Introduction
Rabies is a fatal zoonotic viral disease; it is a threat to half the world's population and kills >59 000 people each year, most of them children [Reference Hampson1, Reference Hampson2]. Globally, >29 million people annually undergo post-exposure prophylaxis (PEP) for rabies [3]. Biting or scratching wounds, and licking of broken skin and mucous membranes are the common modes of virus transmission from rabid animal saliva to humans and other animals [4].
The rabies virus is believed to be capable of infecting all mammals, although species susceptibility appears to vary. In humans, it is transmitted mostly by canines, cats, mongoose and bats and infrequently by farm animals. Once symptomatic, death is almost unavoidable [4, Reference Chernet and Nejash5]. In addition to fatality in humans, globally an unknown number of domestic ruminants and wildlife also die due to rabies; most of the deaths occur in Asia and Africa [Reference Taylor and Nel6]. Domestic dogs cause >99% of all human cases [4]. In humans, the disease is fully preventable through wound management, prompt administration of PEP and inoculation of rabies immunoglobulin to bite victims [Reference Tao7].
Rabies can be controlled through mass vaccination of domestic dogs [Reference Hampson1, Reference Moges8]. PEP is costly and often not available in rural areas where the disease is more prevalent. In contrast, dog vaccination is relatively more cost-effective and a feasible method to decrease the incidence of human and domestic animal rabies. Considering this, the World Health Organization (WHO) and partners have adopted a goal to eliminate dog-mediated human rabies by 2030 via the control of the disease in dogs [4].
Bangladesh ranks third globally after India and China for rabies mortalities: about 2100 people die per year [Reference Gongal and Wright9, Reference Hossain10]. Children and rural people in Bangladesh are most frequently affected by rabies [Reference Gongal and Wright9, Reference Hossain11]. Dog (90%), cat (6%), jackal (3%) and mongoose (1%) were reported to be responsible for human bites in Bangladesh [Reference Hossain11]. The national rabies control plan aims to reduce the number of human rabies deaths by 50% by 2015 and eradicate it by 2020 through implementing mass dog vaccination and providing free-of-cost PEP in every district [12]. Despite a declining trend of human rabies was reported, the eradication goal has not yet been achieved [Reference Sumon13]. There are about 24.2 million cattle, 1.4 million buffalo, 26.3 million goats and 3.5 million sheep in Bangladesh [14]. About 85% of the national cattle populations are non-descript indigenous and 15% are crossbred [Reference Hamid15]. Holstein Friesian cross cattle - recognised by their morphology and colour (black and white without any hump or horn)- dominate the crossbred population in Bangladesh. The economic loss incurred by rabies in domestic ruminants has not yet been estimated in Bangladesh. However, 14 085 dog bite cases and 3425 domestic ruminant deaths (cattle: 2845; goats: 547; sheep: 13) were reported due to rabies between 2010 and 2012 in Bangladesh [Reference Mondal and Yamage16]. The number of rabid animal bite (RAB) cases in domestic ruminants can be used as a proxy for human cases within a local area [Reference Tenzin17]. Bangladesh Agricultural University Veterinary Teaching Hospital (BAUVTH) − located in Mymensingh district − records and provides PEP to the RAB cases in domestic ruminants. Retrospective analysis of hospital-presented RAB cases in domestic ruminants allows the description of reservoir animal species, identification of demographic and temporal risk factors and assessment of the impact of the national rabies control strategy in the study area. Since this information has not yet been reported, our aims were to describe reservoir animals, demographic and temporal risk factors for RABs in domestic ruminants.
Materials and methods
Data
The RABs case and control data were collected from Bangladesh Agricultural University Veterinary Teaching Hospital (BAUVTH) clinical records. All cases of RAB in domestic ruminants attended at BAUVTH from January 2009 to December 2018 were collected. For each case of RAB, three control records of domestic ruminants were randomly selected. The list of all domestic ruminant cases attending for all other reasons during the 10-year study period (sampling frame) was entered into a spreadsheet (Microsoft Excel 2010). A random number was generated for each control record using the ‘rand’ function of Excel. Cases and controls were not matched and the control records were randomly selected from the sampling frame. The data on RAB cases and controls included the patient number, owner's address, date of examination, farm location name, demographic data, clinical signs, presumptive diagnosis and treatment given. If the address of an owner is within a municipality then it was considered to be an urban area, otherwise rural.
Case and control definitions
RAB cases recorded at BAUVTH were diagnosed based on the history of a rabid animal (abnormal demeanour, biting of multiple animals and humans) bite, biting wound and other signs of rabies (salivation, excitement or dullness). When dead suspect rabid dogs were brought to BAUVTH and domestic ruminants died with suspect rabies at BAUVTH, a diagnosis of rabies was confirmed by histopathology. Briefly, brain stem and cerebellum were collected from the brain of dead animals and preserved in 10% formalin. The fixed tissue samples were processed by a routine paraffin embedding technique. Tissue sections of 4 − 5 μm were stained using Harris Haematoxylin and Eosin method [Reference Bancroft, Layton, Suvarna, Layton and Bancroft18]. The slides were screened for the presence of Negri bodies by microscopy. Controls were defined as those records – excluding those diagnosed as RAB-attended at BAUVTH − during the study period. Control records in which any clinical signs suggestive of rabies were noted were excluded from the study. Controls were selected randomly, irrespective of time or location, because we were interested in exploring time and place as risk factors. We used three controls per case because a ratio of more than three controls per case adds little to the precision of a study [Reference Koop19].
Data analysis
Collected data were cross-checked for missing information or errors and corrected by checking the original case records stored at BAUVTH. Age was converted to a categorical variable. Months of presentation were converted to season. The geographic coordinates of each location (approximate village name) of cases and controls were identified via Google Earth. Some locations had multiple cases and controls which were difficult to visualise. So, only one case and one control were used to visualise those locations. A map of case and control locations was prepared using ArcGIS 10.7.1 (Environmental System Research Institute, USA) and a shapefile of Mymensingh district in Bangladesh (WGS 84). The RAB case–control data were summarised by using the ‘tabpct’ function of the R package ‘epicalc’ [Reference Chongsuvivatwong20]. Summary statistics calculated to include the yearly distribution of rabies cases, and the frequency and distribution of RAB cases and controls for different categories of the explanatory variables. We used Mann–Kendall trend test using the ‘MannKendall’ function of the R package ‘Kendall’ to identify the trend of RAB cases over the period of 2009–2018 [Reference McLeod21].
Univariable mixed-effect logistic regression analyses
First, a univariable mixed-effect logistic regression analysis was performed by including location (geographic coordinates) of cases and controls as random intercept (R package ‘lme4’ [Reference Bates22]. The model used RAB status as the response and demographic, spatial and temporal factors as explanatory variables. Any explanatory variable associated with rabies case status with a P-value of ⩽0.10 was selected for multiple mixed-effect logistic regression analysis. Collinearity among explanatory categorical variables was assessed by calculating a Cramer's π-prime statistic (R package ‘vcd’, ‘assocstats’ function). A pair of categorical variables was considered collinear if Cramer's π-prime statistic was >0.70 [Reference Meyer23].
Multivariable mixed-effect logistic regression analyses
A manual forward mixed-effect multiple logistic regression analysis was performed to identify risk factors for RAB. The best univariate model was selected based on the lowest Akaike's information criterion (AIC) value. Then the remaining variables were added in turn, based on AIC. The final model selected had the lowest AIC. Confounding was checked by observing the change in the estimated coefficients of the variables that remained in the final model by adding a non-selected variable to the model. If the inclusion of this new variable led to a change of >25% of any parameter estimate, that variable was considered to be a confounder and retained in the model [Reference Dohoo, Martin and Stryhn24]. The two-way interactions of all variables remaining in the final model were assessed for significance based on AIC values [Reference Dohoo, Martin and Stryhn24]. The intra-class correlation (ICC) statistic was estimated using the formula$ICC_{Location} = \partial _{Location}^2 /\lpar {\partial_{Location}^2 + \pi^2/3} \rpar$![]() . The 95% confidence interval (CI) of the ICC was bootstrapped using the ‘bootMer’ function of the R package ‘lme4’ [Reference Bates22]. All of the above analyses were performed in R 3.6.0 [Reference Team25].
. The 95% confidence interval (CI) of the ICC was bootstrapped using the ‘bootMer’ function of the R package ‘lme4’ [Reference Bates22]. All of the above analyses were performed in R 3.6.0 [Reference Team25].
Results
Out of 457 RAB cases, recorded during a 10 years period, only eight were goat cases and others were cattle cases. Due to the small number of goat cases, they were excluded from the risk factor analysis. During the study period, 18 rabid dogs and 10 cattle brain samples were examined by histopathology. Sixteen rabid dogs and 8 cattle were confirmed as rabies by observing Negri bodies in brain tissue sections. Figure 1 shows the location of RAB cases and controls in Mymensingh district. Dogs (87.8%) and jackals (12.2%) were most often identified as biting animals. Figure 2 shows the annual number of RAB cases, which ranged from 27 (2009) to 64 (2011). An increasing trend (τ = 0.02) in RAB cases was identified over the period 2009–2018, but this was not statistically significant (P = 1). The monthly distribution of RAB cases (Table 1) shows the highest proportion of cases to be in January (40.4%) and the lowest in September (16.6%).
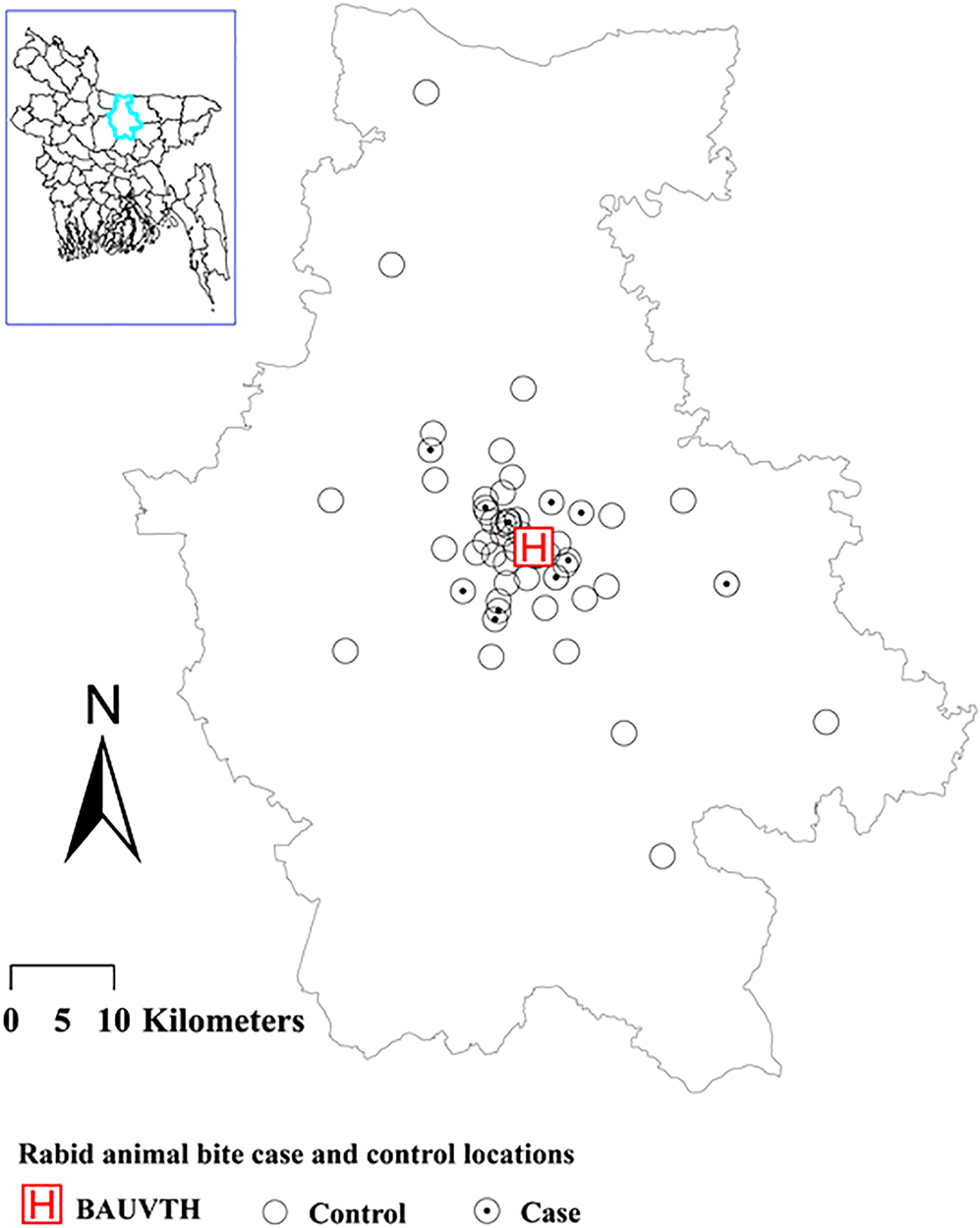
Fig. 1. Map of Mymensingh district showing the RAB cases and control locations.

Fig. 2. Yearly distribution of rabies cases attended to BAUVTH between January 2009 and December 2018.
Table 1. Monthly distribution of RAB cases and controls presented at BAUVTH between January 2009 and December 2018

Bold face indicates highest and lowest values.
Based on the results of univariable logistic regression analyses, the proportions of RAB cases were found to be significantly higher in crossbred animals (4.89 times; P < 0.001 than indigenous; in older animals (>6 months old) (2.77–6.04 times; P < 0.001) than young (up to 6 months); in female animals (1.79 times; P < 0.001) than males; in rural areas (41.34 times; P < 0.001) than urban; and in pre-monsoon (1.81 times; P < 0.001) and winter (1.89 times; P < 0.001) seasons compared to post-monsoon (Table 2).
Table 2. Contingency tables and univariable logistic regression analyses conducted to evaluate the association between explanatory variables and RAB case/control status based on records obtained from BAUVTH between January 2009 and December 2018

%Cases among each category of the risk factor.
No confounding variable was found. All two-way interactions of the variables evaluated in the final mixed-effect model were not statistically significant, so were not included in the final model.
The variance component of the mixed-effect logistic regression for a location as random intercept was estimated to be 0.25 and the ICC coefficient was 0.89 (95% CI: 0.80–0.96). The odds of RAB cases were significantly higher in cattle aged >0.5–2 years (odds ratio (OR) = 2.89; 95% CI: 1.56–5.37), >2–5 years (OR = 3.63; 95% CI: 1.97–6.67) and >5 years (OR = 6.42; 95% CI: 3.39–12.17) compared to those aged <0.5 years.
Crossbred cattle were found to be at higher risk of being bitten by rabid animals (OR = 5.48; 95% CI: 3.56–8.42) compared to indigenous. Similarly, female cattle were more at risk of being bitten by rabid animals (OR = 1.62; 95% CI: 1.15–2.29) than males. Domestic ruminants in rural areas (OR = 39.48; 95% CI: 6.14–254.00) were at a much higher risk of being bitten by rabid animals than those in urban areas (Table 3).
Table 3. Factors retained in a final mixed-effect multivariable logistic regression model of the risk of RAB in domestic ruminants in Bangladesh, 2009–2018

The variance of random effect (location of case and control): 0.25.
AIC: 1182.1.
Discussion
Only rabid dog and jackal were found to be involved in domestic animal bites. Female and older cattle in rural areas were at greater risk of being bitten by rabid animals. Our study suggests that the prevention of rabies in rural dogs and jackals will reduce the incidence of rabies in livestock, and potentially humans, in Bangladesh.
A higher proportion of RAB cases were observed in December, January and July. A similar trend has also been observed in human rabies cases in Bangladesh [Reference Hossain11]. Increased number of rabies cases in December–January might be associated with the breeding season of dogs. During the dog-breeding season, there are increased contact rates between dogs, which lead to frequent fights and increase the risk of virus transmission. In the Indian subcontinent dogs breed once a year [Reference Chawla and Reece26], beginning in September; considering an incubation period of 2–3 months [Reference Singh27], clinical canine rabies incidence reaches a peak in December and January, with a likely subsequent spillover of rabies to livestock. So, domestic ruminants at the highest risk should either be managed indoors following the dog breeding season (December–January) or vaccinated.
A dog bite was most frequent, but jackal bites also were recorded for a substantial proportion of domestic ruminants. Dogs contribute 90% of the human rabies cases in Bangladesh [12], which is very similar to livestock bites (87.8%) in this study. Rabies in Bangladesh is maintained by two interrelated transmission cycles, sylvatic and urban cycles. The sylvatic cycle is mostly maintained by jackals and mongooses [Reference Hossain11, 12]. Vaccination of wildlife has not yet been attempted in Bangladesh. We observed jackals as the dominant wildlife reservoir. One study suggested chicken head as the preferred oral bait vaccine for jackals [Reference Koeppel, Kuhn and Thompson28]. So, the second priority for rabies elimination in Bangladesh should be vaccinating jackals using oral bait vaccines. However, the majority of rabies in both humans and livestock is due to the urban cycle and dog bites, as we also observed. Thus livestock RAB cases can serve as a proxy for − or as an adjunct to − human rabies surveillance. We did not observe any bite cases in domestic ruminants attributed to cats or mongoose, in contrast to a study of human RAB cases [12].
The Bangladesh canine rabies elimination program has focused primarily on PEP and mass dog vaccination to reduce the incidence of human deaths [14]. Since 2011, dog vaccination campaigns have been undertaken in all 64 districts. The aim of this program was to reduce by 50% human rabies deaths by 2015 and to eradicate it by 2020. However, the rabies elimination program was based on district-level activities, which might not have achieved sufficient vaccination coverage of dogs at the village level. Also, we did not observe any declining trend in domestic ruminant bite cases over the ten-year study period in the Mymensingh district. Moreover, we observed that RAB cases were 39-times more likely in rural than an urban area, perhaps indicating less impact of rabies control in rural areas [Reference Hossain10, Reference Taylor29]. Similarly, 82% of human rabies cases in Bangladesh have been reported to be from rural areas [12]. Domestic ruminants are fed by a tethering system in public fields, roadside or rice fields after harvest, thus exposing them to rabid dogs. In contrast, domestic ruminants in urban areas are mainly kept indoors. These factors likely are responsible for the higher risk of RAB in rural areas. The dog-population density in rural areas [Reference Hossain30] and Dhaka city [Reference Tenzin31] has been estimated to be 14 and 52 dogs/km2, respectively. Although the dog-density in rural areas is lower, the proportion of rural dogs that are rabid is likely to be higher perhaps due to more contact between rural dogs and jackals (sylvatic cycle). We recommend that vaccination coverage needs to be higher in village dog populations, and considerably higher than the recommended 70% coverage [Reference Brookes, DuÃàrr and Ward32]. In Bangladesh, mass dog vaccination is a challenge due to the high proportion of roaming dogs that cannot be readily handled for parenteral vaccination. A similar situation exists in India and the oral bait vaccine was found to be feasible, economical and effective for free-roaming dog populations [Reference Gibson33]. We also suggest this approach for rural dog vaccination in the Bangladesh scenario.
Female cattle were found to be more frequently bitten by rabid animals than males. Other authors have also reported similar results [Reference Thiptara34, Reference Islam35].
As for sex, older cattle were also found to be at higher risk for RAB than younger cattle. A similar result has also been reported in cattle by other authors [Reference Thiptara34], but this finding contrasts with a report from Bangladesh [Reference Islam35] − although that study described only 118 dog bite cases and the study duration was only four months. The tethering system used might increase the risk of aged cattle being bitten by rabid animals.
Moreover, crossbred cattle were found to be more frequently bitten by rabid animals than indigenous. It is not clear whether the colour of crossbred cattle is more likely to attract rabid animals or whether it is the manner in which crossbred cattle are managed. Moreover, in contrast to crossbred cattle, indigenous cattle have horns that might protect them from being bitten.
The strengths of the case-control study design are that many risk factors can be evaluated simultaneously [Reference Katz, Wild and Elmore36]. This study design is inexpensive, relatively quick and easy to complete, but the representativeness of the cases and controls may be unknown [Reference Friis37]. Berkson's bias is common in hospital-based case-control studies when hospitalisation depends on exposure. However, when exposures are risk factors for which animals are not hospitalised, rather than for the treatment of the outcome, then Berkson's bias does not occur [Reference Schwartzbaum, Ahlbom and Feychting38]. Our objectives were to identify demographic and temporal risk factors for being a RAB case. If we match on time and breed then we cannot analyse these as risk factors. For this reason, we did not match cases on breed, time and location. In addition, no significant trend in RAB cases was found. Therefore, not matching by year is unlikely to have introduced bias into the study results. It was not possible to confirm all rabid animals as definite rabies cases, which is a limitation of this study. This was not possible because dead rabid animals are not normally submitted to laboratories such as BAUVTH for post-mortem examination. However, the history of biting multiple animals and humans by rabid dogs and jackals, together with clinical signs suggestive of rabies, indicates rabies is maintained in both of these species in Bangladesh. In addition, a high ICC value (0.89) indicates clustering of RAB cases, which is expected based on the epidemiology of rabies in developing countries. There is no organised surveillance system for human and animal rabies in Bangladesh. In this scenario, retrospective analysis of hospital-based RAB cases can generate important knowledge on the epidemiology of rabies in domestic ruminants. RAB cases attending BAUVTH were treated by PEP. Most of the PEP-treated cases were monitored via telephone contact with owners and the majority of these cases did not develop any clinical signs of rabies. Only a small percentage of PEP-treated cases developed clinical signs of rabies and died either due to delay in treatment or location of biting site in the head region.
Female, crossbred and older cattle especially in rural areas should either be managed indoors during the dog breeding season or vaccinated. The national rabies elimination program should prioritise rural dogs for a mass vaccination campaign. Jackals and roaming dogs should also be immunised using an oral bait vaccine. Prevention of rabies in rural dogs and jackals will reduce the incidence of this disease in both humans and domestic ruminants.
Acknowledgements
The authors are grateful to the BAUVTH authority for providing the data.
Financial support
A. K. M. Anisur Rahman was supported by the US government as a Fulbright Visiting Scholar.
Conflict of interest
None.
Data
The data that support the findings of this study are available upon request to the corresponding author.








