A wide range of health benefits have been related to the consumption of long-chain n-3 PUFA including improvements in cardiovascular and several inflammatory disorders(Reference Ruxton, Reed and Simpson1). Evidence also suggests that these n-3 PUFA, notably EPA (20 : 5n-3), can benefit skin health, by tempering inflammation and reducing the acute effects of UV radiation such as sunburn, and potentially protecting against longer-term photoageing and photocarcinogenesis(Reference Rhodes, O'Farrell and Jackson2–Reference Pilkington, Watson and Nicolaou6). In the UK, as of 2004, the recommended daily consumption of long-chain n-3 PUFA for a healthy adult was increased from 100–200(7) to 450 mg(8) as a result of the emergence of new evidence as to the diversity of potential benefits of n-3 PUFA(Reference Ruxton, Reed and Simpson1, 8).
EPA and DHA (22 : 6n-3) are believed to be the most biologically active n-3 PUFA, their activities including modulation of lipid messengers and transcriptional activation(Reference Ziboh, Miller and Cho9, Reference Deckelbaum, Worgall and Seo10). These long-chain n-3 PUFA compete with the long-chain n-6 PUFA arachidonic acid (AA, 20 : 4n-6) for metabolism by cyclo-oxygenases and lipoxygenases, resulting in the production of less pro-inflammatory eicosanoids(Reference Massey and Nicolaou11). The respective short-chain n-3 and n-6 PUFA α-linolenic acid (ALA, 18 : 3n-3) and linoleic acid (LA, 18 : 2n-6) are regarded as essential fatty acids, as they cannot be synthesised endogenously by humans. Ingested ALA is obtained largely from plant sources and can be metabolised endogenously to the longer-chain n-3 PUFA(Reference Pilkington, Rhodes, Krutmann and Humbert12); however, the conversion of ALA to its long-chain metabolites is inefficient: up to 21 and 9 % for EPA and DHA, respectively(Reference Burdge and Wootton13). Thus, the most effective way of naturally obtaining long-chain n-3 PUFA is through the consumption of oily fish and seafood(Reference Whelan and Rust14). In recent years, food products fortified with n-3 PUFA, such as milk, margarine, eggs and bread, have also provided other sources of these bioactive fats(Reference Metcalf, James and Mantzioris15, Reference Shapira, Weill and Loewenbach16).
Several methods of estimating n-3 PUFA nutrition have been described(Reference Øverby, Serra-Majem and Andersen17). Erythrocytes and plasma are often used to assess n-3 PUFA levels, but few other human tissue targets are as easily sampled. FFQ have become useful tools for quantifying nutrient intakes from diet as they are non-invasive and easily implemented into studies of large populations. Studies examining the relationship between n-3 PUFA intake estimated from matched FFQ data and blood samples have mostly reported good correlations between these assessments (correlation coefficients between 0·4 and 0·6)(Reference Øverby, Serra-Majem and Andersen17). To date, however, there have not been any comparisons of these assessments with concentrations measured in the skin despite the reported relevance of skin n-3 PUFA content to photoprotection and potentially other aspects of skin health, as invasive skin assessment (biopsy) is not generally feasible in population studies.
The principal aim of the present study was to perform a three-way matched assessment of EPA nutrition, in order to assess the value of FFQ and erythrocyte measurements as indicators of the skin ‘target organ’ content. Using a multidisciplinary approach, we quantified n-3 PUFA intake from a self-reported FFQ, and correlated this with concentrations measured in erythrocytes and directly in dermal samples from the same adult volunteers in the UK.
Methods
Study subjects and design
This analysis used baseline nutritional data collected from participants in a double-blind, randomised, placebo-controlled nutritional study of n-3 PUFA supplementation. Women who consumed more than two fish meals per week were excluded a priori in order to reduce undue baseline variation in n-3 PUFA intake among study participants. Eligible participants were healthy white Caucasian women aged 18–60 years with sun-sensitive skin (sun-reactive skin type II, i.e. skin susceptible to sunburn, with minimal tanning) and confirmed Ni-sensitivity (cutaneous allergy, evident most usually to Ni-plated jewellery). Women were excluded if they were pregnant, unable to eat fish or gelatin, took photoactive medication (e.g. non-steroidal anti-inflammatory drugs), consumed n-3 PUFA supplements or more than two meals per week of oily fish, had sunbathed or used a sunbed in the past 3 months, or reported a history of atopy, skin cancer, photosensitivity disorder or cardiac disease. Participants were recruited through the Contact Dermatitis Investigation Unit of Salford Royal NHS Foundation Hospital, Manchester, UK or by advertisements placed in the hospital, the University of Manchester and local newspapers. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the North Manchester Research Ethics Committee. Written informed consent was obtained from all subjects.
Dietary assessment
Dietary intake was assessed using a self-administered, semi-quantitative FFQ modified from the validated Nurses' Health Study questionnaire (USA)(Reference Willett, Sampson and Stampfer18) and the Nambour Skin Cancer Study questionnaire (Australia)(Reference Marks, Hughes and van der Pols19, Reference McNaughton, Hughes and Marks20). Only minor modifications were made to the Australian version (validated for PUFA intake using the method of triads(Reference McNaughton, Hughes and Marks20)) in order to ensure that the FFQ used in our present study reflected UK dietary components. There were 147 food items on the questionnaire and participants were asked to report the frequency with which a specified serving of each food item was consumed. Serving sizes were expressed either as standard measurements (i.e. one cup, one teaspoon, etc.) or in natural units (i.e. one egg, one slice, etc.). Frequency options ranged from ‘never’ to ‘4+ times per d’. Additional questions collected information on trimming of visible fat from meat, types of fats and oils used on foods and in cooking, as well as on specific foods consumed that were fortified with n-3 PUFA. Questions regarding self-administered dietary supplements were also included.
Participants reported the average frequency with which they consumed food items in the past 6 months. Average daily intake was calculated by expressing the frequency response of a food item as a proportion of daily use, multiplied by the serving size and by the nutrient content of the food. The McCance and Widdowson's Composition of Foods Integrated Dataset was used to obtain nutrient compositions for UK food items(Reference McCance21). When nutrients could not be obtained from the McCance 2002 database, items were selected from Australian databases(Reference Mann, Sinclair and Percival22–24). n-3 PUFA contents of additional foods including margarine/spreads, bread and eggs were obtained from manufacturers' information.
In addition to the FFQ, women were asked regarding their height and weight for estimation of BMI and about their current or former smoking status.
Biological sampling
Blood samples were taken from the antecubital fossa of participants and collected in EDTA monovette tubes (Sarsstedt) and centrifuged for 15 min at 1500 rpm; the erythrocyte fractions were collected and stored at − 70oC until analysis. A random half of the study subjects had 5 mm punch skin biopsies taken from an unexposed site of upper buttock skin under local anaesthesia (2 % lignocaine without adrenaline). Skin biopsies were snap-frozen in liquid N2 and stored at − 70°C for analysis.
Erythrocyte and skin biopsy analysis
Skin samples were defrosted on ice. Only dermal tissue samples were available for this analysis and these were incubated in chloroform–methanol (4 ml) (2:1, v/v) containing butylated hydroxytoluene (BHT) (0·01 % (w/v) overnight at 4oC and homogenised using a blade homogeniser. Lipids were extracted using a further two volumes of chloroform–methanol–BHT as described before(Reference Green, Anyakoha and Yadid25). Erythrocyte samples (1 ml) were defrosted on ice and lipids were also extracted with chloroform–methanol–BHT (4 ml). The solvent was removed under N2 and the lipid extract was stored at − 20oC before analysis.
Fatty acid methyl esters were prepared using BF3 in methanol, and analysed by GC (GC-flame ionisation detector) on a BPX70 GC capillary column (SGE Europe Limited), using He as the carrier gas. Heneicosaenoic acid (C21 : 0) was used as the internal standard (ACS reagent, Sigma-Aldrich) with a thirty-seven fatty acid methyl esters mixed standard (Supelco), the reference for identification of fatty acids. The detection limit for this method was 5 μg per sample, and all measured n-3 PUFA levels were well above this lower boundary. Results for both erythrocytes and dermis were expressed as percentage of total fatty acids in each sample. The PUFA examined included the principal long-chain n-3 PUFA EPA and DHA, the principal short-chain n-3 PUFA ALA, and the principal long- and short-chain n-6 PUFA, AA and LA, respectively.
Statistical analyses
Energy intakes from the FFQ were assessed to identify any women with extreme or implausible values (>14 700 and < 2100 kJ/d) using Willett's criteria(Reference Willett26). Fatty acid intakes were also adjusted for energy intake using the residual method(Reference Willett, Sampson and Stampfer18). Spearman correlation coefficients for each PUFA were used to assess the linear relationship between each of the parameters (FFQ and erythrocytes, FFQ and dermis, erythrocytes and dermis). Significance was set at P< 0·05. Correlations were also performed stratified by age (21–40; 41–60 years), and, when available, by BMI ( < 25, ≥ 25 kg/m2) and by smoking status (never or ever smoked) to assess possible effect modification by these factors. Agreement between the PUFA measurements, in ranked thirds, from FFQ, erythrocytes and dermis was assessed by comparing percentage exact agreement and gross misclassification (disagreement across two tertiles) to random expected values. All statistical analyses were performed using Statistical Analysis Systems software package version 9.2 (SAS Institute).
Results
A total of seventy-nine eligible volunteers were enrolled for the study, one woman withdrew, and baseline information for analysis was thus obtained from seventy-eight volunteers, with a mean age of 42·8 (sd 10·0) years (range 21–60 years) and an average BMI (available for sixty-one women) of 26·7 (sd 5·0) kg/m2 (range 19·4–44·2 kg/m2) (Table 1). Of the seventy-eight participants, seventy-five completed and returned their FFQ, and, of these, four people were omitted from further FFQ-related analyses as they had implausibly high energy intakes. The mean daily energy intake of the remaining participants (n 71) was 7889 (sd 2390) kJ. Overall, seventy-two participants provided blood samples, and all thirty-nine volunteers randomised to providing skin samples did so. Mean values and standard deviations were calculated for total and specific n-3 PUFA intakes using FFQ data (energy adjusted), blood samples and skin biopsies (Table 2). Average total EPA, DHA and ALA intake from FFQ was 171 (sd 168), 236 (sd 243) and 850 (sd 260) mg/d. Energy-unadjusted means from FFQ were similar (data not shown). From the tissue measures, mean EPA and DHA (percentage of total fatty acids) were highest in erythrocytes, while mean ALA was highest in dermis.
Table 1 Baseline population characteristics of women recruited for double-blind, randomised, placebo-controlled nutritional study (n 78) (Mean values, standard deviations, ranges, number of women and percentages)

* Missing data n 5 (6 %).
† Missing data n 17 (22 %).
‡ Missing data n 3 (4 %).
§ Mean daily nutrient intake values energy-adjusted and exclude people with values >14 700 kJ (n 4).
Table 2 Principal long- and short-chain n-3 PUFA assessed from FFQ, erythrocytes and dermal tissue (Mean values and standard deviations)
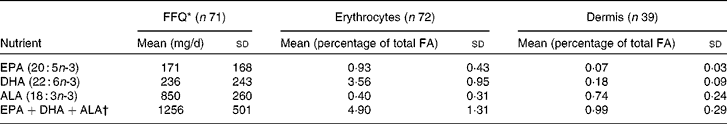
FA, fatty acid; ALA, α-linolenic acid.
* Intakes from FFQ energy-adjusted.
† Sum of EPA, DHA and ALA.
Several significant correlations were observed between the amount of long-chain n-3 PUFA in FFQ, erythrocytes and dermis. FFQ data for EPA showed the most substantial significant correlation coefficients with erythrocytes and dermal data (r 0·57, P< 0·0001 and r 0·33, P= 0·05, respectively) (Table 3; Fig. 1(a–c)). We observed a significant correlation between FFQ and erythrocytes for DHA (r 0·50, P< 0·0001) and for the sum total of EPA+DHA+ALA (r 0·27, P= 0·03) (Table 3; Fig. 2(a–c)). None of the three-way assessments showed significant correlations for ALA. The only significant inverse correlation observed was between erythrocytes and dermal EPA+DHA+ALA (r − 0·39, P= 0·02). When the influence of age, body weight and smoking status on FFQ and erythrocyte correlations (there was an insufficient number of dermal samples to permit stratified analyses) was assessed, correlations remained relatively consistent across categories for EPA and DHA. FFQ and erythrocyte correlations for the sum of the n-3 PUFA fluctuated according to age ( < 40 years or >40 years), BMI ( < 25 or ≥ 25 kg/m2) and smoking habit (ever or never), but only changed by age group for ALA (not shown).
Table 3 Spearman correlation coefficients for principal long- and short-chain n-3 PUFA assessed from FFQ, erythrocytes and dermal tissue

ALA, α-linolenic acid.
* Significant correlation (P< 0·05).
† Sum of EPA, DHA and ALA.

Fig. 1 Bivariate plots of EPA levels from (a) FFQ (mg/d) v. erythrocytes (percentage of total fatty acid (FA)), (b) FFQ v. dermis (percentage of total FA) and (c) erythrocytes v. dermis, with Spearman correlation coefficients and P values. (a) r 0·57, P< 0·0001; (b) r 0·33, P< 0·05 and (c) r 0·45, P< 0·008.

Fig. 2 Bivariate plots of DHA levels from (a) FFQ (mg/d) v. erythrocytes (percentage of total fatty acid (FA)), (b) FFQ v. dermis (percentage of total FA) and (c) erythrocytes v. dermis, with Spearman correlation coefficients and P values. (a) r 0·50, P< 0·0001; (b) r 0·18, P< 0·29 and (c) r 0·09, P< 0·62.
When agreement between ranked thirds of n-3 PUFA nutrition by FFQ, erythrocytes and dermis was assessed (Table 4), EPA had the best agreement between all measures, with over half of subjects classified into the exact third of EPA intake and low gross misclassification. Agreement between ranked thirds of DHA also approached 50 %, though a higher proportion of women were misclassified. Overall, assignment to the same or adjacent ranked third category of EPA and DHA was 79–94 %. Exact agreement was lower than expected for the sum of the n-3 PUFA and ALA.
Table 4 Percentage agreement between tertile assignments according to principal long- and short-chain n-3 PUFA assessed from FFQ, erythrocytes and dermal tissue
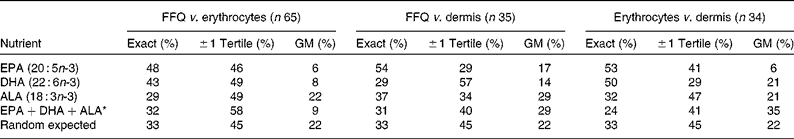
GM, gross misclassification; ALA, α-linolenic acid.
* Sum of EPA, DHA and ALA.
All analyses were repeated for LA, AA, the sum of LA+AA and the ratio of total n-6:n-3. There were no significant correlations between the n-6 PUFA measures, and only the FFQ and erythrocyte correlation was significant for n-6:n-3 (r 0·42, P= 0·0004). Exact agreement of ranked thirds and gross misclassification were highly variable (not shown).
Discussion
In the present study, we have demonstrated that the assessment of dietary EPA intake using an FFQ, and corresponding circulating levels, are in turn significantly and well correlated with the measurement of dermal content. These findings are consistent with evidence of the functional significance of EPA in skin(Reference Kim, Cho and Lee4, Reference Pilkington, Watson and Nicolaou6, Reference Rhodes, Shahbakhti and Azurdia27). This is important new knowledge for those concerned with promotion of skin health through improved nutrition, since previous studies have not examined these parameters synchronously, and confirms the importance of dietary intake of EPA and DHA on their bioavailability in a clinically relevant target tissue.
Among female study populations similar to ours, average daily intakes of EPA and DHA have been reported as 61–280 and 109–460 mg/d, respectively, from FFQ(Reference Andersen, Solvoll and Drevon28–Reference Andersen, Solvoll and Johansson31); the FFQ intakes measured in our present study fell within these ranges. Our mean ALA intakes were only slightly lower than those reported previously (1030–1200 mg/d)(Reference Andersen, Solvoll and Drevon28, Reference Hodge, Simpson and Gibson30). Current recommended daily intake of total long-chain n-3 PUFA for healthy adults is 450 mg in the UK(8). The relatively low intake of EPA and DHA by the women in this study (half the UK recommendation) might be partly explained by the eligibility criterion of consuming less than two serves of fatty fish a week. In addition, consumption of fatty fish in general has declined markedly in the past century and, although a comparatively minor source of n-3 PUFA, lamb and beef consumption in the UK has also declined over the past 20 years(Reference Sanders32); thus these trends may also have contributed to the women's lower-than-recommended n-3 PUFA consumption.
Various methods have been used for the assessment of bioavailable n-3 PUFA including sampling of erythrocytes, platelets, plasma, serum, cholesterol esters, phospholipids(Reference Andersen, Solvoll and Drevon28, Reference Arab33, Reference Kobayashi, Sasaki and Kawabata34), adipose tissue(Reference Andersen, Solvoll and Johansson31, Reference Hunter, Rimm and Sacks35, Reference Tjonneland, Overvad and Thorling36) and buccal mucosa(Reference Connor, Zhu and Anderson37). Erythrocyte measurements are considered better markers of circulating blood PUFA levels than serum or plasma measurements, as they reflect an intake period of several weeks and contain a high proportion of PUFA(Reference Arab33). Of the studies reporting on the validity of FFQ in reference to blood concentrations, only a few reported erythrocyte data as opposed to serum or plasma phospholipid fatty acids. Our correlation coefficients for intake from FFQ compared with erythrocyte concentrations were slightly higher than those previously reported (0·37–0·40 and 0·16–0·39) for EPA and DHA, respectively(Reference Sullivan, Williams and Meyer29, Reference Zhang, Wang and Chen38), and consistent with the high validity coefficients for FFQ and erythrocyte measures of long-chain n-3 PUFA observed in the analysis of Swierk et al. (Reference Swierk, Williams and Wilcox39). The apparent tighter fit of the correlations at lower levels and dispersion at higher levels in the correlation figures was probably due to the study exclusion criteria recruiting a population of relatively low n-3 PUFA consumers. Few participants consumed high levels of n-3 PUFA such that these data points appear more as relative outliers. That a correlation was not observed in the present study between FFQ and blood ALA content is probably due to the estimated 30 % lost in metabolic oxidation of dietary ALA into carbon dioxide for energy(Reference Burdge and Wootton13).
Detailed descriptions of n-3 PUFA content of human skin are sparse in the literature, and the currently reported series appears by far the largest to date. Previous smaller human volunteer studies have reported a low skin content of EPA, ranging from 0·05 % mol in whole-skin biopsies (n 14)(Reference Rhodes, Shahbakhti and Azurdia27) to 1·1 % mol in skin-shave biopsies comprising largely epidermis (n 6)(Reference Rhodes, O'Farrell and Jackson2). The present findings are consistent with these reports, the dermis exhibiting an EPA content within this range. The lead-time for dietary n-3 PUFA to affect skin content is uncertain, but it has been observed that daily supplementation with 4 g EPA capsules resulted in an 8-fold increase in skin content of EPA after 3 months(Reference Rhodes, Shahbakhti and Azurdia27). Our findings complement these results by demonstrating that even low dietary EPA intake appears to be reflected in the skin.
Human n-3 PUFA supplementation studies have additionally demonstrated the role of EPA in decreasing the skin's UV-induced inflammatory response. Specifically, EPA has been shown to increase the skin's threshold to sunburn erythema, and to reduce both basal and UV-induced cutaneous levels of the eicosanoid PGE2(Reference Rhodes, O'Farrell and Jackson2–Reference Pilkington, Watson and Nicolaou6, Reference Rhodes, Shahbakhti and Azurdia27, Reference Shahbakhti, Watson and Azurdia40), a key mediator of the erythema and also involved in skin cancer promotion(Reference Rhodes, Belgi and Parslew41). This is consistent with the known competition of EPA with AA for metabolism by cyclo-oxygenase, leading to reduced PGE2 production(Reference Ziboh, Miller and Cho9). A range of human studies also supports that n-3 PUFA may protect against the longer-term photodamage of skin carcinogenesis and ageing(Reference Kim, Cho and Lee4, Reference Rhodes, Shahbakhti and Azurdia27, Reference van der Pols, Xu and Boyle42). The recent revision of dietary guidelines to intake of 450 mg long-chain n-3 PUFA per d was done to align with existing recommendations of two weekly servings of fatty fish(8); however, the adequacy of these new dietary recommendations for skin health remains unknown. Little is known about the threshold n-3 PUFA intake required for protection against adverse skin health outcomes. This requires examination in future studies, particularly in view of the continued escalation in skin cancer incidence in many white-skinned populations(Reference Garbe and Leiter43); a dietary approach to prevention could have widespread impact at a population level(Reference Black and Rhodes5).
The present study's limitations include potential for error in the accuracy of PUFA intake measurements from self-reported FFQ due to issues of recall by the participants. Further, although a similar version of the FFQ was validated in an Australian population(Reference McNaughton, Hughes and Marks20), the updated FFQ may not be fully valid when used in our UK-based population. We also acknowledge that, as a dietary measurement tool, any FFQ may not be fully valid and reliable(Reference Kristal, Peters and Potter44). On the other hand, the McCance database(Reference McCance21), used to identify the nutrient composition of food items in this study, is the database most widely accepted and used in the UK and as such provides the most accurate PUFA data available for the intake frequencies provided from the FFQ. A limitation of our dermal PUFA data was that a large amount of variation remained unaccounted for when comparing these data with FFQ intake and erythrocyte content. Metabolic differences between individuals in both uptake and transport of n-3 PUFA to the skin may explain some of this variation, but future investigative studies are needed in order to address this issue. Furthermore, the variability of n-3 PUFA levels in skin from different anatomic body sites remains unknown, although our EPA data were consistent with previous reports(Reference Rhodes, O'Farrell and Jackson2, Reference Rhodes, Shahbakhti and Azurdia27). The number of skin samples was also insufficient to precisely evaluate how age, BMI and smoking habits may influence dermal n-3 PUFA content in relation to dietary content. Finally, our present study participants were all females; so it is not known how our results apply to males, and since study participants were volunteers, their representativeness of similarly aged healthy UK women is unknown.
In summary, concentrations of EPA in dermal and erythrocyte samples from these healthy women showed significant correlations with EPA consumption, demonstrating that FFQ intake estimates provided a good measure of both the circulating and skin bioavailability of this long-chain n-3 PUFA. Further research in a more diverse population is required to extend these findings and to determine the threshold of n-3 PUFA intake required to sustain skin benefit.
Acknowledgements
The authors are grateful to the volunteers who participated in the present study. This study was funded by a grant from the Association for International Cancer Research (L. E. R. and A. N., no. 08-0131). S. C. W. and A. C. G. were supported by a fellowship from the Medical Research Council, UK (no. 89912). None of the authors had a personal or financial conflict of interest. The authors' contributions to the study were as follows: S. C. W. managed the data, performed statistical analysis and wrote the manuscript; S. M. P. assisted with data collection and wrote the manuscript; K. A. M. and N. M. I. A.-A. carried out laboratory analyses; T. I. I. and M. C. H. assisted with data management and statistical analysis and wrote the manuscript; S. B. recruited volunteers and collected the data; A. N. supported laboratory analyses and manuscript writing; L. E. R. designed the study and wrote the manuscript; A. C. G. assisted with interpretation of the results and writing of the manuscript.








