Dietary guidelines have been implemented or are being developed by more than 100 countries( 1 ). Consistent with other countries( 1 ), the Australian Dietary Guidelines recommend consuming a wide variety of foods each day, based on the five food groups (vegetables; fruit; grain cereals (mostly wholegrain); lean meats and alternatives; dairy foods and alternatives), limiting ‘discretionary’ foods containing high levels of saturated fat, added salt or sugars and limiting alcohol( 2 ). However, few Australians meet these recommendations, with less than 10 % of adults eating the recommended five servings of vegetables daily, while discretionary foods contribute more than 35 % of adults’ daily energy intake( 3 ). There are similar findings around the world, with increasing levels of people consuming suboptimal diets inconsistent with their country’s dietary guidelines( Reference Raulio, Ovaskainen and Männistö 4 – Reference Haack and Byker 6 ).
Food labelling has been recognised as an important area for policy action to improve dietary intakes at a population level( Reference Hawkes, Jewell and Allen 7 ). Information provided on food labels can facilitate comparisons of products and the selection of healthier choices by consumers( Reference Cowburn and Stockley 8 ). One aspect of food labelling that may be influential in food choice are claims.
Food companies often use claims on food labels to market the addition of nutrients to foods or to inform consumers of certain health benefits or other nutritional characteristics of products( 9 ). Claims on food labels can be influential, causing consumers to believe that products with claims on their labels are healthier than those without( Reference Kaur, Scarborough and Rayner 10 ). Highly processed foods fortified with specific positive nutrients, such as iron or calcium, are commonly sold in Australia( Reference Stanton 11 ). These foods are usually less healthy than whole food alternatives and may contribute to poorer-quality diets( Reference Stanton 11 ).
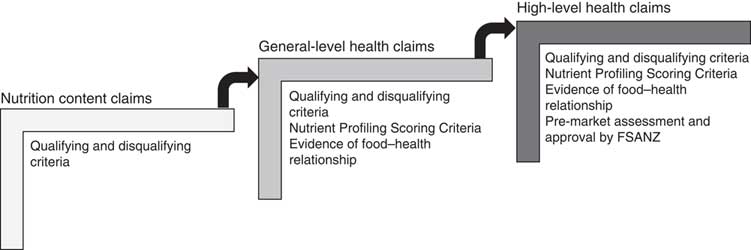
Food labelling is regulated through the Australia New Zealand Food Standards Code (the ‘Code’ hereafter). Food Standard 1.2.7 (the ‘Standard’ hereafter) on nutrition, health and related claims permits three types of claim on food labels: (i) nutrition content claims highlighting the presence or absence of a nutrient, e.g. ‘contains calcium’; (ii) general-level health claims stating, suggesting or implying that a food or property of that food has a health effect, e.g. ‘contains calcium for strong bones’; and (iii) high-level health claims referring to a serious disease or biomarker of a serious disease, e.g. ‘contains calcium to prevent osteoporosis’( 12 ).
These claims are regulated in a stepwise manner (Fig. 1). Nutrition content claims must meet defined qualifying criteria for the nutrient being claimed, to ensure the product actually does contain (or does not contain, in the case of reduced/low nutrient claims) that nutrient( 12 ). In addition to this, general-level health claims must meet Nutrient Profiling Scoring Criteria based on the energy, saturated fat, sugars, sodium, fibre and fruit, vegetable, nut and legume content of the food( 13 ), and there must be sufficient, good-quality evidence of the claimed food–health relationship( 12 ). High-level health claims must meet the requirements for general-level health claims and must be pre-approved by Food Standards Australia New Zealand (FSANZ)( 12 ). The present paper focuses specifically on the self-substantiation process for food–health relationships for general-level health claims.
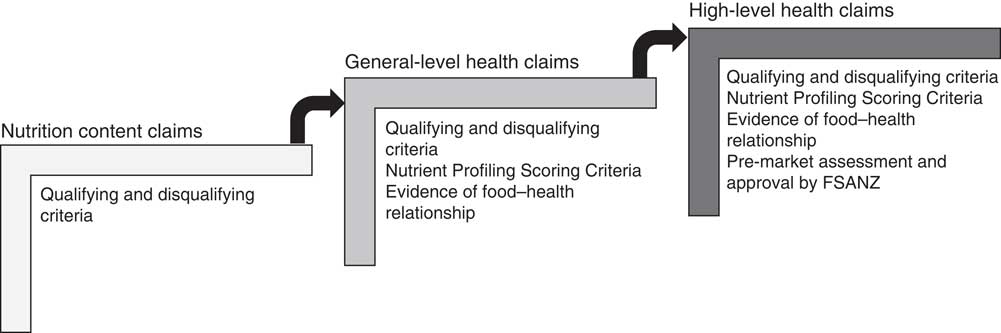
Fig. 1 Stepwise regulation of claims under the Australia New Zealand Food Standards Code( 12 ) (FSANZ, Food Standards Australia New Zealand)
In Australia, the development of food labelling policy and regulatory standards are separated. Overarching food policy is set by the Australia and New Zealand Ministerial Forum on Food Regulation( 14 ). The Forum develops food regulatory policy and policy guidelines, while FSANZ is tasked with setting food standards to support these policies( 15 ). In addition, the enforcement of these standards is the responsibility of state government enforcement agencies not FSANZ( 16 ). These agencies vary from state to state. For example, the New South Wales Food Authority handles the enforcement of the Code in that state, whereas in Western Australia, it is local governments’ responsibilities( 16 ). The personnel enforcing the Code are diverse with a range of professional backgrounds, who may not have the nutrition expertise to adjudicate on the specifics of food labelling.
After a lengthy consultation period, and a three-year implementation period, the health claim standard came into effect in Australia in January 2016. Prior to this, only high-level health claims and nutrition content claims relating to fatty acids were regulated by the Code( 17 ), with other nutrition content claims self-regulated by an industry code of practice( 18 ).
While the new Standard provides greater regulation, there are concerns about the level of protection for consumers and support for public health principles. For example, if there is no previous systematic literature review (SLR) published on a food–health relationship, food companies are able to self-substantiate the evidence by conducting their own systematic review( 12 ). The process for conducting the SLR is set out by FSANZ and guided by the following principles: systematic, transparent, comprehensive, based on evidence from human subjects (as opposed to animal and in vitro studies alone) and evidence showing causality( 12 , 19 ).
To be able to make general-level health claims, the food company must ‘notify’ FSANZ of the food–health relationships on which the claims are based, certify that they have conducted an SLR in accordance with the requirements of the Code, and retain the dossier of evidence( 12 ). Any relationships that have been notified appear on the FSANZ website( 20 ). The notified existence of an SLR is sufficient for claims to appear in the market based on these food–health relationships and, unlike high-level health claims, there is no pre-market approval required.
There is no requirement for FSANZ or an enforcement agency to assess the industry-provided SLR unless a complaint is made. Public health and consumer groups voiced their concern about the self-substantiation process prior to the implementation of the Standard( Reference Metherell 21 – Reference Foundation, Australia and Australia 23 ). The current system relies on state and local government enforcement agencies being adequately resourced to assess the industry-provided dossiers of evidence to decide whether there is sufficient, good-quality evidence for the food company to make the claim. However, enforcement agencies may not have the time to enforce all food labelling issues, or the necessary skills to assess the evidence, or they may prioritise other food regulatory issues over health claims( Reference Condon-Paoloni, Yeatman and Grigonis-Deane 24 ). Therefore, the food industry can potentially make claims based on poor-quality research if no complaints are received.
Health policy and systems research is defined as research aiming to improve how societies organise themselves to promote, restore or maintain health( 25 ). One aspect of health policy and systems research is implementation research, which evaluates whether implemented policies are having the intended impact( Reference Remme, Adam and Becerra-Posada 26 ). However, this type of research is often criticised as lacking rigour( 27 ), and little has been published on the implementation of policies( Reference Ghaffar, Gilson and Tomson 28 ), including self-substantiation of health claims regulations.
In response to the concerns about both self-substantiation as a process and the lack of implementation research in this field, the current project was undertaken by Cancer Council NSW, a community-based organisation with public health and nutrition policy expertise, with the aim of systematically monitoring the implementation of the self-substantiation process in health claims regulation in Australia. To the authors’ knowledge, the project is the first of its kind and provides evidence of the effectiveness of self-substantiation in food labelling.
Methods
The present project was designed to provide input into the regulatory process, highlight areas of concern, and draws on health systems research which looks at the formal content and instruments of health policy, as well as the forces influencing decision making( Reference Ghaffar, Gilson and Tomson 28 ). A governance structure was implemented for the research project, including an advisory panel of food policy practitioners and academics with specialist expertise in nutrition, public health and/or food policy, who provided strategic input into project development and guidance on specific food–health relationships. Although the project is ongoing, herein we report the results obtained between July 2013 and September 2017.
A summary of the process can be found in Fig. 2 and has been guided by the systems that regulate food labelling in Australia. The FSANZ website was monitored monthly by the project team for notifications of any new food–health relationships. Once a notified food–health relationship made by an Australian company appeared on the FSANZ website, the level of evidence for the food–health relationship was independently assessed by the project team by scoping the published literature for existing systematic reviews. If a previous SLR had been conducted and a link between the nutrient and the health effect established, then no further action was taken as the relationship was deemed to be substantiated. Relationships notified by food companies based in New Zealand were excluded from this project as these foods may not be for sale in Australia.
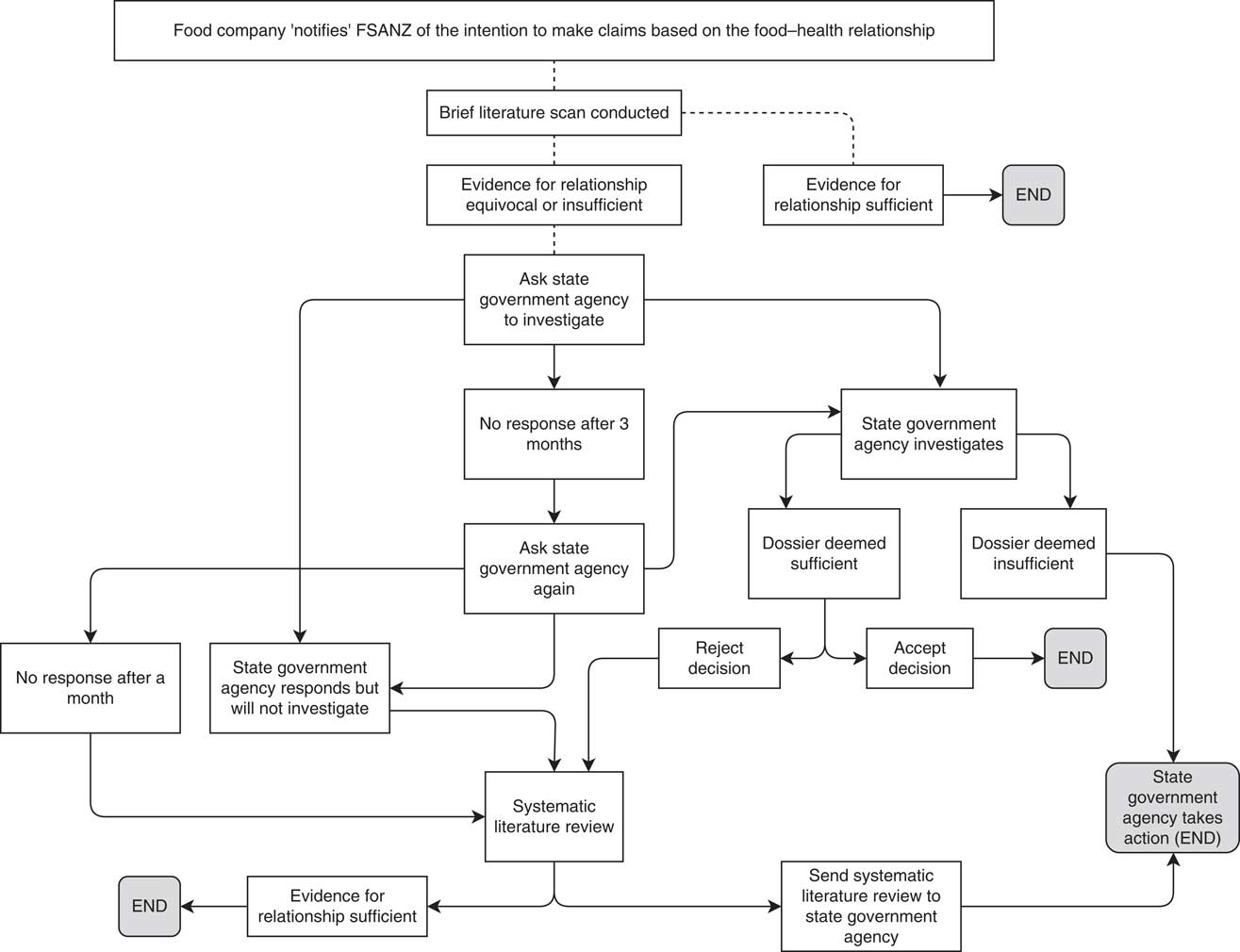
Fig. 2 The process for monitoring food industry self-substantiation of health claims (FSANZ, Food Standards Australia New Zealand)
Where the evidence was equivocal or there was little published evidence, the relevant state government agency responsible for food regulation was contacted and asked to investigate. If the state government agency failed to investigate, or the response from the agency was unsatisfactory, a systematic review of the peer-reviewed evidence supporting the food–health relationship was conducted by the project team and provided to the state government agency. Any systematic reviews undertaken were conducted using the same method specified by FSANZ and outlined in Schedule 6 of the Code( 29 ). These requirements include quality assessment of included studies( 29 ). As it was developed for systematic reviews used for substantiating health claims, the quality appraisal tool developed by Health Canada( 30 ) was used to assess included studies.
The project was established to test the self-substantiation system, and not to target the food companies that notified food–health relationships. As such, the investigations proceeded only through the state government authority process.
Results
The first notified food–health relationship appeared on the FSANZ website on 4 September 2013. During the study period, there were sixty-seven food–health relationships notified by thirty-eight different food companies. Four relationships were excluded as they were notified by New Zealand-based companies. Of the Australian-notified food–health relationships, thirty-three relationships (52 %) from twenty companies were deemed to have enough published evidence to substantiate the relationship and were not investigated further. Three relationships were removed from the FSANZ website before being assessed by the project team. At the time of publication, one relationship was being assessed by the project team. The remaining twenty-six food-health relationships were lodged with the state government agencies for investigation, including two whose determinations were still outstanding at time of publication.
Over the course of the project a total of nine food–health relationships (13 %) were removed from the FSANZ website. Six of these were lodged with state agencies for investigation but subsequently removed.
Investigations
The twenty-seven different food–health relationships that were referred to the state government agencies for assessment came from nine different companies, and these were investigated by four different state government agencies. A summary of the food–health relationships and the outcomes can be found in Table 1. The health conditions that were the subject of the claims were diverse with three claims related to weight loss, two for immune function, two for gut function and digestion, and two for blood glucose control. Improving appetite, energy, sexual performance, skin care and muscle/joint function also appeared as claims.
Table 1 Results of the investigations by state enforcement agencies on self-substantiated food–health relationships made by food companies in Australia, July 2013–September 2017

NHMRC, National Health and Medical Research Council; SLR, systematic literature review.
The length of time for state governments to investigate and adjudicate on the food health relationships ranged from 2 months to 30 months.
The first food–health relationship that was investigated by state enforcement agencies resulted in FSANZ developing a tool for the enforcement agency to assess the SLR held by companies. The Getting Your Claims Right guide( 31 ) provided a checklist for enforcement agencies to determine whether the SLR was conducted in accordance with the requirements set out in the Code( 12 ). This checklist informed the subsequent investigations by state enforcement agencies.
However, this checklist has limitations. When a food–health relationship on green tea catechins, caffeine and weight loss in overweight and obese adults was notified to FSANZ, the enforcement agency initially stated that the SLR demonstrating the relationship had met the criteria set out in Standard 1.2.7( 12 ). After assessing the quality of the studies and the totality of their results, the authors were not convinced that there was sufficient, good-quality evidence to support the food–health relationship. Therefore, we conducted an SLR and concluded that there was no consistent evidence of the relationship. The state enforcement agency requested permission to share our SLR with the food company. After the food company was provided with this independent SLR, it withdrew the notified relationship and claims from the company’s own website. However, the relationship remained on the FSANZ website meaning the company had not formally withdrawn it from FSANZ.
A series of relationships on apple cider vinegar were investigated by an enforcement agency. However, on follow-up with the agency, we were advised that the confidentiality clauses in that state’s Food Act did not permit the complainant to receive any updates on the complaint. Despite this, the relationships in question were removed from the FSANZ website.
Food–medicine interface
Several notified food–health relationships were for products that may sit on the medicine side of the food–medicine interface. The food–medicine interface is the regulatory overlap between certain foods and medicines( 32 ). One notified relationship specifically mentions that the subject of the relationship is tablets (‘Nature’s Way Super Maca Tablets help the body cope with stress’), yet this was deemed to be a food product by the enforcement agency. In all, there were fifteen different food–health relationships (56 % of the relationships investigated) that were for products that may be considered on the medicine side of the food–medicine interface, as determined by the Food–Medicine Interface Guidance Tool( 33 ).
Non-notified food–health relationships
During the course of investigating notified food–health relationships, we found that often other food–health relationships had not been notified. For example, one company notified three food–health relationships. On investigating the company’s website, there were other health claims being made based on other unnotified food–health relationships. This highlights another problem with the self-substantiation process. When companies do not notify FSANZ of all the food–health relationships and yet make claims based on these unsubstantiated relationships, these may go unnoticed by the state government enforcement agencies.
Discussion
The present project to monitor the implementation of the self-substantiation of food-health relationships in Australia was successful in ensuring that more food–health relationships notified by the food industry were investigated by relevant state government enforcement agencies. The process, although slow, appears to be effective in generating a response and action from the enforcement agencies.
The limitations of SLR are well recognised. Studies with negative findings are often not published and duplicate publication bias of papers from the same study can lead to the importance of the results of an SLR being overemphasised( Reference Egger, Dickersin and Smith 34 ). Similarly, the omission of citations can also result in a biased SLR( Reference Egger, Dickersin and Smith 34 ), as was the case found when reviewing the evidence relating to green tea catechins and caffeine and weight loss. While adopting a checklist approach to reviewing the conclusions of an SLR provides clarity and a framework for the enforcement agency staff, the omissions from the company-led SLR were not evident until a second SLR was presented, including additional papers omitted from the first SLR.
The results of an SLR may also be biased by who conducted and funded the review( Reference Egger, Dickersin and Smith 34 ). Commercial interests have been shown to influence SLR outcomes( Reference Egger, Dickersin and Smith 34 ), and in other fields industry-funded research is more likely to be favourable to the products tested compared with non-industry funded research( Reference Lundh, Lexchin and Mintzes 35 ). As health claims are marketing tools that can increase sales of products carrying them( Reference Kaur, Scarborough and Rayner 10 ), it can be argued that the food industry has a significant conflict of interest by being allowed to self-substantiate food–health relationships. Another point of concern is the way that the individual enforcement agencies deal with the complaints generated by the process. While some state government agencies were transparent with their investigations and provided timely and detailed responses, others would not provide any information to the complainant. If complaints that are generated by the public are not responded to, this may cause the public to lose confidence in the system. Enforcement agencies should ensure that complainants are provided with feedback on the progress and outcome of their complaint.
Although every company wanting to make claims needs to notify FSANZ and hold a dossier of evidence to substantiate the claim( 12 ), unless a food company requests the notification to be removed from the FSANZ site, it will stay on the list of notified food–health relationships, even when state government agencies make a ruling that the evidence provided was insufficient or that claims based on the food–health relationships cannot be made. This may be problematic if companies fail to notify based on other companies having already done so. Further, this may also create an impression that notified claims are approved by FSANZ. Determining if either of these were happening was beyond the scope of the current project, and this should be an area for future investigation.
More than half of the ‘foods’ that had food–health relationships notified to FSANZ were products that may sit on the medicine side of the food–medicine interface( 32 ). Medicines in Australia are regulated under the Therapeutic Goods Act, Regulations and Orders( 36 ). The processes for having a product added to the Australian Register of Therapeutic Goods is more onerous than notifying through the Code( 37 , 38 ) and can have substantial fees attached( 39 ). It is possible that companies are trying to avoid the more complex processes of therapeutic goods regulation by applying through the Code instead.
Given that enforcement of the Code is often reactive rather than proactive( Reference Williams, Yeatman and Ridges 40 ), the system relies on complaints from the public and other food companies to highlight potential issues. Claims based on unsubstantiated food–health relationships may remain in the marketplace if complaints are not made and investigated. This is concerning as it has long been established that health claims on food labels drive purchases of those foods( Reference Kaur, Scarborough and Rayner 10 , Reference Levy and Stokes 41 , Reference Ippolito and Mathios 42 ). Further, the presence of claims may prevent consumers from seeking more information on the nutritional value of the product and therefore lead them to make poorer dietary decisions based on these claims( Reference Verrill, Wood and Cates 43 ). Allowing claims that are not underpinned by evidence is potentially misleading to consumers, who may perceive foods with claims as healthier than those without claims( Reference Williams 44 , Reference Dixon, Scully and Wakefield 45 ). It is predicted that the demand for processed foods will continue to rise( Reference Di Nunzio 46 ), and the consumption of minimally processed core foods, such as fruit and vegetables, is falling( 3 ). This may contribute further to Australia’s increasing overweight and obesity rates. Consumers may mistakenly believe they will receive health benefits from eating foods carrying claims on unsubstantiated food–health relationships. It is important that unhealthy processed foods are not permitted to carry unsubstantiated claims, as this may further drive consumption.
The self-substantiation process has been raised as an option for regulation of another aspect of the Code: nutritive substances and novel foods( 47 ). Some of the state government enforcement agencies themselves have noted their concern at the self-substantiation process( 48 ). Given the limitations of the self-substantiation process highlighted in the present paper, it is recommended that FSANZ implement pre-market approvals of health claims, including thorough investigation of the SLR underpinning the food–health relationship. This will not only ensure that the food–health relationships are substantiated by the evidence, but also remove the reliance on consumer complaints for the monitoring process, reducing the burden on state government enforcement agencies. We acknowledge that the recommendation for pre-market approval shifts resourcing implications from monitoring to earlier in the enforcement process; however, these resource costs are justifiable to optimise nutritional intake and therefore public health outcomes.
There is limited published evidence of the effectiveness or otherwise of food policy interventions. The current study presents an analysis of the issues of industry self-substantiation in food labelling and provides the first published evidence of the success of the Standard. Further research should be conducted analysing the impact that these claims have on consumer behaviour, as well as ongoing monitoring of the Standard to ensure unsubstantiated claims are identified and raised with state government agencies. Research could also be undertaken on whether self-substantiated health claims in overseas jurisdictions are appropriately evidence-based.
Health policy and systems research is often criticised for not being generalisable to other settings or countries( 25 ). However, the present study is of significance internationally. Many countries, including the USA( Reference Food and Administration 49 ), Canada( 50 ) and countries of the European Union( 51 ), rely on evidence supplied by the food company wanting to make claims based on the relationships they are substantiating. It is reasonable that the issues identified herein will also apply in other countries. Therefore, there is the potential that food manufacturers across the world are making claims based on unsubstantiated evidence.
Conclusions
Supporting public health objectives to reduce chronic disease related to unhealthy diets and associated overweight and obesity should be a priority of the food regulation system. To ensure that SLR underpinning food–health relationships are scientifically rigorous, as well as reducing regulatory burden on state food enforcement agencies, pre-market approval of food–health relationships should be introduced by FSANZ, with the appropriate resources made available. This will have the benefit of increasing consumer confidence in the regulatory process, as well as preventing potentially misleading general-level health claims on food labels. In the meantime, continued monitoring of the implementation of the health claims standard will ensure any future dubious claims are investigated.
Acknowledgements
Acknowledgements: The study team would like to thank Elaine Ho for her assistance with SLR and the project advisory panel for their guidance throughout. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: L.W.-C. and C.H. formulated the research question and designed the study. K.C. and C.H. provided strategic oversight to the study. L.W.-C., W.W. and C.H. conducted the study. L.W.-C. analysed the data and wrote the manuscript. W.W., C.H. and K.C. provided comment on the analysis and extensive input into the manuscript. Ethics of human subject participation: The study did not require ethical approval as it was an observational study and had no human subjects.