Malformations of the central nervous system (CNS), some of the most prevalent congenital anomalies, represent an important source of morbidity and mortality in children(Reference Wallingford, Niswander and Shaw1). In low-income countries, an estimated 29 % of neonatal deaths related to visible congenital abnormalities are attributable to neural tube defects (NTD), which are also associated with lifelong disability(Reference Blencowe, Cousens and Modell2). Besides NTD, congenital hydrocephalus (CH) is another common major birth defect of the CNS, and 60 % of CH is complicated with NTD(Reference Liu, Jin and Li3). CH, which is characterised by an imbalance in cerebrospinal fluid secretion/absorption that results in the accumulation of fluid in the brain with consequential pathophysiology(Reference Jimenez, Naz and Miyan4,Reference Kahle, Kulkarni and Limbrick5) , is an important cause of neurological morbidity and mortality in children(Reference Jeng, Gupta and Wrensch6). The global prevalence of CH has remained stable over the past decade at 0·81 per 1000 births(Reference Isaacs, Riva-Cambrin and Yavin7).
Periconceptional supplementation with folic acid (FA) among women of childbearing age can prevent NTD(Reference Berry, Li and Erickson8), although the preventive effects on CH have been less studied. Animal studies have revealed that a deficiency in 10-formyl-tetrahydrofolate-dehydrogenase, a folate binding protein, in the nucleus of the brain and liver is linked to decreased DNA methylation, which could be a key factor in the developmental deficits associated with congenital and neonatal hydrocephalus(Reference Jimenez, Naz and Miyan4,Reference Naz, Jimenez and Sanjuan-Vilaplana9) . Although the causes and mechanisms underlying the development of CH are not the same as those involved in NTD, folate and vitamin B12 have fundamental roles in the functioning of the CNS and in the prevention of disorders that affect its development(Reference Reynolds10).
Most CH is accompanied by NTD, and thus CH with non-NTD has been less studied. There are few reports of CH malformation according to maternal characteristics. Using a large-scale population-based surveillance study, we explored the epidemiology of CH and analysed preventive effects of FA supplementation on CH in China.
Methods
Methods of the original study have been described previously(Reference Berry, Li and Erickson8,Reference Liu, Li and Ye11) . Briefly, the Chinese Ministry of Health conducted a public health campaign to prevent NTD in twenty-one counties in southern China (located in Zhejiang and Jiangsu province) and northern China (located in Hebei province) in the early 1990s. A well-organised pregnancy monitoring system (PMS) was established to collect principal records on prenatal care and delivery. Data on the demographic variables and most of the covariates came from this system, with local maternal clinicians collecting the information. All women in the project counties who were preparing for marriage or who became pregnant were registered. The enrolled women were advised to take a pill containing 400 µg FA every day, starting at the time they registered with the PMS and continuing until the completion of their first trimester of pregnancy. Details on FA supplementation were recorded by local health care workers(Reference Berry, Li and Erickson8,Reference Liu, Li and Ye11) . Detailed forms were used to record the number of tablets women took and the dates of all menstrual periods. Mothers’ clinicians distributed bottles of the tablets, supervised the participants, retrieved the bottles and counted the remaining tablets every month. All births at 20 complete gestational weeks, including live births, stillbirths and pregnancy terminations, and all structural congenital anomalies regardless of gestational week were recorded(Reference Li, Moore and Li12). To be included in the study, women had to be residents of the county, be registered with the PMS between October 1993 and September 1995 and deliver with a known outcome by 31 December 31 1996. Women who did not live in the county, did not give birth in the county or did not provide oral consent were excluded.
The project was approved by the institutional review boards of the US Centers for Disease Control and Prevention and Peking University Health Science Center. All women who took pills provided oral informed consent.
Folic acid use: definition and compliance
The classification and patterns of FA consumption were the same as previously reported(Reference Berry, Li and Erickson8). Women who took FA pills at any time from the registration period until the end of the first trimester of pregnancy were classified as FA users. Women who did not agree to take FA or who were registered during the second trimester of pregnancy (i.e., did not have the opportunity to start taking FA by the end of the first trimester) were considered non-users. Three patterns of FA exposure were determined according to when women started and stopped taking FA: (1) periconceptional: initiation before the last menstrual period (LMP) and termination within the first trimester, (2) preconceptional: initiation and termination before the LMP and (3) postconceptional: initiation after the LMP and termination within the first trimester. Compliance was calculated as the number of pills actually consumed divided by the number of pills assigned. A subgroup of women who took more than 80 % of their assigned pills was defined as the high-compliance group(Reference Berry, Li and Erickson8).
Identification of congenital hydrocephalus
Congenital anomalies of the CNS were identified through a birth defects surveillance system(Reference Berry, Li and Erickson8,Reference Li, Moore and Li12) . Live-born infants with birth defects were included in the surveillance system if they had a gestational age of at least 20 weeks. Information on all pregnancies, even those < 20 weeks’ gestation that were electively terminated after a prenatal diagnosis of any birth defect, was also collected. Details of diagnoses were described previously; briefly, diagnoses of suspected defects were made based on photographs of affected fetuses and infants taken at birth or termination of pregnancy and on reviews of reports by several clinicians(Reference Liu, Li and Ye11). Diagnoses of birth defects were made according to the International Classification of Diseases, Ninth Revision, Clinical Modification. CH was diagnosed in accordance with code 742.3, while non-NTD CH refers to CH without anencephaly (740), spina bifida (741) or encephalocele (742·0)(Reference Liu, Jin and Li3).
Statistical analysis
The main effect of FA supplementation (exposure) on CH was estimated. Because our previous study revealed that only 40 % of CH was non-NTD CH(Reference Liu, Jin and Li3), to comprehensively reflect CH status we included both CH and non-NTD CH in our study. As there were no reports in the literature on relative risk, we used the preventive effect of FA on NTD (i.e., the OR of the exposure group relative to the control group), 0·8(Reference Berry, Li and Erickson8), to estimate the incidence of CH in the control group at 0·81 per 1000 births(Reference Isaacs, Riva-Cambrin and Yavin7). Assuming α = 0·05 (bilateral) and β = 0·10, according to case: control = 1:2, significant difference between two groups was 0·2 %, the sample sizes of the exposure group would be 68 766 and sample size in control group was 137 532. A total of 247 831 participants in our study could meet the statistical requirements. The sample size was calculated with PASS (version 20.0.3; NCSS).
We compared means using t tests and distributions using χ 2 tests. We calculated the rate of CH (i.e., the number of cases per 1000 pregnancies of at least 20 weeks’ gestation) according to patterns of FA intake. We estimated risk ratios by dividing the risk for CH among the fetuses or infants of all women who took FA by the risk among the fetuses or infants of women who did not take FA. We also estimated risk ratios by dividing the risk among the fetuses or infants of women with periconceptional/preconceptional/postconceptional FA by the risk among the fetuses or infants of women who did not take FA. In logistic regression, the dependent variable was CH and the independent variable was FA supplementation; the main potentially confounding variables adjusted for in logistic regression included maternal age at delivery, ethnicity, education, occupation, parity and region. We ran the logistic regression separately for CH and non-NTD CH. Missing data for continuous variables (age and BMI) were replaced by the mean of the corresponding variable. Missing data for categorical variables (parity, ethnicity, education and occupation) were replaced by the most common value. Model assumptions and goodness of fit were evaluated with the omnibus test (–2 log likelihood) and Hosmer and Lemeshow test. All data were analysed with SPSS (version 24.0; IBM).
Results
Among the 247 831 participants, the mean age was 25·4 (sd 3·2) years; 85 % of participants were younger than 30 years old. A total of 12·9 % of subjects were from the north and 87·1 % were from the south. Among both CH and non-NTD CH groups, 62 % of mothers were farmers, 55–56 % of mothers had finished junior high school and there were more male than female infants (Table 1). A total of 52·5 % of women took FA during the periconceptional period. The proportion of FA supplementation was higher in the north (58·2 %) than the south (51·7 %). Women who took FA supplements were more likely to be primiparous and to have higher education. A full description of the subjects is available elsewhere(Reference Liu, Li and Ye11).
Table 1 Description of CH by maternal characteristics in China, 1993–1996
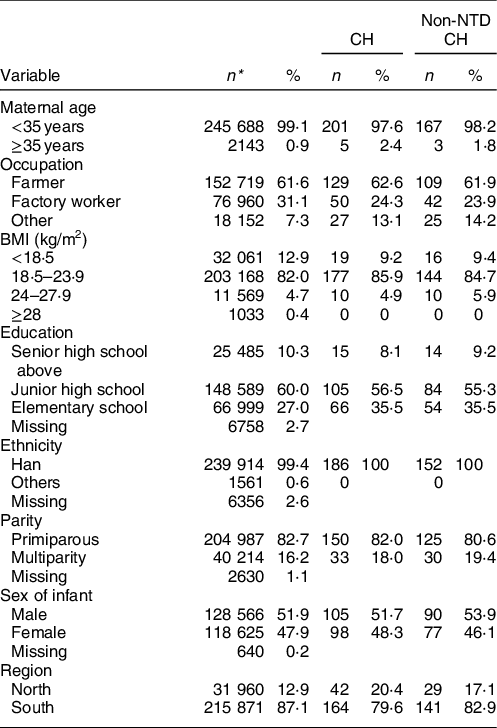
CH, congenital hydrocephalus.
* Due to missing data for variables, sum of values was not equal the total numbers.
A total of 206 cases of CH and 170 cases of non-NTD CH were recorded in the study. The prevalence of CH was significantly higher in the north (1·31 per 1000 births) and among women older than 35 years (2·33 per 1000 births) than in the south and among younger women (0·76 and 0·82 per 1000 births, respectively). Differences in the prevalence of non-NTD CH among these groups did not reach statistical significance (Table 2).
Table 2 Prevalence of CH in China, 1993–1996, n (per 1000 births)*
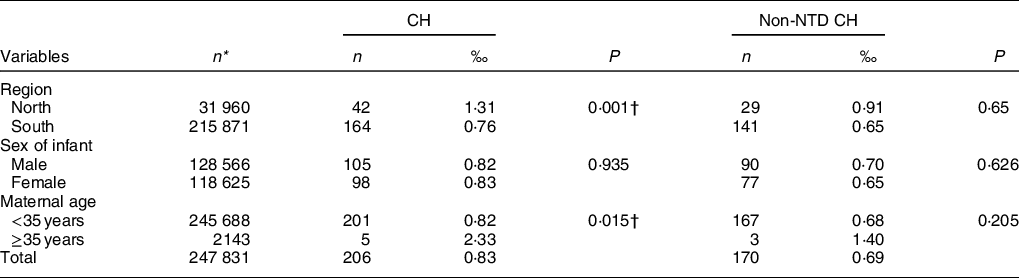
CH, congenital hydrocephalus.
* Due to missing data for variables, sum of values was not equal the total numbers.
† P < 0·05, comparison between regions or maternal age groups.
The prevalence of CH was lower among women who took FA tablets than those who did not, although the difference was not significant. The prevalence of both CH (0·54 per 1000 births) and non-NTD CH (0·38 per 1000 births) was significantly lower in the periconceptional FA supplementation group than among non-users (1·72 and 1·12 per 1000 births for CH and non-NTD CH, respectively; Table 3). No significant difference for this effect was observed in the south. The data indicated that women in the high-compliance (≥ 80 %) FA supplementation group had a lower prevalence of CH compared with women in the low-compliance (< 80 %) group and non-users, although the difference was not statistically significant. Our study size had 99·9 % power (α = 0·05) to detect a decrease of 10 % over the unexposed rate of 0·81 per 1000 for CH.
Table 3 Prevalence of CH by folic acid supplementation and compliance in China, 1993–1996, n (per 1000 births)
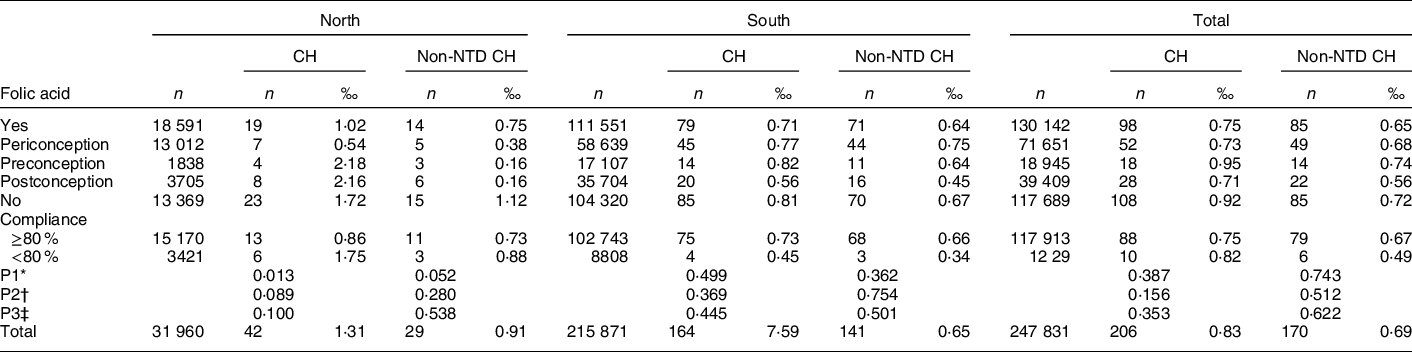
CH, congenital hydrocephalus.
* P1: comparison among periconceptional, preconceptional, postconceptional FA supplement and no FA supplement.
† P2: comparison between FA supplement and non-FA supplement.
‡ P3: comparison between compliance ≥80 %, <80 % and non-FA supplement.
Results for model assumptions revealed that the model had good fit, with both the –2 log likelihood and Hosmer and Lemeshow tests showing P > 0·05 (online supplementary material, Supplemental Table 1). No overall preventive effect of FA on CH was observed in China. When we analysed further by region, we found that FA supplementation during the periconceptional period significantly prevented CH (OR = 0·29, 95 % CI 0·12, 0·69) and non-NTD CH (OR = 0·34, 95 % CI 0·12, 0·97) in the north. Further analyses showed that higher compliance with FA supplementation (≥ 80 %) significantly reduced the risk for CH (OR = 0·25, 95 % CI 0·09, 0·66) and non-NTD CH (OR = 0·33, 95 % CI 0·10, 1·03) in the north (Table 4).
Table 4 RR and 95 % CI for CH by folic acid supplementation and compliance in China, 1993–1996
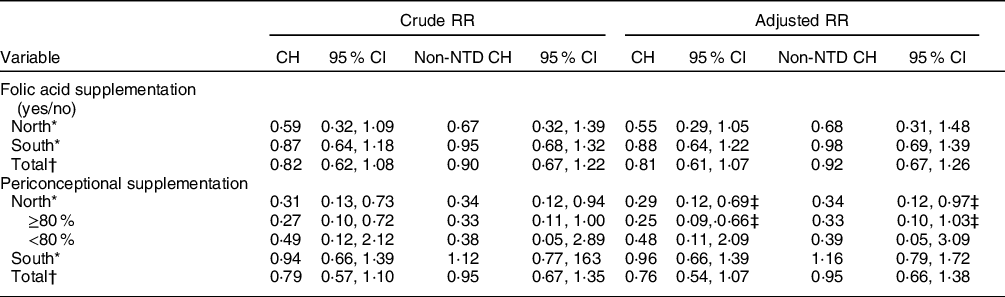
RR, risk ratio; CH, congenital hydrocephalus.
* Adjusted for age at pregnancy, BMI, parity, ethnicity, education, occupation.
† Adjusted for age at pregnancy, BMI, parity, ethnicity, education, occupation, and region.
‡ Refers to model 1–4, with good fit (see Model assumption details in online supplementary material, Supplemental Table 1).
Discussion
Although there is a consensus that periconceptional FA supplementation can reduce NTD, few studies have explored its effects on CH, one of the most common congenital CNS abnormalities besides NTD. Our study provides new evidence that periconceptional FA supplementation can prevent CH in northern China, where there is a high prevalence of NTD.
Using data from a large population-based surveillance system and rigorous diagnosis of birth defects, we reported the epidemiology of CH and non-NTD CH. CH was the most common CNS abnormality after NTD. In our study, NTD accounted for 58 % (275/474) of all CNS abnormalities, followed by CH (36 %, 170/474). Prevalence rates in the current study were 0·83 and 0·69 per 1000 live births for CH and non-NTD CH, which is close to the global average (0·81 per 1000 live births)(Reference Isaacs, Riva-Cambrin and Yavin7), but higher than in European countries (0·47 per 1000 live births)(Reference Garne, Loane and Addor13) and the USA/Canada (0·68 per 1000 live births)(Reference Jeng, Gupta and Wrensch6) while it is lower than Africa (1·45 per 1000 live births) and Latin America (3·16 per 1000 live births)(Reference Dewan, Rattani and Mekary14). These prevalence rates are also higher than reports of around 0·7 per 1000 births in China for 1996–2004(Reference Dai, Zhou and Miao15), 0·62 per 1000 births in 2005 and 0·42 per 1000 births for isolated CH in 2012(Reference Yi, Wan and Deng16). The latter data are from a hospital-based birth defect surveillance system that only included births of 28 weeks’ gestation or greater, whereas our study included all births at 20 complete gestational weeks and all structural abnormalities regardless of gestational week. Population-based surveillance systems that include large numbers of pregnant women can provide more complete data on outcomes(Reference Berry, Li and Erickson8) and give more accurate estimations of the epidemiology of CH. As the primary aim of this large cohort study was to identify NTD, abnormality of CNS was specially checked and recorded, non-NTD CH was recorded accordingly. The preventive effect of FA on NTD is well known, whereas the effect of CH is less frequently reported. Thus, our study adds important evidence on this topic, especially non-NTD CH was analysed separately and explored.
The prevalence of CH was significantly higher in the north than in the south: 1·31 compared with 0·76 per 1000 births, respectively. This is in line with the well-documented difference in rates of NTD between the two regions, which could be due in part to differences in dietary FA intake(Reference Berry, Li and Erickson8). As the original study did not collect information on diet, and it is impossible to examine individual blood sample in a large-scale population study, folate concentrations were examined in a cross-sectional survey in 2003 of women in their first trimester of pregnancy from one county and one city in both the north and south. Results showed that women in the north had less than half the folate concentration of women in the south (440·0 v. 910·4 mmol/l, respectively)(Reference Ren, Zhang and Hao17).
Periconceptional FA supplementation can prevent CH in northern China. Starting to supplement before the LMP and continuing until the full first trimester was key; early discontinuation and late onset did not have preventive effects on CH. As most CH was followed by NTD, periconceptional FA supplementation would prevent NTD and the subsequent CH. Our study provides new evidence that non-NTD CH is also preventable with periconceptional FA supplementation. As FA supplementation has a strong impact on plasma folate concentrations, massive FA supplementation in northern China has significantly improved folate concentrations and reduced CH over time(Reference Liu, Jin and Li3). The cause as well as the heterogeneity of its mechanisms needs to be explored further. Earlier supplementation (before the LMP), increased supplementation frequency and more total days of supplementation are associated with increasing folate concentrations(Reference Liu, Gao and Zhang18). The current nationwide programme should ensure that supplementation begins before pregnancy and continues for a long enough time.
The causes of and mechanisms underlying the development of CH are not well understood. Genetic mutation(Reference Hu, Wang and Liu19), folate imbalance(Reference Cains, Shepherd and Nabiuni20) and abnormal folate metabolism(Reference Naz, Jimenez and Sanjuan-Vilaplana9) have all been reported as risk factors for CH. An animal study revealed that FA deficiency induces antenatal hydrocephalus in rat(Reference Stempak21). In previous studies, FA supplementation was estimated through administrative data while lack of individual FA supplementation data in one study(Reference Jeng, Gupta and Wrensch6) and another study revealed that folate fortification status was not associated with the incidence of hydrocephalus(Reference Isaacs, Riva-Cambrin and Yavin7). Our previous study, which relied on indirect evidence of birth defects and massive FA policy, revealed that FA supplementation was correlated with decreasing CH(Reference Liu, Jin and Li3). Using strict surveillance data on FA supplementation from a large population, the study provided direct, first-hand evidence of the preventive effect of FA on CH as well as non-NTD CH among women with folate insufficiency.
The present study has several strengths. First, it was based on a well-organised, population-based PMS that used quality control measures to ensure the collection of high-quality data on subjects and the diagnosis of birth defects. Birth defects were recorded regardless of gestational week, a more accurate process than hospital-based surveillance (which only covers ≥28 weeks). A previous study revealed that pre-perinatal prevalence (< 28 gestational weeks) was higher than perinatal prevalence (> 28 gestational weeks) for both non-NTD and total CH(Reference Liu, Jin and Li3), which reflects an underreporting in hospital-based surveillance. Second, in our study, reliable data on FA supplementation were documented prospectively before the outcome of the pregnancy was known(Reference Berry, Li and Erickson8). All FA supplements were supplied by the community intervention project; there were no over-the-counter FA supplements in China at that time. Detailed information on FA supplementation was recorded prospectively. If women consented to take FA, the pills were distributed at the time of registration. At the end of each month, health care workers recorded the dates of all menstrual periods and how many pills remained in each bottle. Supplementation started at the time of registration with the PMS and stopped at the end of the first trimester. FA supplementation was ultimately confirmed by local health care workers, who checked the number of pills taken at monthly home visits. Third, FA supplementation (400 µg/d) was the only intervention in the study. There was no market supply of FA supplements or multivitamins that included FA in the early 1990s in the study area, and there was no massive FA supplementation or food fortification among women of childbearing age in China(Reference Li, Ye and Zhang22). Our study thus provides evidence of the effect of FA only on CH among the largest population studied to date.
Limitations of the study should also be noted. First, we did not collect information on the dietary folate intake of the women, which could have influenced the results. However, the study population had low folate levels, and there was no other FA fortification besides the current intervention. Second, we did not explore the effects of other potential confounding factors associated with FA use. For example, the women were not randomly assigned to the FA supplementation or no supplementation groups(Reference Berry, Li and Erickson8), and systematic differences between the two groups may have influenced FA use. Women who did not take FA were slightly older than those who did and were more likely to have been pregnant before, while further stratified analysis according to age and the number of previous pregnancies did not influence the result. However, multivariate analyses adjusted by age and parity did not reveal different results. Other lifestyle factors such as smoking or alcohol consumption, husband characteristics may be considered in future study. Third, the supplementation period in the current study was from the time of registration in the PMS to the end of the first trimester of pregnancy, so we could only observe the potential effect of FA supplementation in the first trimester. Whether continued supplementation in the second or third trimesters would have preventive effects on CH requires further study.
Despite these limitations, our large sample size and use of population-based data as well as the individual data on FA supplementation reveal a correlation between periconceptional FA supplementation (until the first trimester) and CH. In conclusion, periconceptional FA supplementation does not significantly prevent CH overall. However, in northern China of common maternal folate insufficiency, there is some evidence. Further study of the causes and underlying mechanisms of CH is needed to develop preventive measures.
Acknowledgements
Acknowledgements: The authors thank the staff members and participating women of the original trial. The authors also thank the anonymous reviewers for helpful comments. Financial support: This work was supported in part by the National Key Research and Development Program, Ministry of Science and Technology of the People’s Republic of China (grant No. 2016YFC1000501) and Natural Science Foundation of China (No. 81373014 and No. 81202265). The original project was supported by a cooperative agreement between the US Centers for Disease Control and Prevention and Peking University (grant no. U01 DD000293). Conflict of interest: None. Authorship: J.F.L. conceptualised the study, analysed the data, drafted the initial manuscript, and reviewed and revised the manuscript. Z.L. and R.Y. conceptualised and designed the study, and critically revised the manuscript; A.R., R.Y. and J.M.L. coordinated and supervised data collection, reviewed and revised the manuscript. All authors read, reviewed and approved the final manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the institutional review boards of the US Centers for Disease Control and Prevention and Peking University Health Science Center. Verbal informed consent was obtained from all subjects. Verbal consent was witnessed and formally recorded.






