INTRODUCTION
Sex chromosome aneuploidies (SCAs), genetic disorders characterized by an abnormal number of X and/or Y chromosomes, occur collectively at a rate of approximately 1/400 births (Nielsen & Wohlert, Reference Nielsen and Wohlert1991). While several SCA variants exist, Klinefelter (47,XXY), Trisomy X (47,XXX), and 47,XYY syndromes are the most common. Prior research has demonstrated that individuals with SCAs are at increased risk for experiencing impairments on verbal fluency tasks (see discussion below). These tasks, which are among the most frequently administered in neuropsychology (Ardila, Ostrosky-Solis, & Bernal, 2006), require individuals to rapidly state words within a specific time frame that either fit into a semantic category (semantic fluency) or start with a particular letter (phonemic fluency) while simultaneously following a set of rules. Successful completion of these tasks requires that individuals have a well-developed, interconnected lexicon and use effective strategies for identifying as many novel words as possible while not repeating previously stated words. Thus, these tasks tap into multiple cognitive domains (Unsworth, Spillers, & Brewer, 2010; Henry, Messer, & Nash, Reference Henry, Messer and Nash2015) and may provide insight into impaired cognitive processes associated with different SCAs.
One cognitive domain that is relevant for verbal fluency tasks (Whiteside et al., Reference Whiteside, Kealey, Semla, Luu, Rice, Basso and Roper2016) and is an area of challenge for individuals with SCAs (for review, see Leggett, Jacobs, Nation, Scerif, & Bishop, Reference Leggett, Jacobs, Nation, Scerif and Bishop2010) is language. Other cognitive domains of interest include episodic memory, processing speed, and different executive functions (e.g., working memory, initiation; Strauss, Sherman, & Spreen, Reference Strauss, Sherman and Spreen2006). This latter set of skills, executive functions, are also reported to be impaired youth with SCAs (Lee et al., Reference Lee, Wallace, Clasen, Lenroot, Blumenthal, White and Giedd2011, Reference Lee, Anand, Will, Adeyemi, Clasen, Blumenthal and Edgin2015; Ross, Zeger, Kushner, Zinn, & Roeltgen, Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009). As verbal fluency has been found to be associated with important functional outcomes in other clinical populations (activities of daily living: Cahn-Weiner, Boyle, & Malloy, Reference Cahn-Weiner, Boyle and Malloy2002; social abilities: Bowie et al., Reference Bowie, Leung, Reichenberg, McClure, Patterson, Heaton and Harvey2008; and academics: Daneman, Reference Daneman1991; Nathan & Abernathy, Reference Nathan and Abernathy2012), further research into the nature of fluency impairments in different SCAs may shed light on cognitive skills that are related to real-world outcomes in these groups.
Few studies have characterized verbal fluency performance in youth with supernumerary SCAs. However, limited research indicates that the presence of a single supernumerary X chromosome is adversely related to performance on phonemic and semantic fluency in females with 47,XXX (Bender, Linden, & Harmon, Reference Bender, Linden and Harmon2001; Bender, Linden, & Robinson, Reference Bender, Linden and Robinson1989). Similarly, the addition of an X chromosome in males with 47,XXY has been found to be associated with poorer verbal fluency performance in some studies (phonemic: Bender et al., Reference Bender, Linden and Robinson1989; Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009; semantic: Bender et al., Reference Bender, Linden and Harmon2001). Still, inconsistency in the 47,XXY literature exists, as some studies have failed to find differences between males with 47,XXY and controls (semantic: Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009). Research on verbal fluency in males with a supernumerary Y chromosome is scarce. The one published study reported impaired phonemic but not semantic fluency performance in males with 47,XYY relative to typical controls (Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009). However, effect sizes for both conditions were large suggesting more general verbal fluency impairments.
Despite preliminary evidence that individuals with SCAs present with impaired verbal fluency, most studies have been limited to youth with SCA trisomies. No studies have examined verbal fluency across the full spectrum of supernumerary SCAs, including youth with tri-, tetra-, and pentasomies. Moreover, existing studies have not consistently contrasted how phonemic and semantic fluency performance varies as a function of an additional X or Y chromosome.
Thus, the current research aimed to fill these gaps in the literature by answering the following questions:
1. How does the presence of an extra X chromosome impact fluency performance, and does performance worsen with increasing supernumerary X chromosome number? Consistent with prior research (e.g., Lee et al., Reference Lee, Wallace, Clasen, Lenroot, Blumenthal, White and Giedd2011), it was hypothesized that the presence of an extra X chromosome would be associated with an overall decrement in verbal fluency performance. Moreover, greater impairments with each additional X chromosome were anticipated, consistent with research on supernumerary X effects on overall cognitive (Polani, Reference Polani1977) and language (Lee et al., Reference Lee, Wallace, Adeyemi, Lopez, Blumenthal, Clasen and Giedd2012) functioning.
2. How does the presence of an extra Y chromosome impact verbal fluency performance? Consistent with prior research (Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009), we anticipated that the presence of an extra Y chromosome would be associated with a decrement in verbal fluency performance.
3a. Are there differences in the effects of an extra X versus Y on verbal fluency performance among males; 3b. Does the presence of both an extra X and an extra Y chromosome (48,XXYY) result in greater impairments in verbal fluency than the presence of an extra X (47,XXY) or extra Y (47,XYY) in isolation? Because prior research has documented somewhat different cognitive profiles for those with supernumerary X versus Y SCAs (Lee et al., Reference Lee, Wallace, Adeyemi, Lopez, Blumenthal, Clasen and Giedd2012; Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009), we hypothesized that there would be differential X and Y chromosome effects on verbal fluency. However, given inconsistencies in the literature, it was difficult to predict whether an extra X or Y would impact phonemic versus semantic fluency more or less. Lastly, we anticipated that males with both an additional X and additional Y would be more impaired than those with an extra X or Y in isolation, given that prior research indicates that increasing sex chromosome number is associated with increasing impairment (Lee et al., Reference Lee, Wallace, Adeyemi, Lopez, Blumenthal, Clasen and Giedd2012; Polani, Reference Polani1977).
METHOD
Participants
Seventy-nine youth with SCA participated in this study as part of a larger research program conducted at the National Institute of Mental Health (NIMH) Intramural Research Program. These youth were recruited from parent organizations and by means of the NIMH Web site. Inclusion criteria for the SCA group were as follows. Participants must have (a) had a supernumerary SCA diagnosis confirmed by karyotype, and (b) been free of a comorbid neurological disorder or acquired head injury (e.g., TBI, hydrocephalus) that would impact gross brain development.
In this study, SCA groups were divided based on the number of extra X or Y chromosomes rather than by karyotype. As such, the +1X group included those with 47,XXX and 47,XXY. Because of the rarity of tetra- and pentasomy SCAs, youth with 2 and 3 additional X chromosomes were combined into one group, which we refer to as the +2/3X group. This group consisted of individuals with 48,XXXX, 48,XXXY, and 49,XXXXY. The +1Y group included males with 47,XYY, and +1X,+1Y group included males with 48,XXYY. See Table 1 for demographic information.
Table 1 Demographic information, IQ, and verbal fluency scores by study group and karyotype
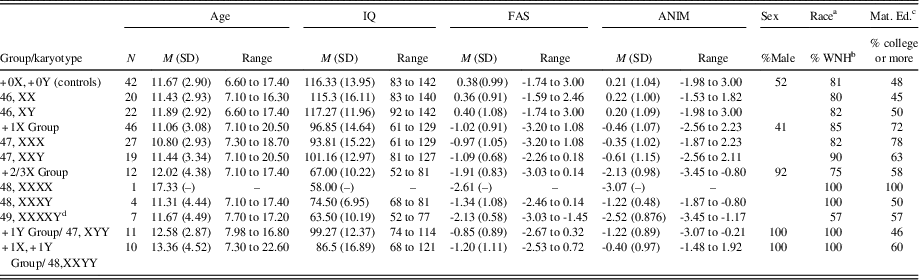
a The ethnic/racial composition of the SCA group was as follows: white, non-Hispanic (87.3%), black (1.3%), white, Hispanic (5.1%), Asian (2.5%), and other (3.8%); The ethnic and racial composition for the control group was as follows: white, non-Hispanic (81%), black (7.1%), white, Hispanic (7.1%), Asian (2.4%), and other (2.4%).
b White, non-Hispanic.
c Maternal Education: percent with college education or higher.
d IQ data were missing for one participant with 49, XXXXY.
Before grouping males and females with supernumerary X chromosomes together for analyses, we compared the performance of the 47,XXY (n = 29) and 47,XXX (n = 27) groups on verbal fluency measures. Sex differences were not found on phonemic or semantic fluency (Phonemic: 47,XXY M = -1.09 ± .68; 47,XXX M = -.97 ± 1.05; [t < 1; p > .6]; Semantic: 47,XXY M = -.61 ± 1.15; 47,XXX M= -.35 ± 1.02; [t < 1; p > .4]). This group comparison could not be made for those with +2/3X, as there was only one female in this group (who had 48,XXXX). Thus, for parsimony in analyses, males and females with supernumerary X chromosomes were combined into groups.
Participants in the SCA sample included those with prenatal and postnatal diagnoses: +1X (70% prenatal, 30% postnatal), +2/3X (100% postnatal), +1Y (46% prenatal, 54% postnatal), and +1X,+1Y (100% postnatal). Given the subtle physical and cognitive phenotype associated with sex chromosome trisomies, youth with these disorders can go undiagnosed (Bojesen, Juul, & Gravholt, Reference Bojesen, Juul and Gravholt2003). Consequently, youth with postnatal diagnoses may present with more severe impairments than those with prenatal diagnoses, as these impairments may have led to their eventual diagnosis. To examine this possibility, analyses that included youth with +1X or +1Y were re-run with just the prenatal subsample to ensure consistency in findings. Results were largely the same. Thus, study findings are from analyses of youth with both pre- and postnatal diagnoses.
Because the youth with tetra- and pentasomies in our sample all had postnatal diagnoses, we could not complete analogous analyses with a prenatal sample only. However, we were less concerned about ascertainment bias in these groups, particularly in those with +2/3X group, as these SCA variants tend to be associated with greater physical dysmorphology and cognitive impairments. Thus, they are less likely to go undetected by medical providers.
A control group of 42 typically developing (TD) males and females who were part of other studies at the National Institutes of Health (NIH) and recruited with the help of the NIH Healthy Volunteer office were also included. This group, referred to as the +0X,+0Y group, was matched to the SCA group on age, racial/ethnic background, and maternal education. Inclusion criteria for this group included being free of any psychiatric, neurological, and/or learning disorders.
Information regarding age, sex ratio, IQ, maternal education, and racial composition of the groups is summarized in Table 1. As desired, groups did not differ on age (p > .25), maternal education (% ≥ college education; p > .18), or racial composition (%White, non-Hispanic; p > .26). Groups did, however, differ on IQ (F[4,119] = 32.15; p < .001), such that TD controls had higher IQ scores than all of the SCA groups (all ps < .001), and the +2/3X group had lower IQ scores than all other groups (ps < .05). These findings were expected, as reductions in overall intellectual functioning are a characteristic of SCAs (Polani, Reference Polani1977). Lastly, groups differed on sex ratio (p < .001) as expected, as karyotypes involving an extra Y occur only in males.
Procedures
The NIH Combined Neuroscience Institutional Review Board approved this study. Participants over the age of majority and parents of minors signed consent forms. Participants under the age of majority also provided assent. Study procedures, including the completion of verbal fluency and an abbreviated intellectual assessment, were completed at the NIH Clinical Center.
Measures
Verbal fluency task
Participants completed both phonemic and semantic fluency conditions. For phonemic, participants stated as many words as they could that started with a F, A, or S during three, 60-s intervals. For semantic, participants stated as many animals as they could during a 60-s interval. Across both conditions, participants were told to avoid repeating words. In the phonemic condition, participants were also instructed to avoid using proper nouns (e.g., names, brands, etc.) and providing minor variations of the same word (e.g., fall, fell). To compare phonemic and semantic fluency performance using the same scale, age-standardized Z-scores for these conditions were generated using norms from Gaddes and Crockett (Reference Gaddes and Crockett1975), Halperin, Healey, Zeitchik, Ludman, and Weinstein (Reference Halperin, Healey, Zeitchik, Ludman and Weinstein1989), and Tombaugh, Kozak, and Rees (Reference Tombaugh, Kozak and Rees1999)Footnote 1 . These Z-scores were the primary outcome variables used in the current investigation.
Intelligence (IQ) test
IQ was estimated using the four-subtest Wechsler Abbreviated Scale of Intelligence (Wechsler, Reference Wechsler1999) for all but one participant who completed the Wechsler Preschool and Primary Scale of Intelligence—Third edition (Wechsler, Reference Wechsler2002).
Statistical Plan
Question 1 focused on whether the presence of an extra X chromosome impacts verbal fluency performance and whether increasing supernumerary X chromosome number relates to greater impairments. For this question, we included all participants without an extra Y chromosome and completed a 3 × 2 mixed model analysis of variance (ANOVA), with one between-subjects factor (Extra X number: +0X,+1X, +2/3X) and one within-subjects factor (fluency condition: phonemic, semantic). The second question examined extra Y effects on verbal fluency and included only males from the following groups: +0X,+0Y (TD males: XY) and +1Y (47,XYY). A 2 × 2 mixed model ANOVA was completed with one between-subjects factor (Extra Y number: +0Y, +1Y) and one within-subjects factor (fluency condition). The third question contrasted the effects of a supernumerary X versus Y chromosome on fluency in males. To evaluate this, a 2 × 2 × 2 mixed model ANOVA, with two between-subjects factors (presence of an extra X; presence of an extra Y) and one within-subjects factor (fluency condition) was used. This analysis included males with +0X,+0Y (XY), +1X (47,XXY), +1Y (47,XYY), and +1X, +1Y (48,XXYY).
For primary analyses that examined the effects of an extra X, extra Y, fluency condition, or interaction (of any kind), a false discovery rate (FDR; Benjamini & Hochberg, Reference Benjamini and Hochberg1995) experiment-wise adjustment for multiple tests was completed. For the 13 effects that were being examined, the FDR adjusted p-value was .023. Effects that survived FDR correction are noted in a summary table which is described in the Results section. For all aforementioned analyses, in the event of a significant interaction (e.g., group*condition), tests of simple effects that compared performance between pairs of groups and within a group (on condition) were completed with Bonferroni adjustment for multiple comparisons.
Because it was difficult to evaluate normality in the study’s small groups (+2/3X group, n = 12; 47,XYY group, n = 11), analyses involving these groups were re-run using one of the following non-parametric tests: Kruskal-Wallis (to evaluate differences among groups when more than two groups were compared); Mann-Whitney U (to evaluate differences between pairs of groups); and Wilcoxon Signed-Rank test (to evaluate within group differences between fluency conditions). Non-parametric results for the questions involving these groups were consistent with results from parametric tests. Because of this convergence in findings and the desire to examine group by verbal fluency condition interactions, only parametric test (mixed model ANOVA) results are reported in the manuscript.
Lastly, before completion of primary analyses, we examined data for outliers (i.e., scores > 3 standard deviations from the sample mean). Our analyses revealed one outlier for phonemic fluency (Z = 3.28) and one for semantic fluency (Z = 4.39). Both were from the TD group. To examine the possible influence of these outliers on study findings, we conducted all primary analyses with and without them included. Results were largely the same. Rather than include or exclude outliers, we elected to take an intermediate approach. Consistent with methods suggested by others (e.g., Ghosh & Vogt, Reference Ghosh and Vogt2012; Tabachnick & Fidell, Reference Tabachnick and Fidell2007), we assigned less extreme Z scores to these outliers (Z scores of 3). Because this approach permitted including these individuals while making their scores less influential, primary study results include these two outliers with their adjusted scores.
RESULTS
Phonemic and semantic fluency scores for all groups are summarized in Table 1. Table 2 summarizes findings for the three study questions and displays which study findings survived experiment-wise correction for multiple tests using a FDR adjustment.
Table 2 Summary of Main Study Findings
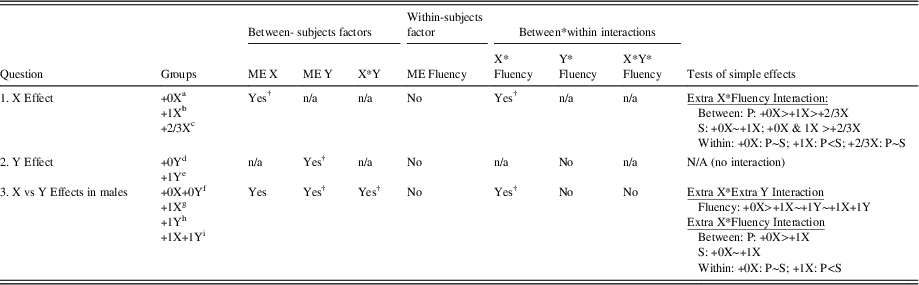
Note. Karyotypes included in different groups were as follows: a+0X (46,XX, 46,XY); b+1X (47,XXY, 47,XXX); c+2/3X (48,XXXX, 48,XXXY, 49,XXXXY); d+0Y (46,XY); e+1Y (47,XYY); f+0X+0Y (46,XY); g+1X (47,XXY); h+1Y(47,XYY); i+1X+1Y (48,XXYY)
†Survives FDR correction for multiple tests (13 primary tests; adjusted p-value=.02); Tests of simple effects are Bonferroni corrected. (See text for details).
ME = main effect; P = Phonemic; S = Semantic.
In the sections that follow, we outline results by question.
Q1. How does the presence of an extra X chromosome impact fluency performance, and does performance worsen with increasing supernumerary X chromosome number?
Groups: +0X (XX, XY), +1X (47,XXX, 47,XXY), +2/3X (48,XXXX, 48,XXXY, 49,XXXXY).
Results: The 3 × 2 mixed model ANOVA revealed a main effect of extra X (F[2,97] = 43.17; p < .001; η2 p = .47), such that having additional supernumerary X’s was associated with poorer performance overall. However, the main effect of extra X was qualified by a significant extra X*fluency condition interaction (F[2,97] = 5.36; p < .01; η2 p = .10). Between-group tests of simple effects revealed that all groups differed from one another on phonemic and semantic fluency (+0X>+1X>+2/3; ps < .008; Bonferroni adjustment for six tests). Tests of within-group simple effects revealed that the interaction was driven by the fact that the profile of scores on phonemic and semantic fluency differed by group. Specifically, phonemic and semantic fluency scores were similar within the +0X and +2/3X groups (ps > .35). However, they differed in the +1X such that semantic fluency was less impaired than phonemic fluency (p < .016; Bonferroni adjustment for 3 tests). See Figure 1.
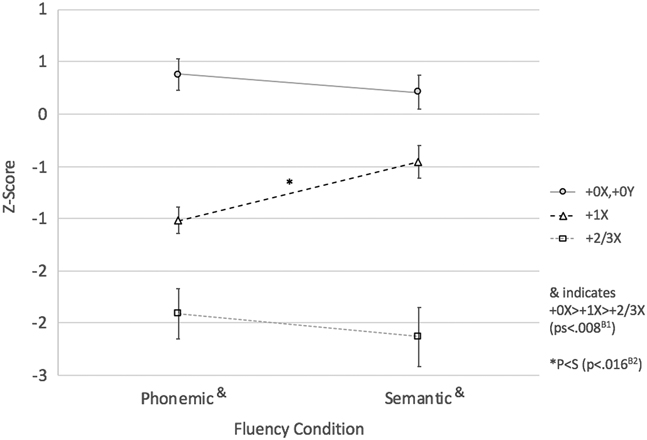
Fig. 1 Effect of extra X on phonemic and semantic fluency performance. B1 p < .008 (Bonferroni correction for six tests). B2 p < .016 (Bonferroni correction for three tests).
Q2. How does the presence of an extra Y chromosome impact fluency performance?
Groups: +0X,+0Y (XY), +1Y (47,XYY).
Results: A 2 × 2 mixed model ANOVA revealed a main effect of extra Y, such that males with an extra Y chromosome performed worse than controls overall—that is, across conditions (F[1,31] = 18.96; p < .001; η2 p = .38). However, no extraY*fluency condition interaction was found (p > .6) nor was there a main effect of fluency condition (i.e., there was no evidence of differences in phonemic and semantic fluency scores across the groups; p > .19). Thus, tests of simple effects to evaluate within and between group differences were not completed (see Figure 2).
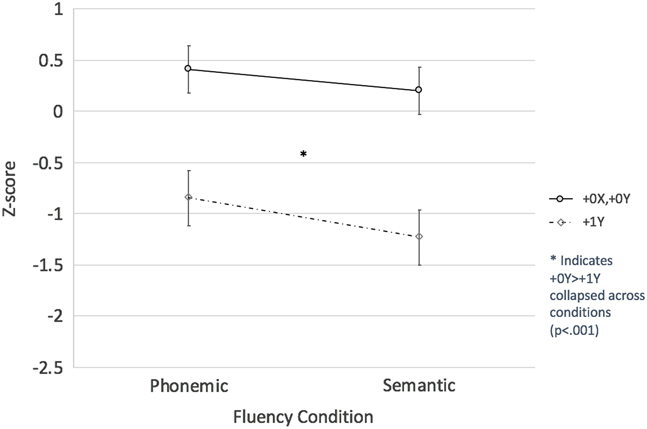
Fig. 2 Extra Y Effect on Phonemic and Semantic Fluency Performance
Q3a. Are there differences in the effects of an extra X versus Y on fluency among males?
Q3b. Does the presence of both an extra X and an extra Y chromosome (48,XXYY) result in greater impairments in verbal fluency than the presence of an extra X (47,XXY) or extra Y (47,XYY) in isolation?
Groups: +0X,+0Y males (XY), +1X males (47,XXY), +1Y males (47,XYY), +1X,+1Y males (48,XXYY).
Results: To evaluate questions 3a and 3b, a single 2 × 2 × 2 mixed model ANOVA was completed; however, results are organized below by study question.
Q3a. Consistent with analyses from Q1 in which an extra X was evaluated in isolation (in youth with a somewhat different set of karyotypes), the 2 × 2 × 2 mixed model ANOVA revealed a main effect of extra X (F[1,58] = 4.31; p < .05; η2 p = .07), such that the addition of an extra X was associated with an overall decrement in verbal fluency performance. Again, this main effect was qualified by an extra X*fluency condition interaction (F[1,58] = 9.18; p < .01; η2 p = .14). Between group tests of simple effects revealed that when compared to those without an extra X (i.e., typical males, XY, and males with 47,XYY), those with an extra X (i.e., males with 47, XXY and 48, XXYY) performed less well on phonemic fluency (p < .025; Bonferroni adjustment for 2 comparisons), but similarly on semantic fluency (p > .3). Within-group tests of simple effects revealed that phonemic fluency was more impaired than semantic fluency in youth with an extra X (p < .025; Bonferroni adjustment for 2 comparisons). This was not the case for those without an extra X (p > .2).
Consistent with analyses for Q2, when the effects of an extra Y were evaluated in isolation (in youth with a somewhat different set of karyotypes), the 2 × 2 × 2 mixed model ANOVA also revealed a main effect of extra Y (F[1,58] = 8.47; p < .01; η2 p = .13), such that an extra Y was associated with an overall decrement in performance on verbal fluency. Stated another way, lower scores (collapsed across fluency conditions) were found for those with an extra Y (i.e., males with 47, XYY and 48, XXYY) compared to those without (i.e., typical males, XY, and males with 47, XXY). Again, there was no Y*fluency interaction (p > .8) nor was there a main effect of fluency (p > .2).
Q3b.The results of the 2 × 2 × 2 mixed model ANOVA also revealed an extra X*extra Y interaction (F[1,58] = 9.85; p < .01; η2 p = .15). Between-group tests of simple effects revealed that those with +0X,+0Y (i.e., typical males) outperformed all three SCA groups (ps < .008; Bonferroni adjustment for six comparisons) on verbal fluency overall (collapsed across conditions). However, contrary to expectations, overall verbal fluency performance of males with +1X,+1Y (48, XXYY) was similar to that of males with +1X (47, XXY) and males with +1Y (47, XYY) who in turn did not differ from one another. Finally, there was no extra X*extra Y*fluency condition interaction (p > .4) (see Figure 3).
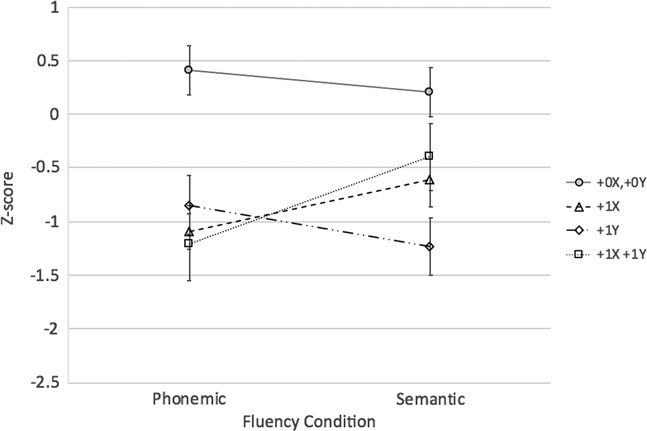
Fig. 3 Extra X versus Y Effects on Fluency Performance among Males. Note: +0X,+0Y > all groups on verbal fluency (collapsed across conditions); p<.008 (Bonferroni correction for 6 tests)
DISCUSSION
The current study sought to characterize verbal fluency performance in youth with SCA tri-, tetra-, and pentasomies. We tested whether the presence of one or more supernumerary sex chromosomes resulted in impaired performance on both phonemic and semantic fluency and whether performance varied as a function of supernumerary X versus Y chromosome status. In addition, we evaluated how verbal fluency performance was impacted by increasing supernumerary X chromosome number and contrasted performance of males with +1X,+1Y (48,XXYY) to those with either an extra X (47,XXY) or an extra Y (47,XYY) in isolation.
First, with regard to supernumerary X chromosome influences, our findings indicate that while an extra X exerts a downward influence on verbal fluency overall, semantic fluency appears less impaired than phonemic fluency in youth with +1X. These findings are largely consistent with prior research examining verbal fluency in 47,XXY and 47,XXX considered separately (e.g., Bender et al., Reference Bender, Linden and Robinson1989; Bender et al., Reference Bender, Linden and Harmon2001; Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009). However, as mentioned, some studies have failed to detect group differences in verbal fluency for males with 47,XXY relative to TD controls (Semantic: Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009). In considering these inconsistencies, it should be noted that effect sizes for group differences in aforementioned studies were medium to large, suggesting that group differences were present but were not statistically significant due to smaller samples.
Consistent with our prior work on language functioning (Lee et al., Reference Lee, Wallace, Adeyemi, Lopez, Blumenthal, Clasen and Giedd2012), we found evidence that increasing supernumerary X chromosome number was associated with increased verbal fluency impairments. Specifically, TD youth outperformed youth with X chromosome trisomies, who in turn outperformed youth with X chromosome tetra- and pentasomies on both phonemic and semantic fluency.
Unlike X chromosome effects, only a main effect of extra Y was revealed. There was no Y*fluency condition interaction (nor was there a main effect of verbal fluency condition), suggesting that an extra Y impacted phonemic and semantic fluency similarly. This finding is generally in line with past research (e.g., Ross et al., Reference Ross, Zeger, Kushner, Zinn and Roeltgen2009) in which medium to large effect sizes on semantic and phonemic fluency have been found relative to controls.
Lastly, to contrast X and Y chromosome effects in males only using a 2 (Extra X: 0 or 1) by 2 (Extra Y: 0 or 1) design, performance of the +0X,+0Y (XY), +1X (47,XXY), +1Y (47,XYY), and +1X,+1Y (48, XXYY) groups was contrasted. Findings replicated those reported above that examined these effects individually in a different subset of participants with SCAs. Specifically, in this subset of males, an extra Y was associated with an overall decrement in performance while an extra X was associated lesser semantic than phonemic fluency impairments. While not surprising, these results were not inevitable given differences in the analytic approach and subset of SCA participants included to answer this question. However, they provide further support for possibly differential effects of supernumerary X and Y chromosomes on verbal fluency performance.
Given the limited existing literature comparing subtleties in the neuropsychological phenotype among males with an extra X versus Y, it is difficult to make conclusions about what aspects of the cognitive phenotype for these different disorders are driving these findings. One possibility is that differences in the nature of language impairments experienced by these groups are impacting performance. For example, those with an additional X may have a narrower language impairment that influences phonological more than semantic processing, similar to individuals with dyslexia (Cavalli, Duncan, Elbro, Ahmadi, & Cole, 2017) or isolated speech sound disorder without concomitant global language learning difficulties (Raitano, Pennington, Tunick, Boada, & Shriberg, Reference Raitano, Pennington, Tunick, Boada and Shriberg2004).
Consistent with this possibility is research that has demonstrated phonemic but not semantic fluency impairments in individuals with dyslexia (e.g., Smith-Spark, Henry, Messer, & Ziecik, Reference Smith‐Spark, Henry, Messer and Zięcik2017). In contrast, males with an extra Y may have a more global language impairment, impacting phonological and semantic processing more broadly, similar to youth with developmental language disorders (Bishop & Snowling, Reference Bishop and Snowling2004; Claessen, Leitão, Kane, & Williams, Reference Claessen, Leitão, Kane and Williams2013) and youth with reading impairments impacting both phonological decoding and comprehension (Snowling & Hulme, Reference Snowling and Hulme2012).
Alternatively, differences in executive control between the groups could be a contributing factor, as some research has suggested that phonemic fluency task performance may be particularly impacted by executive demands (Luo, Luk, and Bialystok, Reference Luo, Luk and Bialystok2010). However, research on this topic is inconsistent, as other studies (Shao, Janse, Visser, & Meyer, Reference Shao, Janse, Visser and Meyer2014) have reported no differences in the relations between executive abilities and phonemic and semantic fluency performance. Given that there is some research to suggest that males with an extra Y have higher rates of attention-deficit/ hyperactivity disorder (ADHD) than males with an extra X (Tartaglia, Ayari, Hutaff-Lee, & Boada, Reference Tartaglia, Ayari, Hutaff-Lee and Boada2012), greater executive dysfunction impairments may characterize 47,XYY and thus could contribute to overall decrements in fluency performance that did not vary by condition. Future studies should begin to disentangle the linguistic and executive underpinnings of verbal fluency in youth with supernumerary X versus Y SCAs. More nuanced descriptions of the cognitive underpinnings of performance on complex tasks such as verbal fluency in youth with SCAs may inform the implementation of more nuanced interventions for this group.
Lastly, we found an extra X*Y interaction, such that males with +1X,+1Y did not differ from males with an isolated extra X or Y when verbal fluency was considered across conditions, that is, the presence of both an extra X and extra Y did not reduce performance in an additive or multiplicative manner. This finding was unexpected, as prior research suggests that increasing sex chromosome number is associated with poorer performance on measures of language and intellectual functioning (Lee et al., Reference Lee, Wallace, Adeyemi, Lopez, Blumenthal, Clasen and Giedd2012; Polani, Reference Polani1977). It is difficult to interpret this unexpected finding, given that limited research exists contrasting cognitive abilities in youth with different SCA variants. Thus, future research is needed to begin to understand how differing numbers of supernumerary X and Y chromosomes impact cognition and the brain more broadly. This topic is discussed next.
Taken together, this study’s results point to both general and specific effects of supernumerary X versus Y chromosomes on verbal fluency. Generally, the presence of an extra X or Y chromosome is associated with poorer verbal fluency performance. Prior studies of brain anatomy in our cohort have identified convergent supernumerary X- and Y-chromosome effects on a large swath of cortical thickness in the frontal lobe as well as proportional cortical thickness of several left-lateralized brain regions central to canonical language networks (Raznahan et al., Reference Raznahan, Lee, Greenstein, Wallace, Blumenthal, Clasen and Giedd2014). The converged effects documented for supernumerary X and Y chromosome dosage on verbal fluency overall echo this anatomical convergence in X/Y effects, suggesting a potential link between patterns of changes in brain and behavior in SCAs.
However, when considering youth with chromosomal trisomies in particular, it appears that an extra X chromosome influences semantic fluency less than phonemic fluency. This pattern was not seen in males with 47,XYY, as only a main effect of extra Y was found; no Y*condition interaction or main effect of fluency was detected. As the literature on neuroanatomical differences associated with supernumerary X and Y chromosomes is limited, it is difficult to make hypotheses about the neural basis of these subtle verbal fluency differences. Further work is needed to directly assess the degree to which anatomical differences are correlated with cognitive differences in those with supernumerary X and Y SCAs.
This study has several limitations. First, like most recent studies of youth with SCAs (e.g., Tartaglia et al., Reference Tartaglia, Wilson, Miller, Rafalko, Cordeiro, Davis and Ross2017; van Rijn & Swaab, Reference van Rijn and Swaab2015), some of the participants in our sample were postnatally ascertained, which may have biased results. However, inclusion of this group cannot account for our findings, as results were largely consistent when analyses were re-run with only the prenatal subsample. Another limitation of the current research is the small sample sizes of the tetra- and pentasomy groups. Given the rarity of these conditions, small sample sizes are inevitable for single-site studies. Thus, future research may benefit from combining samples across sites to increase sample size for rarer SCAs. Third, we were not able to examine the contributions of testosterone insufficiency (or supplementation) on performance in males with supernumerary X chromosomes, as testosterone replacement therapy is routinely recommended for adolescent males with these conditions (Wikström & Dunkel, Reference Wikström and Dunkel2011). Lastly, as briefly summarized in the introduction, research suggests that verbal fluency tasks measure multiple domains of cognition, including language and executive abilities (e.g., Henry & Crawford, Reference Henry and Crawford2004, Reference Henry and Crawford2005; Henry et al., Reference Henry, Messer and Nash2015; Ruff, Light, Parker, & Levin, Reference Ruff, Light, Parker and Levin1997). Thus, it is difficult to make strong conclusions about more nuanced cognitive underpinnings of the verbal fluency impairments faced by youth with different SCAs. Thus, future research should explore the cognitive correlates of verbal fluency tasks in these groups.
Acknowledging these limitations, the current study’s findings contribute to the literature on genetic influences on cognition and suggest that genes on the X and Y chromosomes may be important to examine when investigating the genetic underpinnings of verbal fluency impairments, and likely both language and executive function impairments, in other developmental disorders. To truly understand these relationships, future research should combine genetic expression studies, detailed cognitive phenotyping, and neuroimaging. Additionally, murine models of SCAs (e.g., Raznahan et al., Reference Raznahan, Lue, Probst, Greenstein, Giedd, Wang and Swerdloff2015) may provide insights into the genetic underpinnings of atypical brain development in these groups.
Lastly, another avenue for future research involves an examination of factors that influence individual differences in verbal fluency performance within SCA groups, as there was significant variability in fluency performance within the different SCA groups. Thus, future research should add to the small literature investigating factors that relate to these individual differences (e.g., Bender, Linden, & Robinson, Reference Bender, Linden and Robinson1987; van Rijn, Barneveld, Descheemaeker, Giltay, & Swaab, Reference van Rijn, Barneveld, Descheemaeker, Giltay and Swaab2018) to identify possible biological and psychosocial factors that could be targets of treatment in future research.
Taken together, findings from our study provide further support that youth with SCAs are at an increased risk for presenting with impairments in verbal fluency and related executive function and language tasks. These challenges are consistent with the comorbid learning and psychiatric conditions that occur at higher rates in youth with SCAs. Specifically, prior research demonstrates that children with SCAs are substantially more likely than TD peers to have ADHD (Tartaglia et al., Reference Tartaglia, Ayari, Hutaff-Lee and Boada2012), dyslexia (Simpson et al., Reference Simpson, Addis, Brandler, Slonims, Clark, Watson and Fairfax2014), language disorders (Simpson et al., Reference Simpson, Addis, Brandler, Slonims, Clark, Watson and Fairfax2014), and other psychiatric/cognitive impairments (Bruining, Swaab, Kas, & van Engeland, Reference Bruining, Swaab, Kas and van Engeland2009; Rovet, Netley, Bailey, Keenan, & Stewart, Reference Rovet, Netley, Bailey, Keenan and Stewart1995). For example, Tartaglia et al. (Reference Tartaglia, Ayari, Hutaff-Lee and Boada2012) found that 58% of their sample (47,XXY, 47,XXX, 47,XYY, 48,XXYY) met criteria for ADHD and 74% met criteria for a learning disability.
Still, there is considerable variability within groups for each of these conditions. Consequently, further research is needed to identify neuropsychological and neuroanatomical/ physiological predictors of risk for these comorbid conditions in youth with SCAs. As studies of youth without SCAs have shown that verbal fluency is a useful measure to differentiate children with typical development from those with ADHD (Abreu et al., Reference Abreu, Argollo, Oliveira, Cardoso, Bueno and Xavier2013) or dyslexia (Moura. Simões, & Pereira, Reference Moura, Simões and Pereira2015), this measure may prove to be a clinically useful early indicator of risk for developing these conditions in children with SCAs. Thus, future research on relations between verbal fluency, language, reading, and attentional deficits in youth with SCAs is needed. Such research may permit the early identification of youth with SCAs who are likely to struggle most in these domains and allow for the implementation of targeted and potentially preventative interventions that could improve real world outcomes in individuals with SCAs.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Mental Health (1 ZIA MH002794-13; Protocol ID 89-M-0006). The authors report no conflict of interest. We thank the families who made this research possible.







