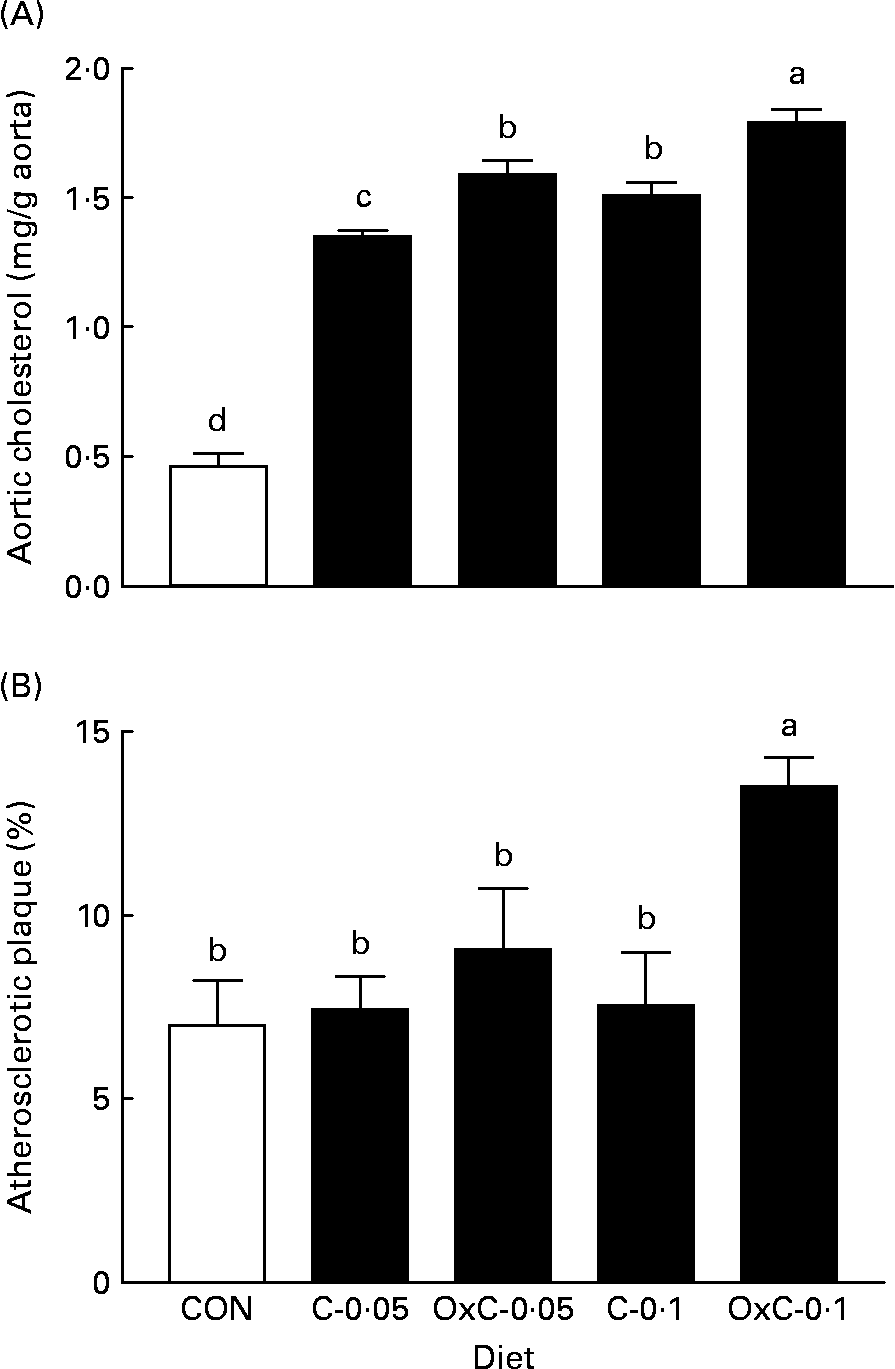
Fig. 2 Effect of dietary oxidised cholesterol on cholesterol content (A) and atherosclerotic plaque (B) in the aorta in hamsters. CON, control diet; C-0·05, diet containing 0·05 % non-oxidised cholesterol; C-0·1, diet containing 0·1 % non-oxidised cholesterol; OxC-0·05, diet containing 0·05 % oxidised cholesterol mixture; OxC-0·1, diet containing 0·1 % oxidised cholesterol mixture. Values are means (n 10), with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05).
Aortic relaxation
The effect of dietary oxidised cholesterol on aortic function was examined by measuring aortic contraction–relaxation ability. The concentration–response curve of phenylephrine–acetylcholine was constructed (Fig. 3). Compared with the control group, both the non-oxidised and oxidised cholesterol groups showed greater inhibition on acetylcholine-induced relaxation. In contrast to the non-oxidised cholesterol groups, only the OxC-0·1 group had inhibition on acetylcholine-induced relaxation significantly greater than the C-0·1 hamsters (Fig. 3).
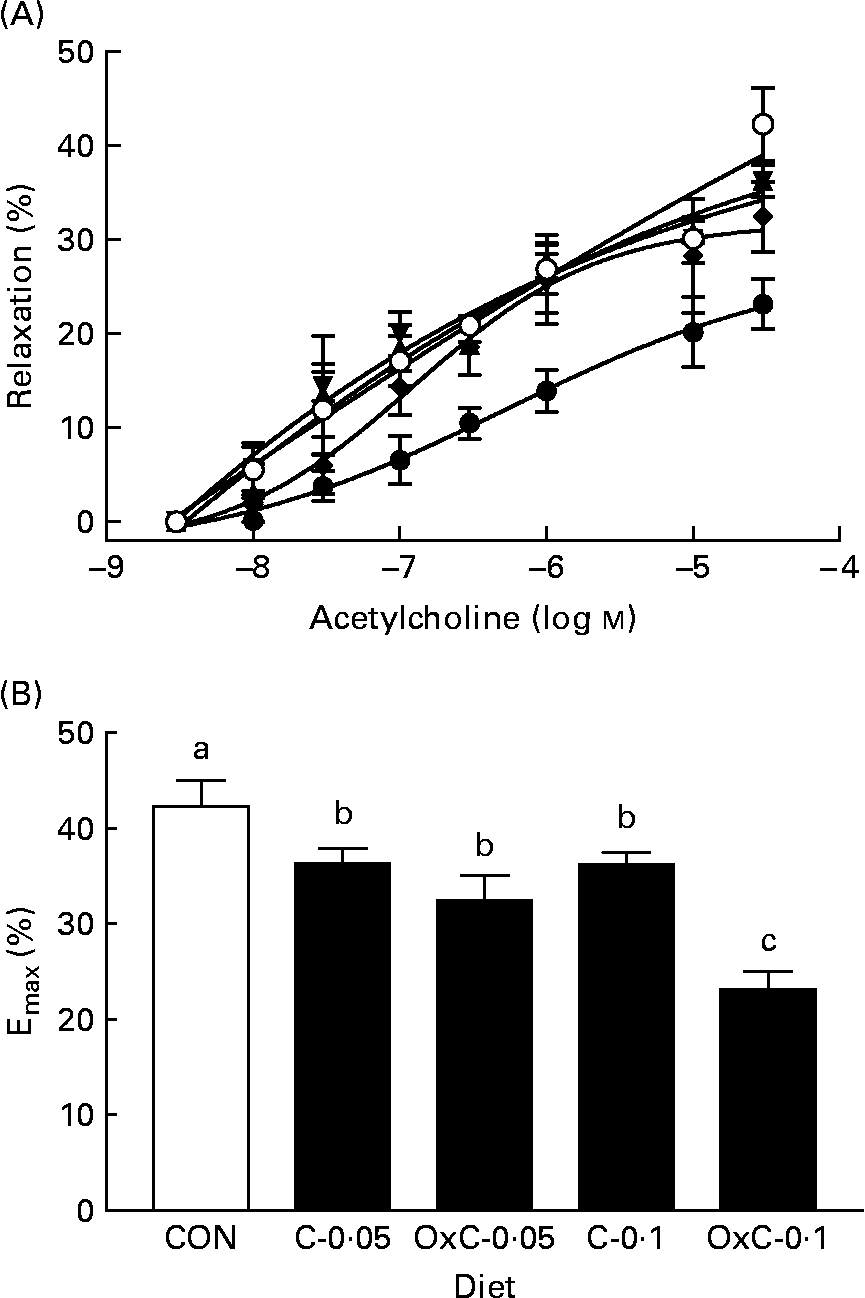
Fig. 3 Effect of dietary oxidised cholesterol on acetylcholine-induced relaxation (A) and maximum relaxation (B) in aorta rings. (○), Control diet; (▲), diet containing 0·05 % non-oxidised cholesterol; (▾), diet containing 0·1 % non-oxidised cholesterol; (♦), diet containing 0·05 % oxidised cholesterol mixture; (●), diet containing 0·1 % oxidised cholesterol mixture. Values are means (n 10), with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters are significantly different (P < 0·05).
Faecal neutral and acidic sterols
Addition of both non-oxidised and oxidised cholesterol into the diet caused greater faecal excretion of both neutral and acidic sterols compared with the control diet (Table 3). However, no difference in faecal excretion of both neutral and acidic sterols was seen between the non-oxidised and oxidised cholesterol groups.
Table 3 Faecal excretion of neutral and acidic sterols (mg/hamster per d) in hamsters fed the control (CON) and the experimental diets containing 0·05 % non-oxidised cholesterol (C-0·05), 0·10 % non-oxidised cholesterol (C-0·1), 0·05 % oxidised cholesterol (OxC-0·05) and 0·10 % oxidised cholesterol (OxC-0·1) for 6 weeks
(Mean values and standard deviations)
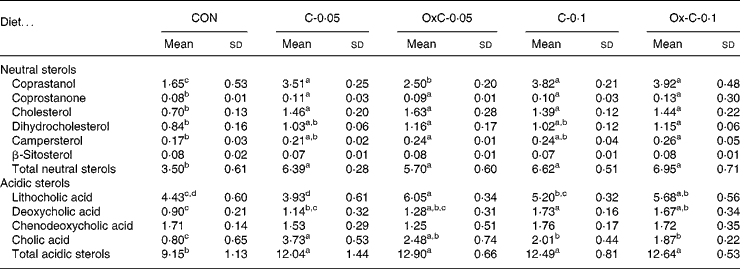
a,b,c,d Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
Discussion
The present results clearly demonstrated that dietary oxidised cholesterol had a greater hypercholesterolaemic activity than cholesterol itself in hamsters. The analysis of lipoprotein profile showed that, accompanied with elevation in serum TC, incorporation of oxidised cholesterol in the diet increased proportionally HDL-C and non-HDL-C levels, thus having no effect on the non-HDL-C:HDL-C ratio, compared with non-oxidised cholesterol in the diet. The Golden Syrian hamster was chosen in the present study because its cholesterol metabolism is similar or close to that in humansReference Sujiyama, Odaka, Itokawa, Ishikawa, Tomari and Ikeda20. The present result was in agreement with that in an earlier report by Podrez et al. Reference Podrez, Kosykh, Lankin, Novikov, Volgushev, Viktorov and Rapin21, who found that the rabbits fed a diet containing 5 % of oxidised cholesterol derivatives had a 5-fold increase in serum cholesterol level compared with non-oxidised cholesterol-fed rabbits. In another study in rats, Al Kanhal et al. Reference Al Kanhal, Ahmad, Al Othman, Al Orf and Al Murshed6 examined the toxicity of oxidised cholesterol and found that the addition of 1 % oxidised cholesterol in the diet could significantly elevate serum TC by 8 % greater than dietary 1 % non-oxidised cholesterol, although the authors did not intend to examine the serum cholesterol-raising activity of oxidised cholesterol v. non-oxidised cholesterol. Quantitatively, we observed that oxidised cholesterol elevated serum TC by 12–25 % greater than the corresponding non-oxidised cholesterol. In this regard, greater hypercholesterolaemic activity associated with oxidised cholesterol in hamsters than in rats can be explained by the observation that rats are hyporesponsive and have greater bile acid excretion capacity than hamsters in response to dietary cholesterol loadingReference Horton, Cuthbert and Spady22.
Measurement of both atherosclerotic plaque and aortic cholesterol provides direct evidence that oxidised cholesterol is more atherogenic. The present result showed clearly that dietary oxidised cholesterol significantly increased atherosclerotic plaque and cholesterol content in the aorta compared with dietary non-oxidised cholesterol (Fig. 2). It had been shown that oxidised cholesterol in the diet could be absorbed in the small intestine and incorporated into chylomicrons, VLDL, HDL and LDL in human subjectsReference Fraley and Tsimilas23, Reference Staprans, Pan, Rapp and Feingold24. Similarly, rabbits fed an oxidised cholesterol diet had an increase in fatty streak lesion in the aortaReference Staprans, Pan, Joseph and Feingold14. Oxidised lipoproteins, particularly oxidised LDL, are atherogenic and play a key role in pathogenesis of CHDReference Fraley and Tsimilas23. However, the origin of oxidised lipoproteins in vivo is not clear but at least part of circulating oxidised LDL originates from the dietReference Staprans, Pan, Rapp and Feingold24, deposits in endothelial cells and initiates atherosclerotic plaqueReference Fraley and Tsimilas23, Reference Staprans, Pan, Rapp and Feingold24. The present results demonstrated that oxidised cholesterol in the diet was not only more hypercholesterolaemic but also more atherogenic compared with cholesterol itself.
No study to date has examined the effect of dietary oxidised cholesterol on functionality of arteries in vivo. The present study found that both non-oxidised and oxidised cholesterols significantly inhibited acetylcholine-induced relaxation of the aorta in hamsters (Fig. 3). However, 0·1 % oxidised cholesterol in the diet was more potent in inhibition than non-oxidised cholesterol, suggesting that dietary oxidised cholesterol could cause greater endothelial damage. Endothelium plays a key role in regulating cardiovascular function. The presence of oxidised lipoproteins is believed to be an early event in the pathogenesis of endothelial dysfunction-associated CVDReference Gutierrez, Ballinger, Darley-Usmar and Landar25, Reference Ryoo, Lemmon, Gupta, White, Nyhan, Shoukas, Romer and Berkowitz26. The present result was in agreement with that of Zhao & TackettReference Zhao and Tackett27 and Chan et al. Reference Chan, Lougheed, Laher and Steinbrecher28, who found that oxidised LDL significantly inhibited acetylcholine-induced relaxation and increased contractile responses to vasconstrictors when blood vessels were incubated with oxidised LDL. Most likely, dietary oxidised cholesterol could incorporate into lipoproteins and produce oxidised LDL, which accumulated on the endothelium to promote atherosclerotic plaque and impair endothelium-dependent vasorelaxationReference Staprans, Pan, Joseph, Grunfeld and Feingold15, Reference Staprans, Pan, Rapp and Feingold24.
Dietary oxidised cholesterol not only increased serum TC but also reduced the liver cholesterol level compared with non-oxidised cholesterol (Table 2). When 0·05 % oxidised cholesterol was added in the diet, no difference was observed in liver cholesterol content between the oxidised cholesterol and non-oxidised cholesterol groups. However, OxC-0·1 hamsters accumulated hepatic cholesterol significantly less than the C-0·1 hamsters. In this regard, it is known that oxidised cholesterol is much more potent than non-oxidised cholesterol in the inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase in human fibroblastsReference Brown and Goldstein29. Individual oxidised cholesterol derivates have been studied with regard to their potency in inhibiting 3-hydroxy-3-methyl-glutaryl-CoA reductase in a large number of mammalian cellsReference Schroepfer30, finding that half-maximal inhibitory concentration (IC50) ranged from 0·1 to 25 μm. In addition, reduction in 3-hydroxy-3-methyl-glutaryl-CoA reductase activity associated with oxidised cholesterol feeding compared with non-oxidised cholesterol was also seen in hensReference Naber, Allred, Winget and Stock31. In the present study, dietary oxidised cholesterol led to the accumulation of COP in the liver (Table 2). On one hand, stronger inhibition on 3-hydroxy-3-methyl-glutaryl-CoA reductase by COP in hamsters fed the OxC-0·1 diet could reduce cholesterogenesis in the liver, leading to lesser cholesterol accumulation in the liver than that in hamsters fed the corresponding non-oxidised cholesterol diet. On the other hand, feeding oxidised cholesterol may not necessarily decrease the secretion of hepatic VLDL and instead it may increase its secretion, as one report demonstrated that VLDL secretion by hepatocytes from oxidised cholesterol-fed rabbits was dramatically increased in comparison with those from non-oxidised cholesterol-fed rabbitsReference Podrez, Kosykh, Lankin, Novikov, Volgushev, Viktorov and Rapin21.
Greater potency in elevating serum TC associated with the oxidised cholesterol feeding compared with non-oxidised cholesterol in the diet suggested a shift in balance among dietary intake, synthesis and catabolism. On one hand, the removal of LDL-cholesterol is mainly mediated by LDL receptors in the liver. Although the present study did not provide direct evidence that dietary oxidised cholesterol inhibited LDL receptors, several studies on cell lines have demonstrated that most COP caused suppression on levels of 125I-labelled LDL binding in human fibroblastsReference Brown and Goldstein29, mouse teratocarcinoma cellsReference Goldstein, Brown, Krieger, Anderson and Mintz32 and human epithelioid carcinoma cellsReference Schneider, Basu, McPhaul, Golstein and Brown33. Inhibition on LDL receptor activity was reported to be associated with a decrease in mRNA level of LDL receptors in HepG2 cellsReference Srivastava, Ito, Hess, Srivastava and Schonfeld34. It was most likely that dietary oxidised cholesterol caused a relative decrease in LDL receptor activity, led to an inefficient removal of LDL-cholesterol from the circulation and serum TC was thus raisedReference Brown and Goldstein29. On the other hand, although it is speculative, dietary COP may increase plasma oxysterols, induce up regulation of scavenger B receptor 36 in monocytes/macrophages, result in an increased uptake of modified LDL and thus lead to an ensuing increase in both vascular cholesterol and COPReference Schroepfer30, Reference Nicholson, Han, Febbraio, Silversterin and Hajjar35. In fact, the present study found that oxidised cholesterol feeding led to greater accumulation of cholesterol and atherosclerosis plaque in the aorta (Fig. 2).
The current recommendation for daily cholesterol intake is less than 300 mg, and in some countries the food label must present the amount of cholesterol in foods because cholesterol increases plasma TC and is associated with the risk of CHD. Of significance, if the data in the present study can be applied to humans, with human diets containing not only cholesterol but also oxidised cholesterol, the present study emphasises that the oxidised cholesterol is much more hypercholesterolaemic.
Acknowledgements
We thank the Hong Kong Research Grant Council for supporting this research (project no. CUHK 4586/06M).








