Fructans are reserve carbohydrates comprising fructose molecules linked or not to a terminal sucrose molecule. They may have a linear or branched structure, with different bonds between fructose monomers: (1) β(2,6) bonds found in levan-type fructans; and (2) β(2,1) bonds found in inulin-type fructans (Roberfroid & Delzenne, Reference Roberfroid and Delzenne1998). Inulin-type fructans are commonly found in almost all species in the Asteraceae family, many of which are of economic importance, such as Chicorium intybus and Helianthus tuberosus (Carvalho & Figueiredo-Ribeiro, Reference Carvalho, Figueiredo-Ribeiro, Lajolo, Saura-Calixto, Penna and Menezes2001).
Yacon (Smallanthus sonchifolius [Poepp & Endl.] H. Robinson, Asteraceae) is a perennial plant originally from the Andes Mountains. It grows successfully between 800 and 3500 m above sea level, although it is also grown at sea level in New Zealand and in the USA. There are also reports of its cultivation in other countries in South America (Argentina, Colombia, Peru and Venezuela) and Europe (Germany, Italy, France and Czech Republic) (Valentová & Ulrichová, Reference Valentová and Ulrichová2003). Yacon has been cultivated in south-eastern Brazil as a crop since 1991 from August to September, yielding up to 100 t/ha (Vilhena et al. Reference Vilhena, Câmara and Kakihara2000). Its tuberous roots are sweet and crispy and can be eaten either raw or cooked (Zardini, Reference Zardini1991) and are widely used, together with its leaves, to brew a medicinal tea. More recently, its physico-chemical properties have contributed to its use in the development of beverages and bakery products (Quinteros, 2001; Moscatto et al. Reference Moscatto, Borsato, Bona, De Oliveira and Hauly2006).
Its underground system consists of two different types of edible reserve organs: the tuberous roots, the actual commercialized crop; and the rhizophores, the organs of vegetative reproduction. Both accumulate high amounts of fructans and other soluble carbohydrates, such as fructose, glucose and sucrose. Nevertheless, variations in the concentration of such carbohydrates and in the chain length of fructans may occur during plant growth and after harvesting (Itaya et al. Reference Itaya, Carvalho and Figueiredo-Ribeiro2002). Studies have shown that the best period to harvest yacon in tropical regions is between the 31st and the 35th week after cultivation, regarding the concentration of fructans and their proportion in relation to mono- and disaccharides (Oliveira & Nishimoto, Reference Oliveira and Nishimoto2004). Furthermore, yacon plants present a high hydrolytic activity at maturation phase of the tuberous roots, contributing to the predominance of a low degree of polymerization (DP < 10; fructooligosaccharides (FOS)) over high DP fructans (inulin) (Itaya et al. Reference Itaya, Carvalho and Figueiredo-Ribeiro2002).
FOS have been classified as prebiotic ingredients since they are selectively fermented by the microflora in the large intestine, leading to a modulation in the composition of the natural ecosystem (Roberfroid et al. Reference Roberfroid, Van Loo and Gibson1998). Studies carried out in animals and man have demonstrated that fructans, through their gastrointestinal effects, may indirectly affect the immunological function and the metabolism of carbohydrates, lipids and minerals (Roberfroid & Delzenne, Reference Roberfroid and Delzenne1998; Van Der Heuvel et al. Reference Van Der Heuvel, Muys, Van Dokkum and Schaafsma1999; Buddington et al. Reference Buddington, Kelly-Quagliana, Buddington and Kimura2002). Although the small intestine is the major site for mineral absorption, the large intestine plays an important role in mineral absorption as well, since some dietary fibres (DF; including fructans) are fermented by bacteria in the colon. SCFA, produced as a result of the increase in the metabolic activity of the intestinal microflora, lower the luminal pH and thereby increase mineral solubility. Both low pH and SCFA result in the hypertrophy of the mucosal cells, leading to enlargement of the surface area in the intestine and enhanced mineral absorption (Roberfroid & Delzenne, Reference Roberfroid and Delzenne1998). Moreover, a direct coupling of SCFA to the minerals may lead to an increase in their uptake by the intestinal cells (Lutz & Scharrer, Reference Lutz and Scharrer1991). Finally, an increase in the expression of calbindin-D9k (Ohta et al. Reference Ohta, Motohashi, Ohtsuki, Hirayama, Adachi and Sakuma1998; Raschka & Daniel, Reference Raschka and Daniel2005; Nzeusseu et al. Reference Nzeusseu, Dienst, Haufroid, Depresseux, Devogelaer and Manicourt2006) and other genes involved in Ca transport in the large intestine (Raschka & Daniel, Reference Raschka and Daniel2005) has been reported as a result of fructans ingestion. These effects are reflected in increases in bone mineralization as well (Kruger et al. Reference Kruger, Brown, Collett, Layton and Schollum2003; Zafar et al. Reference Zafar, Weaver, Zhao, Martin and Wastney2004; Nzeusseu et al. Reference Nzeusseu, Dienst, Haufroid, Depresseux, Devogelaer and Manicourt2006), possibly related to a decrease in osteoclastic resorption (Kruger et al. Reference Kruger, Brown, Collett, Layton and Schollum2003; Zafar et al. Reference Zafar, Weaver, Zhao, Martin and Wastney2004; Nzeusseu et al. Reference Nzeusseu, Dienst, Haufroid, Depresseux, Devogelaer and Manicourt2006).
The potential prebiotic effect of fructans and the presence of potentially antioxidant phytochemicals (such as chlorogenic, caffeic and ferulic acids) from yacon tuberous roots have been reported (Pedreschi et al. Reference Pedreschi, Campos, Noratto, Chirinos and Cisneros-Zevallos2003; Valentová & Ulrichová, Reference Valentová and Ulrichová2003). Recently, a study evaluated the toxical effects in rats fed yacon root flour offered 340 and 6800 mg FOS/body weight per d over 4 months, and no adverse effects were observed (Genta et al. Reference Genta, Cabrera, Grau and Sanchez2005). Since yacon roots are a rich source of FOS, they may potentially be used as a novel source of prebiotic components in diet supplements. Moreover, yacon roots may be an alternative to more traditional fructan sources such as Chicorium intybus and Helianthus tuberosus.
In the present study, we have evaluated the effects of supplementing a diet with fructan-rich yacon flour on Ca and Mg intestinal absorption and balance as a result of its caecum fermentation in rats. We then assessed its effects on bone mineral density (BMD) and content and femur biomechanical properties as a measurement of mineral bioavailability. To establish the possible relation of mineral absorption and caecum enlargement, morphometry of the caecal mucosa (number of crypts, crypt depth and bifurcated crypts) was investigated.
Materials and methods
Plant material
Approximately 70 kg yacon tuberous roots from São Sebastião Farm, in the city of Ibiúna (São Paulo, Brazil) were harvested 8 months after cultivation and stored for 2 d at room temperature and humidity, under dim light. They were then washed with flushing water, kept in cotton-fibre bags and autoclaved (121 °C, 20 min), for inactivation of fructan hydrolases and phenol oxidases present in Asteraceae species (Itaya et al. Reference Itaya, Carvalho and Figueiredo-Ribeiro2002). The autoclaved samples were blended, freeze-dried (Liotécnica Ind. Com. Ltda, Embu, São Paulo, Brazil) and powdered to obtain the flour.
Animals and diets
Twenty-four 5-week-old male Wistar rats, initially weighing 65–70 g, were obtained from the colony of the Faculty of Pharmaceutical Sciences, University of São Paulo. The experimental protocol was approved by the Commission on Ethics in Animal Experiments of the Faculty of Pharmaceutical Sciences of the University of São Paulo, according to the guidelines of the Brazilian College on Animal Experimentation. All rats were housed in individual stainless-steel wire-mesh cages under a controlled temperature (22 ± 2 °C) with a 12 h light–dark cycle (lights on 07·00–19·00 hours). The experimental diets supplemented with yacon flour were modified from AIN-93G (Reeves et al. Reference Reeves, Nielsen and Fahey1993; Table 1) and presented final FOS concentrations of 5 % (50 g/kg diet) and 7·5 % (75 g/kg diet). The amounts of FOS and soluble sugars (glucose, fructose, sucrose) present in the yacon flour were subtracted from those of sucrose and starch. The control and FOS diets were formulated in order to provide 7·5 g Ca/kg diet. The following factors were used for energy calculations: 4, for carbohydrates and proteins, 9 for lipids and 1 for fructans (Roberfroid et al. Reference Roberfroid, Gibson and Delzenne1993).
Table 1 Composition (g/kg) of the experimental diets
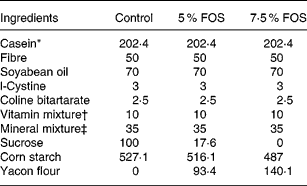
FOS, fructooligosaccharides.
* Casein protein = 92·94 % (N × 6·25).
† AIN-93-VX vitamin mixture (Reeves et al. Reference Reeves, Nielsen and Fahey1993).
‡ Modified from a mineral mixture (AIN-93G-MX; Reeves et al. Reference Reeves, Nielsen and Fahey1993) supplying (g/kg mixture): CaCO3, 538·18; KH2PO4, 196; NaCl, 74; K2SO4, 46·6; K3C6H5O7.H2O, 70·78; MgO, 24; ferric citrate, 6·06; ZnCO3, 1·65; MnCO3, 0·63; CuCO3, 0·3; KIO3, 0·01; Na2SeO4, 0·01 025; ammonium tetra-hydrated paramolybdate, 0·00 795; Na2SiO3, 1·45; KCr(SO4)2.12H2O, 0·275; H3BO3, 0·0815; NaF, 0·0635; NiCO3, 0·0318; LiCl, 0·0174; NH4VO3, 0·0066; sucrose, 39·846.
Experimental design
All animals were fed the same control diet for 5 d and thereafter either the control or the FOS diets for 27 d. The food intake was determined daily and body weight recorded every 2 d. Food and demineralized water were offered ad libitum. The feed efficiency was determined as the weight gain/g food intake at the end of the experiment. Faeces and urine were collected along three periods of 5 d (starting on the 4th, 10th and 16th days of experiment), pooled according to the collection period and stored at − 20 °C.
At the end of the experiment, the rats were fasted for 12 h before being killed. The animals were anaesthetized with a 1:2 (v/v) mixture of ketamine (10 mg/kg) and xylazine (25 mg/kg) and their livers, spleens and kidneys were removed, rinsed in saline solution (0·85 % NaCl, w/v) and weighed. The caecum was removed with its contents, weighed, placed on ice (Lu et al. Reference Lu, Gibson, Muir, Fielding and O'Dea2000) and cut open along the small curvature. Aliquots of the contents were frozen at − 20 °C and tissue samples were fixed in Bouin's reagent (picric acid–formalin–glacial acetic acid, 75:2:15, by vol.) and stored in 70 % ethanol. The hind limbs were removed and the bones were defleshed, weighed and stored at − 20 °C until analysis.
Chemical composition of yacon flour and experimental diets
The following components were determined: moisture content, ash and total lipids (Instituto Adolfo Lutz, 1995); protein by micro-Kjeldahl method (Association of Official Analytical Chemists, 1995) (conversion factor of 6·25) and soluble and insoluble DF by enzymatic-gravimetric method, using 2-(4-morpholino)ethanesulphonic acid–Tris buffer (Lee et al. Reference Lee, Prosky and De Vries1992). Fructans and soluble sugars (glucose, fructose and sucrose) were determined by an adaptation of the Hoebregs method (Hoebregs, Reference Hoebregs1997). In this method the sample is enzymatically treated with lyophilized amyloglucosidase (A-7420; Sigma Chemical Co., St Louis, MO, USA) and inulinase (Fructanase Mixture, MegazymeTM, Bray, Ireland; cat. E-FRMXLQ), and the released sugars are determined by anion-exchange chromatography with pulsed amperometric detection. The hydrolysis by amyloglucosidase was not performed due to the low starch contents in the yacon roots (Capito, 2001). Ca and Mg were measured by atomic absorption spectrophotometry (Polarized Zeeman AAS, Hitachi Z-5000; Hitachi, Tokyo, Japan), employing a hollow cathode lamp at 422·7 and 202·6 nm and slits of 0·7 and 1·3 nm, respectively, after wet digestion (HNO3–H2O2, 5:1, v/v) and addition of 0·1 % (w/v) lanthanum as La2O3. The working standard solutions were prepared by diluting CaCl2 (Titrisol; Merck) and MgCl2 (Tritisol; Merck, Darmstadt, Germany).
Mineral balance
Dry faeces were milled and the powdered samples, as well as urine samples, were utilized for Ca and Mg analyses. Apparent absorption and balance were calculated by the following equations:
Caecal pH and histology
Caecal contents were centrifuged at 11 000 g in two 30 min cycles and the pH measured with a pH test strip (Merck) in the supernatant; moisture was determined in an aliquot of the remaining caecal contents. For histological examination, the tissue fixed fragments were included in paraffin and approximately 5 μm-thick cuts were obtained and stained with haematoxylin and eosin. Only crypts cut lengthwise were used to measure the depth with the crypts divided into arbitrary zones according to the lines of an integrating ocular (Zeiss No. 2). These lines limited spaces or zones which enabled us to calculate the number of spaces within each crypt. Each space was then taken as a standard measurement. At least thirty crypts per animal were used. For the determination of the number of bifurcating crypts, the crypts with an indentation at the base or presenting longitudinal fission (one crypt mouth and two bases) were considered (Maskens, Reference Maskens1978). The calculation was carried out by determining the number of the bifurcating crypts per microscopic field, considering the same criteria used in the measure of the depth of the crypts. To estimate the total number of crypts per microscopic field, also the obliquely sectioned crypts were considered. At least twenty microscopic fields per animal were analysed.
Bone mineral content, density and biomechanics
Ca and Mg concentrations in previously dried tibia and femur (105 °C, 12 h) of the left limb were determined. BMD of the right femur and tibia was measured by dual-energy X-ray absorptiometry (Freitas et al. Reference Freitas, Moriscot, Jorgetti, Soares, Passarelli, Scanlan, Brent, Bianco and Gouveia2003), using a pDEXA Sabre Densitometer and the pDEXA Sabre software, version 3.9.4 (Norland Medical Systems, Fort Atkinson, WI, USA), both designed for small animals. The regional difference was estimated by evaluation of the regional BMD (25 % proximal, 50 % middle (midshaft) and 25 % distal of each bone). After analyses, the bones were again stored at − 20 °C until biomechanical strength tests were carried out. The storage at − 20 °C does not alter the mechanical properties of the bones (Sedlin & Hirsch, Reference Sedlin and Hirsch1966).
The mechanical properties were evaluated in the mid-diaphyseal region of the right femurs using a three-point bending test (Shimano et al. Reference Shimano, Shimano and Volpon2002) and loading continued to failure, through a TA-TX2 texture analyser coupled to the Texture Expert software, version 1.2 (Stable Micro Systems, Haslemere, UK). For the assay, the bones were thawed at room temperature and rehydrated in saline solution. The bone length (measured from the femur head to the condilus) and diameter (measured in the mid-diaphyseal region) were measured by a caliper. The centre of the bones was fractured under the following conditions: 16 mm sample space, 0·2 mm/s plunger speed and 25 kg load range.
Statistical analysis
Results are expressed as means with their standard deviations and analysed considering a 5 % significance level. The statistical model used was the ANOVA (Neter et al. Reference Neter, Kutner, Natchsheim and Wasserman1996) under a completely crossed planning, where the treatments were defined by the combination of period and group factors and all measurements of period factor repeated. As the effects between group and period factor were not significant (P>0·05), new models without interactions were adjusted. In order to study the variables measured at the end of the experiment, ANOVA with two levels in the group factor were carried out. All statistical analyses were carried out using SPSS for Windows, version 11.5 (SPSS, Chicago, IL, USA).
Results
Chemical composition of yacon flour and experimental diets
Yacon flour contains FOS (55·3 %) as the main storage sugar and also features lower glucose, fructose and sucrose contents (8·9, 13·5 and 13·4 %, respectively), as well as soluble and insoluble DF (3 and 7 %, respectively) (Table 2). The glucose, fructose, sucrose and fructans concentrations in yacon flour were used to calculate the amounts of ingredients in the experimental diets. As considerable amounts of free sugars were observed in the yacon flour, they were subtracted from the total amount of sucrose (5 and 7·5 % FOS groups) or starch (7·5 % FOS group) to be added to the experimental diets. Low contents of Ca and Mg were found in yacon flour (Table 2) and thus the addition of the flour did not significantly alter the content of these minerals in the diets. Chemical analysis of the diets showed Ca and Mg concentrations of 12 170, 11 780 and 12 700 mg Ca/kg, and 480, 520 and 590 mg Mg/kg, in the control, 5 % FOS and 7·5 % FOS diets, respectively. The energy values (kJ/g) of the diets were 16·5, 15·4 and 14·9, in the control, 5 % FOS and 7·5 % FOS diets, respectively.
Table 2 Composition (g/100 g) of the yacon flour*(Mean values and standard deviations)
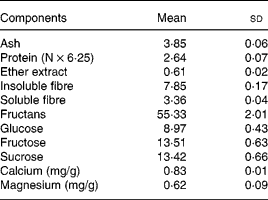
* n 3 except for insoluble and soluble fibre, n 4; fructans, glucose, fructose and sucrose, n 6.
Body weight gain, feed intake and efficiency, and weight of organs
The consumption of the yacon flour-supplemented diets did not affect the growth and development (P>0·10) of the animals during the experimental period (27 d), as the mean feed efficiencies were similar (Table 3). Statistically significant differences (P < 0·05) in the total feed intake and in spleen, liver and kidney relative mean weights (g/100 g body weight) between the experimental groups were not observed. The animals fed diets supplemented with yacon flour showed no gastrointestinal disturbances.
Table 3 Body weight gain, food intake, faecal output and caecal parameters in rats fed a control diet and yacon flour-supplemented diets*(Mean values and standard deviations, n 8)

FOS, fructooligosaccharides.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures, see p. 777–778.
Effects on faecal output and caecal parameters
Table 3 shows the results for dry weight and water content of faeces collected within three experimental periods. A significant increase (P < 0·01) in the faeces mean dry weight was observed during the three experimental periods in animals fed yacon flour, when compared to the control group, although the results have not shown any differences between the FOS groups. Yacon flour-supplemented diet intake resulted in an increase (P < 0·05) in the percentage of water content in the faeces relative to the animals fed the control diet in the three predetermined periods along the experiment.
Yacon flour-fed animals had lower caecal wall and caecal content weights (wet weight in g/100 g body weight) than those fed control diet (P < 0·01). The increase in the wet weight of caecal contents was accompanied by increases in moisture and residue weight obtained after centrifugation of the caecal contents (+57 and +68 %, for the 5 and 7·5 % FOS groups, respectively, P < 0·05) compared to the control group (Table 3). No significant differences in the pH values were observed in the supernatant of the caecal contents among the groups. Diet supplementation with 7·5 % FOS significantly increased (P < 0·05) the number and depth of caecal crypts compared to the control group (Fig. 1). Furthermore, these effects were concomitant with a greater number of bifurcating crypts in animals fed yacon flour (5 and 7·5 % FOS groups) when compared to the control group (Fig. 2).

Fig. 1 The photographs are histological sections of caecal mucosa from rats fed a control diet and yacon flour-supplemented diets (5 and 7·5 % fructooligosaccharides (FOS)) (haematoxylin and eosin; scale bars = 140 μm). (A), Number of crypts per microscopic field; (B), crypt depth (arbitrary units). For details of procedures, see p. 778. Values are means with their standard deviations depicted by vertical bars (n 8). a,bMean values with unlike superscript letters were significantly different (P < 0·05).

Fig. 2 The photograph is a histological section showing a crypt in the bifurcation process (arrow) (haematoxylin and eosin; scale bar = 140 μm). The graph shows bifurcating crypts per microscopic field (%) in the caecum from rats fed a control diet and yacon flour-supplemented diets (5 and 7·5 % fructooligosaccharides (FOS)). For details of procedures, see p. 778. Values are means with their standard deviations depicted by vertical bars (n 8). a,bMean values with unlike superscript letters were significantly different (P < 0·05).
Intestinal absorption and balance of calcium and magnesium
The apparent Ca absorption in the yacon flour groups was statistically higher (P < 0·05) than that from the control group in all experimental periods, and this difference increased from approximately 51 % in the first period to 56 % in the last (Fig. 3(A)). The Ca balance of the FOS groups was statistically different (P < 0·05) from that of the control group (Fig. 3(B)). No significant differences were observed in the Mg apparent absorption among the experimental groups (Fig. 3(C)). However, the 7·5 % FOS group showed a significantly higher Mg balance when compared to the control group (Fig. 3(D)).

Fig. 3 Calcium (A, B) and magnesium (C, D) intestinal absorption and balance of rats fed a control diet (□) and yacon flour-supplemented diets (■, 5 % fructooligosaccharides (FOS);
![]() , 7·5 % FOS). For details of procedures, see p. 778. Values are means with their standard deviations depicted by vertical bars (n 8). a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05).
, 7·5 % FOS). For details of procedures, see p. 778. Values are means with their standard deviations depicted by vertical bars (n 8). a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05).
Effects on bone parameters
No significant differences were observed in the dry weight, length and diameter of femur (Table 4) among the experimental groups. On the other hand, yacon flour corresponding to 7·5 % FOS in the diet caused a statistically significant increase (P < 0·05) in Ca concentration in femur and tibia, when compared to the control group (Fig. 4). No significant differences were observed in Mg concentrations in femur and tibia between the experimental groups (117 (sd 23), 106 (sd 7) and 109 (sd 8) μmol/g, in dry femur, and 120 (sd 16), 107 (sd 11) and 111 (sd 8) μmol/g, in dry tibia, for the control, 5 % FOS and 7·5 % FOS groups, respectively). BMD was evaluated ex vivo using dual-energy X-ray absorptiometry. The animals which consumed FOS presented no statistically significant difference in BMD (P < 0·05) when compared to those from the control group. Table 4 presents results concerning the mechanical properties evaluated in the midshaft region of the femur. An increase in all studied parameter values was observed (peak load, yield load, stiffness, resilience and absorbed energy) in the FOS groups in comparison to the control group, but only values regarding peak load and stiffness presented statistically significant differences (P < 0·05) between the experimental groups.
Table 4 Biomechanical properties of femur in rats fed a control diet and yacon flour-supplemented diets*(Mean values and standard deviations, n 8)
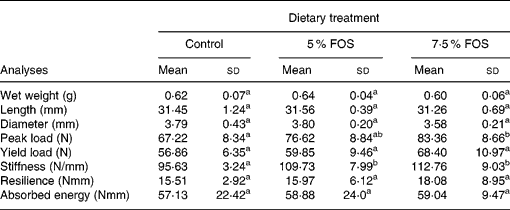
FOS, fructooligosaccharides.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures, see p. 778.

Fig. 4 Calcium concentration in femur and tibia of rats fed a control diet (□) and yacon flour-supplemented diets (■, 5 % fructooligosaccharides (FOS);
![]() , 7·5 % FOS). For details of procedures, see p. 778. Values are means with their standard deviations depicted by vertical bars (n 8). a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
, 7·5 % FOS). For details of procedures, see p. 778. Values are means with their standard deviations depicted by vertical bars (n 8). a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
Discussion
The present results showed that the consumption of yacon flour containing FOS stimulated apparent Ca intestinal absorption as well as Ca and Mg balance, leading to higher bone Ca content and a greater mechanical resistance of the femur at the mid-diaphysis, as evaluated by the peak load and stiffness variables, in comparison to the control group. In addition, an enlargement of the caecum was observed, as demonstrated by increases in the number and depth of caecal crypts, as well as in the number of bifurcated crypts, suggesting an increase in the absorptive surface area of the caecum and probably contributing to mineral absorption. In this context, the positive effects of fructans (FOS and inulin) consumption on mineral absorption in the large intestine have been broadly studied using different experimental protocols (Ohta et al. Reference Ohta, Baba, Takizawa and Adachi1994a , Reference Ohta, Ohtsuki, Takizawa, Inaba, Adachi and Kimura b , Reference Ohta, Ohtsuki, Baba, Takizawa, Adachi and Kimura1995, Reference Ohta, Motohashi, Ohtsuki, Hirayama, Adachi and Sakuma1998; Kruger et al. Reference Kruger, Brown, Collett, Layton and Schollum2003; Zafar et al. Reference Zafar, Weaver, Zhao, Martin and Wastney2004; Raschka & Daniel, Reference Raschka and Daniel2005). However, to our knowledge, this is the first report about the effects of yacon FOS on mineral (Ca and Mg) absorption and bone Ca retention.
The presence of food in the intestine is the most important determinant for the stimulation of cell proliferation, considering that the lack of nutrients in the intestinal lumen (as seen in total parenteral nutrition cases) or the ingestion of DF-free diets leads to atrophy of the mucosa (Wong & Wright, Reference Wong and Wright1999). This atrophy can be reduced by the presence of some type of DF in the diet (Goodlad & Wright, Reference Goodlad and Wright1983). Fructans share some properties with DF such as non-digestibility in the small intestine and fermentability in the large intestine (Schneeman, Reference Schneeman1999) and particular attention has been given to their ability to stimulate the selective growth of some bacterial species, considered beneficial to the host (Roberfroid et al. Reference Roberfroid, Van Loo and Gibson1998). Pedreschi et al. (Reference Pedreschi, Campos, Noratto, Chirinos and Cisneros-Zevallos2003) demonstrated in vitro that yacon FOS have the potential to be fermented by bifidobacteria and lactobacilli species and they classified it as a novel source of prebiotics. In the present study, feeding rats with yacon flour containing FOS induced a significant increase in the caecal wall and contents (Table 3), probably as a result of the increase in biomass due to a greater fermentation in the caecum. Furthermore, this trophic effect has been believed to be mediated by SCFA produced as a result of the increase in the metabolic activity of the intestinal microflora (Rémésy et al. Reference Rémésy, Levrat, Gamet and Demigné1993; Campbell et al. Reference Campbell, Fahey and Wolf1997). Among these SCFA, butyrate has been recognized as the main source of energy for the colonocytes, promoting the health and integrity of the caecal/colonic mucosa (Le Blay et al. Reference Le Blay, Michel, Blottière and Cherbut1999).
Feeding rats with 7·5 % FOS diet resulted in a significant increase (P < 0·05) in the number and depth of caecal crypts (Fig. 1). Moreover, 5 % FOS in the diet was enough to provide a very high incidence of bifurcating crypts in the caecum (Fig. 2). Other studies also have shown a change in the length and density of crypts after consumption of fermentable carbohydrates (Rémésy et al. Reference Rémésy, Levrat, Gamet and Demigné1993; Howard et al. Reference Howard, Gordon, Garlab and Kerley1995). McCullough et al. (Reference McCullough, Ratcliffle, Mandir, Carr and Goodlad1998) demonstrated that fermentable fibres increased both the number of crypts per circumference and the number of branched crypts in the proximal colon of rats. It has been proposed that increased crypt size is the major stimulus for crypts to divide (Totafurno et al. Reference Totafurno, Bjerknes and Cheng1987). Small indentations are observed in the base of a crypt, which then ascend longitudinally and proceed until there are two separate crypts (Totafurno et al. Reference Totafurno, Bjerknes and Cheng1987), a process thought to be related to expansion of crypt stem cell population (Bjerknes & Cheng, Reference Bjerknes and Cheng2005). This process is increased in postnatal development and during recovery of the intestine from injury (Maskens, Reference Maskens1978; Cheng et al. Reference Cheng, Araki, Furuya, Matsuoka, Mashima, Kobayashi and Matsuura2000). In addition, the production of new crypts by fission can provide an alternative or additional means of increasing tissue mass (McCullough et al. Reference McCullough, Ratcliffle, Mandir, Carr and Goodlad1998).
Caecal pH, which is considered an indirect result of SCFA production, was not affected by yacon flour consumption in the present experimental conditions. Furthermore, the values were higher than those reported in the literature as a response to bacterial fermentation of fructans and other fermentable carbohydrates (Levrat et al. Reference Levrat, Rémésy and Demigné1991; Campbell et al. Reference Campbell, Fahey and Wolf1997; Lu et al. Reference Lu, Gibson, Muir, Fielding and O'Dea2000). Wolf et al. (Reference Wolf, Firkins and Zhang1998), after feeding growing rats with different dietary FOS concentrations for 27 d, attributed the high caecal pH values at the end of their experiment to the high buffering ability of the AIN-93G diet. In the present study, animals were deprived of food for 12 h before being killed. According to Le Blay et al. (Reference Le Blay, Michel, Blottière and Cherbut2003), SCFA may vary considerably depending on the period between the last meal and the moment of sample collection, which might thus explain the more elevated pH values observed in the present study. Finally, centrifugation of caecum contents may have contributed to the volatilization of SCFA present in the samples, thus affecting the pH of the soluble phase after processing (Lu et al. Reference Lu, Gibson, Muir, Fielding and O'Dea2000).
The present results showed that the intake of yacon FOS increased Ca apparent intestinal absorption and balance in comparison to the control group, as evaluated in three periods of 5 d each (Fig. 3). Studies have demonstrated the effects of fermentable carbohydrates on Ca absorption, although some conditions like short-term or long-term consumption of these carbohydrates, mineral and carbohydrate levels or their ratio in the diet may affect their ability to promote mineral absorption. Levrat et al. (Reference Levrat, Rémésy and Demigné1991) evaluated the effects of different inulin concentrations (5, 10 and 20 % in the diets) on apparent Ca absorption in rats and observed a dose–response pattern. In another study, Coudray et al. (Reference Coudray, Feillet-Coudray, Tressol, Gueux, Thien, Jaffrelo, Mazur and Rayssiguier2004) evaluated the impact of dietary Ca levels (0·25, 0·50 or 0·75 %) on the stimulatory effect of inulin on Ca absorption and demonstrated that Ca absorption efficiency was inversely correlated with dietary Ca intakes and that the highest effect of inulin was observed in the group receiving the lowest dietary Ca level. Contrarily to the effect on Ca absorption, yacon flour consumption did not affect Mg absorption, despite the significant increase in Mg balance observed in the 7·5 % FOS group. In this respect, studies have been observed that an elevated Ca ingestion may negatively affect Mg absorption (Couzy et al. Reference Couzy, Keen, Gershwin and Marechi1993). Miura et al. (Reference Miura, Matsuzaki, Suzuki and Goto1999) observed a significant (P < 0·05) decrease in the Mg apparent absorption and serum and femur concentrations in growing rats after ingestion of a 1·5 % Ca diet. In the present study, Mg urinary excretion in the control animals was numerically higher (data not shown) than that in the rats consuming a 7·5 % FOS diet, which at last led to a higher Mg retention in the FOS group.
Previous studies have shown that the consumption of fructans not only stimulates mineral absorption in the large intestine, but also increases bone mineral retention (Kruger et al. Reference Kruger, Brown, Collett, Layton and Schollum2003; Zafar et al. Reference Zafar, Weaver, Zhao, Martin and Wastney2004; Nzeusseu et al. Reference Nzeusseu, Dienst, Haufroid, Depresseux, Devogelaer and Manicourt2006). The present results showed that the consumption of 7·5 % yacon FOS caused a significant increase (P < 0·05) in Ca concentration both in femur and in tibia (Fig. 4), as well as a higher BMD when compared to the bones from the control group, although BMD values have not reached statistical significance. Using growing healthy rats, Kruger et al. (Reference Kruger, Brown, Collett, Layton and Schollum2003) observed a significant increase (P < 0·05) in femur BMD after inulin ingestion (DP>23) for 4 weeks, although this effect had not been observed after the consumption of oligofructose (DP between 2 and 8). Whole-body BMD was altered only after 22 weeks in healthy growing rats fed diets supplemented with 5 % and 10 % inulin and 0·2 %, 0·5 % or 1 % Ca (Roberfroid et al. Reference Roberfroid, Cump and Devogelaer2002). In a recent study, Nzeusseu et al. (Reference Nzeusseu, Dienst, Haufroid, Depresseux, Devogelaer and Manicourt2006) verified a greater increase in the area and BMD of the cancellous bone of the proximal tibia and vertebra in growing rats fed inulin than in those fed oligofructose after 3 months, as assessed by peripheral quantitative computerized tomography measurements. The mechanisms involved in the modulatory effects of fructans on bone metabolism have been discussed. Zafar et al. (Reference Zafar, Weaver, Zhao, Martin and Wastney2004) observed that the significant increase in Ca intestinal absorption in rats consuming fructans decreased the bone turnover through suppression of osteoclast reabsorption. In growing rats consuming inulin for 4 weeks, Kruger et al. (Reference Kruger, Brown, Collett, Layton and Schollum2003) observed a significant decrease in urinary excretion of fragments of type 1 collagen, a biochemical marker for bone reabsorption. The anti-resorptive effect of fructans was also confirmed by Nzeusseu et al. (Reference Nzeusseu, Dienst, Haufroid, Depresseux, Devogelaer and Manicourt2006), who demonstrated a significant decrease in serum levels of C-telopeptide.
The resistance of a composite material like bone may be evaluated through the construction of a load × deformation curve, where most of the load applied to the bone is supported by the mineral phase (Shimano et al. Reference Shimano, Shimano and Volpon2002). Bone breaking strength measures are simple to perform and can directly determine loads required to cause fracture (Shimano et al. Reference Shimano, Shimano and Volpon2002). In the present study, a three-point bending test was utilized for evaluating some mechanical properties in the mid-diaphyseal region of the femur, a region essentially comprised of cortical bone. A trend towards an increase of all properties studied was detected in yacon FOS groups, although only the 7·5 % FOS group differed from the control group regarding the peak load and stiffness variables, providing a positive influence of yacon flour on resistence and rigidity of femoral diaphyses. Nevertheless, it should be kept in mind that bone strength also depends on the architectural disposition of bone material (bone geometry parameters, e.g. cross-sectional area, moment of inertia, cortical thickness) and other mineralization unrelated factors such as crystal arrangement and size, the degree of collagen cross-linking, and the amount and distribution of tissue microdamage (Burr, Reference Burr2002). In this view, the previous results of our laboratory showed an increase in the peak and yield load of femur in rats fed 5 % FOS-supplemented diets, suggesting an influence of FOS on organic component (Lobo et al. Reference Lobo, Colli and Filisetti2006).
In summary, the present study showed a stimulatory effect of yacon FOS on Ca intestinal absorption and balance, resulting in a higher bone Ca content and stronger bone structural properties in the femoral midshaft of growing rats. Moreover, we speculate whether the enlargement of the caecal wall caused by bifurcating crypts might have contributed to the increased mineral absorption in the animals fed fructans. This aspect, of course, needs further research. More accurate studies expanding the present findings on bone architecture and intestinal physiology open new perspectives for the use of yacon flour as an ingredient in functional foods, as well as other fructan-containing crops. Clinical trials should be performed in order to verify the effect of yacon consumption on man.
Acknowledgements
Part of this work has been presented in abstract form: FASEB Journal (2004) 18, A150. We thank Anita Hagy (in memoriam) for supplying of the yacon tuberous roots; Dr Cecília Gouveia for her collaboration in the BMD analyses; Dr José Alfredo Gomes Arêas, Dr Luiz Antonio Gioielli and Dr Chiu Chi Ming for their collaboration in the bone biomechanical assays; Fundação de Amparo à Pesquisa do Estado de São Paulo (research project 02/13 407-7) for supporting the research, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for the master's fellowship awarded to Alexandre Rodrigues Lobo. We also thank Álvaro Augusto Feitosa Pereira for the review of the manuscript.










