Approximately one billion people worldwide are afflicted by hypertension, hence there is a great deal of interest in the prevention and treatment of this chronic disorder( Reference Kearney, Whelton and Reynolds 1 ). Some studies have shown that individuals with hypertension produce more reactive oxygen species and have an impaired antioxidant defence system, both of which increase oxidative stress and lead to an ongoing, vicious cycle( Reference Russo, Olivieri and Girelli 2 ). Antioxidants inhibit oxidation reactions, thereby reducing the number of free radicals produced and the amount of damage they can cause. Se, an essential trace element with antioxidant properties, was hypothesized to have a protective effect on hypertension( Reference Rayman 3 ).
Se is a key component of glutathione peroxidase (GPx), an enzyme that prevents the oxidation of lipids and atherosclerotic plaque formation( Reference Wojcicki, Rozewicka and Barcew-Wiszniewska 4 ). GPx also indirectly prevents the aggregation of platelets, thereby inhibiting blood clot formation( Reference Bryant, Bailey and King 5 ). There is a direct relationship between Se and GPx activity when Se concentrations are low, but GPx activity plateaus off at high Se levels( Reference Rea, Thomson and Campbell 6 ). A direct link between Se and hypertension was provided by the role of Se in Keshan disease, a disorder that occurred in regions of China where Se was severely deficient in the soil and diet( Reference Boosalis 7 ). Symptoms of Keshan disease, including hypertension, heart failure and pulmonary oedema, can be relieved by administering Se supplements( Reference Boosalis 7 ). However, various studies have shown that increasing Se levels above the recommended daily intake is not beneficial and can actually cause hypertension, diabetes and hyperlipidaemia( Reference Stranges, Navas-Acien and Rayman 8 ). Randomized trials with Se as part of multivitamin supplementation in Se-deplete areas were shown to reduce gastric cancer, stroke and overall mortality, but did not reduce the risk for hypertension, CVD or cataracts( Reference Huang, Caballero and Chang 9 ).
Given the conflicting results regarding the relationship between Se levels and hypertension, we undertook a systematic evidence review that examines this relationship in studies conducted in numerous countries with various study designs.
Methods
A systematic literature search was conducted in PubMed and OVID of all published articles as of 1 June 2011 that examined the relationship between Se and hypertension in man (Fig. 1). Search terms used were ‘selenium’ AND (‘hypertension’ OR ‘blood pressure’ OR ‘pulse pressure’). All articles that reported on animal studies were excluded. The initial search yielded 291 articles. After excluding articles that were not written in English and those that were irrelevant based on a review of article titles, 111 articles were chosen for review of the abstracts. Review articles, case studies, poster presentations, any remaining animal studies and articles with no usable information were excluded. Of the remaining sixty-one articles, those that measured a different outcome variable other than hypertension or blood pressure, used pregnant female subjects or lacked sufficient data were further excluded. Three studies on paediatric populations were further excluded since blood pressure increases continually until puberty( Reference Shankar, Eckert and Saha 10 ) and there is no standard definition of hypertension in children because their blood pressure fluctuates so much( Reference Scharer 11 , Reference DeSanto, Trevisan and Capasso 12 ). Twenty-five articles met the criteria for full article review and data abstraction.
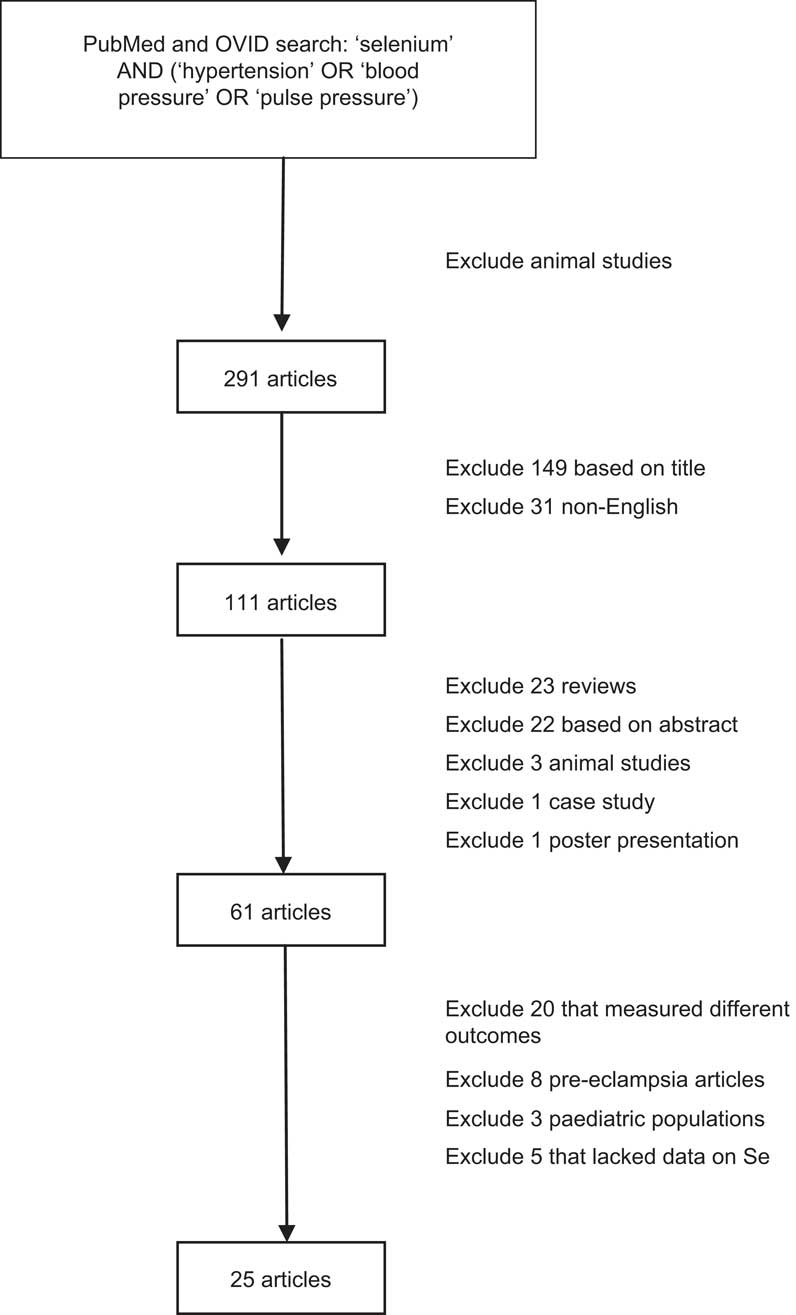
Fig. 1 Literature search on the relationship between selenium and hypertension in man
For each paper reviewed, information regarding the study setting, participant demographic information, exclusion criteria used for the study, interventional dosage for intervention studies, outcome variable and covariates were independently extracted by two reviewers (D.K. and S.G.). The level of Se measured in participants and its relationship to the outcome variable were noted. In addition, we also extracted the same information in articles that measured GPx levels. From the final multivariate model given in each paper, effect size measures, hazard ratios, parameter estimates and total or partial correlation coefficients as well as P values were recorded. The relationships were described as ‘protective’ if higher Se levels were associated with a lower risk of hypertension, ‘none’ if there was no significant relationship between Se and hypertension, or ‘harmful’ if higher Se levels were associated with a higher risk of hypertension. Statistical significance was defined as P < 0·05.
For ease of comparison across study populations, mean serum Se levels were converted to μg/l, if the original levels reported were not in those units. In three studies, the original units for the mean level of Se appeared to be incorrect as they greatly exceeded the maximum values reported in man. These were assumed to be erroneous in units and are denoted in the tables with **. For papers that expressed their mean Se level as a range or provided multiple numbers for different populations (for example, hypertensive and normotensive groups), the average of these values was used when converting the units of mean level of Se to μg/l. In those studies that measured Se levels in different media in the same group of people, we chose to include plasma measures of Se in the tables, as blood samples were the most commonly used measures in these studies.
Since the number of studies reporting randomized trials and prospective analyses was small and some of these studies lacked information necessary for meta-analysis, we conducted meta-analyses on cross-sectional studies and case–control studies where the extracted information permitted such analyses. For cross-sectional studies, outcome measures were converted to correlation coefficients if the original articles reported regression coefficients or other information using the method proposed by Thompson et al.( Reference Thompson, Ekelund and Jebb 13 ). Difference in mean Se levels between cases and controls was used as the outcome measure for case–control studies. Random-effect models in the software Comprehensive Meta Analysis version 2 were used for combining study results.
Results
A total of twenty-five articles reported results on the relationship between Se and hypertension in adult populations. Outcome measures used in these studies included a binary definition of hypertension with the cut-off being 140/90 mmHg, continuous systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure, or a combination of these variables. Table 1 provides summary information of the twenty-five studies grouped by study design. Studies reporting both longitudinal and baseline results were included in the longitudinal section only.
Table 1 Summary of studies on the relationship between selenium levels, hypertension and blood pressure
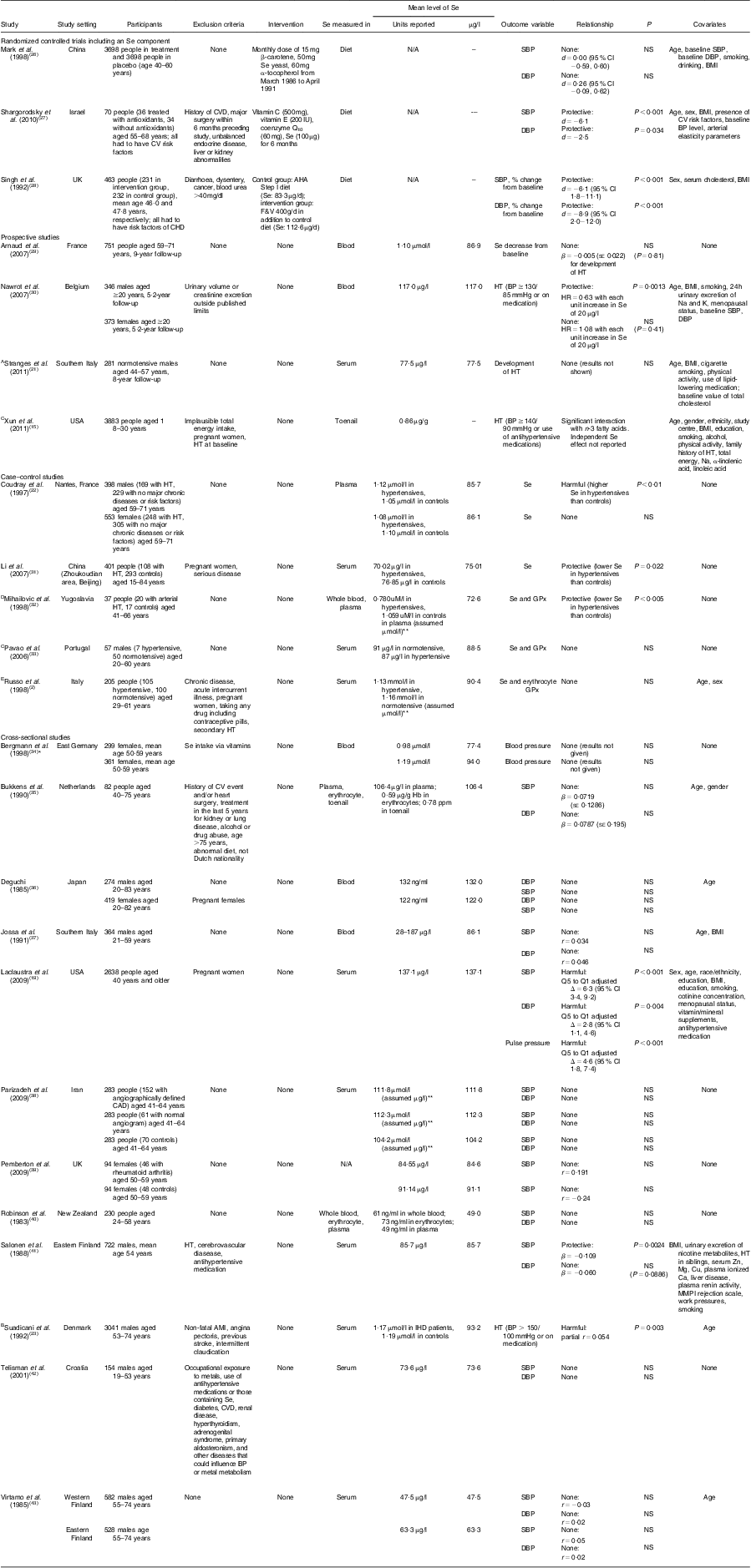
CV, cardiovascular; HT, hypertension; CAD, coronary artery disease, AMI, acute myocardial infarction; BP, blood pressure; N/A, not applicable; AHA, American Heart Association; F&V, fruits and vegetables; SBP, systolic blood pressure; DBP, diastolic blood pressure; GPx, glutathione peroxidise; NS, P ≥ 0·05.
Definition of hypertension was not given unless otherwise noted: ASBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or current antihypertensive drug treatment; Breceiving antihypertensive treatment or having BP ≥ 150/100 mmHg; CSBP ≥ 140 mmHg and DBP ≥ 90 mmHg or current use of antihypertensive medications; Dsecond- or third-degree HT using the classification of the World Association of Cardiologists; E>135/85 mmHg.
Measures of association: d = difference in BP between treatment and control groups; β = regression parameter estimate from linear regression models; HR = hazard ratio; r = correlation coefficient; Δ = difference in BP between two quintile groups (Q5, fifth quintile; Q1, first quintile).
*No numerical data provided.
**Units were assumed to be something other than what was given in the paper.
There were no published randomized controlled trials (RCT) with Se as the only intervention agent. There were three RCT that included Se as part of the intervention in dietary supplementation. One study co-administered Se with vitamin C, vitamin E and coenzyme Q10. Another administered Se in the form of fruits and vegetables so participants also received vitamins A, C and E, carotene, Cu, Mg and dietary fibre. In both cases, SBP and DBP were lowered significantly in the intervention groups. In addition, both of these studies enrolled only subjects carrying cardiovascular risk factors. A third and much larger RCT that administered Se with β-carotene and α-tocopherol, however, found no difference in blood pressure between intervention and control groups.
Four studies utilized the prospective study design where the development of hypertension was the outcome. One study found a significant protective effect of Se on incident hypertension in young adult males, but no significant relationship in females. The remaining three studies reported a non-significant association between Se and hypertension. In addition, one of the three studies reported a significant interaction between n-3 fatty acids and Se on hypertension risk.
Five studies used the case–control study design and compared Se levels between hypertensive subjects and normal controls. Two studies reported significantly lower Se levels in hypertensive subjects for both males and females, one study found significantly higher Se levels in hypertensive subjects but in males only, and the other two studies found no relationship. Figure 2 presents the meta-analysis of these five studies using random-effect models. There is no overall difference in Se levels between hypertensive and normotensive subjects, as the pooled mean difference was estimated to be −0·228 (95 % CI −0·517, 0·060) μg/l.
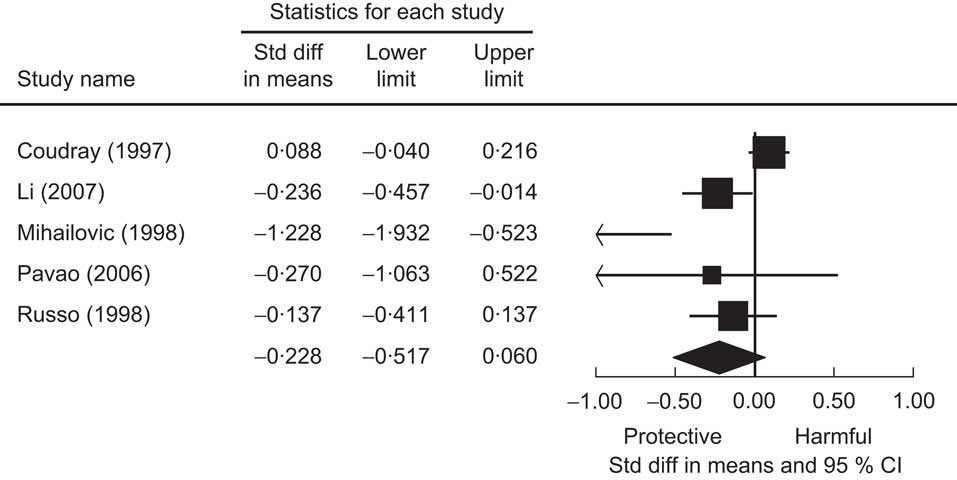
Fig. 2 The association of selenium with hypertension from case–control studies. Random-effects meta-analysis showing the standard difference (std diff; and 95 % confidence interval) in mean plasma selenium level (μg/l) between hypertensive subjects and normal controls; the size of the square indicates the weight of each study in the analysis, the horizontal lines represent the 95 % CI and the diamond represents the pooled mean difference (its width represents the 95 % CI of the pooled mean difference)
Cross-sectional analysis was used in twelve studies. Of these, nine studies found no significant association of Se with either SBP or DBP. Two studies found higher Se levels associated with higher blood pressure, while one study found the opposite relationship. We present a meta-analysis based on these cross-sectional studies in Fig. 3. The pooled correlation coefficient between Se and SBP was not statistically significant (r = 0·01, 95 % CI −0·02, 0·04).
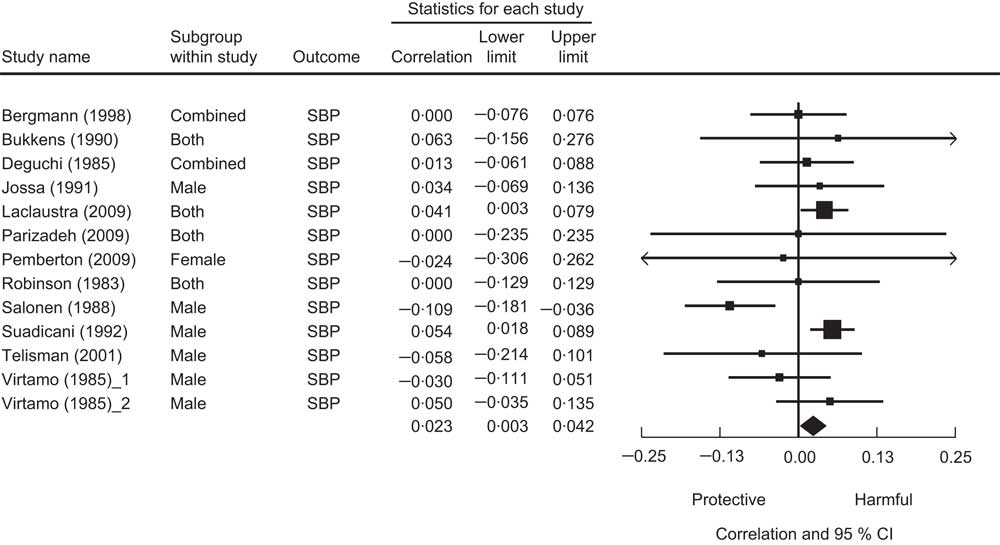
Fig. 3 The association between selenium and systolic blood pressure from cross-sectional studies. Random-effects meta-analysis showing the correlation coefficient (and 95 % confidence interval) between mean plasma selenium level and systolic blood pressure; the size of the square indicates the weight of each study in the analysis, the horizontal lines represent the 95 % CI and the diamond represents the pooled correlation coefficient (its width represents the 95 % CI of the pooled correlation coefficient)
Seven of the twenty-five studies measured GPx levels as well as Se in their study population (Table 2). These studies looked at the relationship between GPx and SBP, DBP, hypertension, or a combination of the three. Of these, only one study showed a protective relationship between Se and hypertension while all others showed no relationship. When examining the relationship between GPx and hypertension in these same studies, three of the four case–control studies showed lower GPx levels in hypertensive subjects than controls and one study that measured GPx in erythrocytes found higher GPx levels in hypertensive subjects than controls.
Table 2 Comparison of selenium and glutathione peroxidase levels in man in relation to hypertension and/or blood pressure
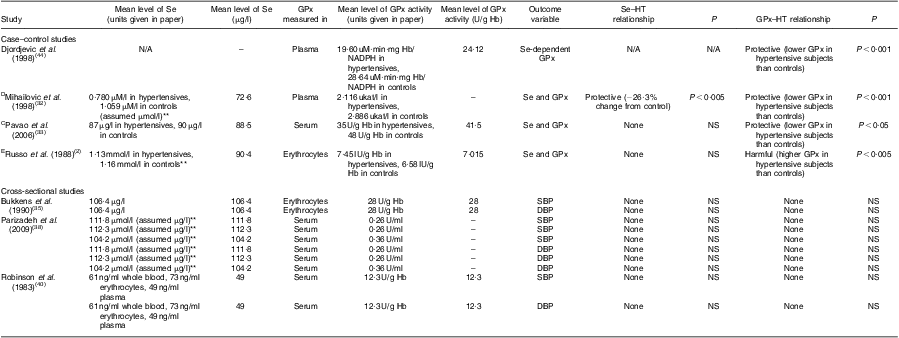
GPx, glutathione peroxidase; HT, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; N/A, not applicable; NS, P ≥ 0·05.
Definition of hypertension was not given unless otherwise noted: CSBP ≥ 140 mmHg and DBP ≥ 90 mmHg or current use of antihypertensive medications; Dsecond- or third-degree HT using the classification of the World Association of Cardiologists; E>135/85 mmHg.
**Units were assumed to be something other than what was given in the paper.
Discussion
The present systematic literature review on Se and hypertension offered no conclusive evidence on a relationship between Se and hypertension. The review also highlighted the limited number of large RCT or prospective studies on Se and hypertension. No RCT with Se as the only intervention agent was published and the three RCT included in the review used different dietary components in addition to Se, making comparisons between studies difficult. Only one out of the four prospective studies reported a significant protective effect of Se on the development of hypertension while the remaining studies found no association between Se and hypertension.
It is likely that the heterogeneity in study design, sample size and demographic characteristics of study participants contributed to the divergence in findings. The present review also underscores the complex relationship between Se and blood pressure. It is possible that the relationship between Se and hypertension is non-linear so that in populations with low Se intake, higher Se may be protective against hypertension; while in those with high Se intake, higher Se may be associated with hypertension risk( Reference Navas-Acien, Bleys and Guallar 14 ).
Since the effect of Se on human health is channelled through GPx activities, it is highly likely that other agents with similar antioxidant properties may interact with Se on the control of blood pressure as reported in one prospective study( Reference Xun, Hou and Daviglus 15 ). In addition, it has been shown that in subjects with low Se intake, vitamin E can protect against hypertension( Reference Thomson and Robinson 16 ). Therefore, it is important that studies examining the relationship between Se and hypertension also measure other important antioxidant levels and explore potential interactive relationships with Se.
In the several studies measuring GPx activities as well as Se, a trend for a positive correlation between Se levels and GPx activity was seen. This was anticipated because Se is a component of GPx( Reference Rotruck, Pope and Ganther 17 ). Increased GPx activity was found to reduce lipid peroxidation, atherosclerotic plaque formation and platelet aggregation( Reference Wojcicki, Rozewicka and Barcew-Wiszniewska 4 , Reference Bryant, Bailey and King 5 ). Therefore, higher GPx activity is thought to be protective against hypertension, a view supported by animal studies. When comparing rats that received a high-Se diet with those that did not, higher Se intake increased GPx activity and reduced the size of myocardial infarct( Reference Tanguy, Morel and Berthonneche 18 ). However, GPx activity plateaus at high Se levels despite a direct relationship between Se and GPx activity when Se concentrations are low( Reference Rea, Thomson and Campbell 6 ), making the effect of high Se level beyond that required for optimal GPx activity uncertain. In a study conducted in a US population with high mean serum Se concentration (137 μg/l), higher Se was found to be associated with higher blood pressure( Reference Laclaustra, Navas-Acien and Stranges 19 ). A possible explanation for the harmful effect of higher Se may be that excess Se overwhelms the liver and kidneys, both of which play an important role in the metabolism and excretion of Se( Reference Suzuki, Hashiura and Matsumura 20 ). Over time, the heart may have to work harder to pump more blood to these organs, leading to hypertension.
Hypertension is suspected to impair the antioxidant defence system( Reference Russo, Olivieri and Girelli 2 ). Hence, a further complication leading to the diverse study findings on Se and hypertension may be a different role for Se in hypertension prevention compared with hypertension treatment. It is possible that the amount of Se for maintaining normal blood pressure may be different from the amount required in hypertensive subjects who may already have a damaged antioxidant defence system affecting the way Se and other antioxidants are metabolized and stored. In the present review, apart from the two prospective studies( Reference Xun, Hou and Daviglus 15 , Reference Stranges, Galletti and Farinaro 21 ) where incident hypertension was the outcome, no other studies examined the relationship between Se and blood pressure in normotensive subjects and hypertensive subjects separately. Given the large percentage of hypertensive subjects in the reviewed studies, the possibility of a reverse causation could be another source contributing to the heterogeneity in results. We also note that a harmful relationship between Se and hypertension was observed from case–control( Reference Coudray, Roussel and Mainard 22 ) and cross-sectional studies( Reference Laclaustra, Navas-Acien and Stranges 19 , Reference Suadicani, Hein and Gyntelberg 23 ).
Many studies included in our review were conducted in small sample sizes, thus suffering from insufficient power to detect a significant relationship between Se and blood pressure or hypertension. We decided to include all studies regardless of sample size in our review to provide the full range of studies conducted on this subject. However, if we were to restrict the review to the nine largest studies with a minimum sample size of 700, we would still have five studies finding no association, two finding higher Se to be protective and another two studies finding higher Se to be harmful for hypertension. It seems that the divergence in results remains even if we only consider studies with large sample sizes.
Our review points to a potential gender difference in the relationship between Se and hypertension. In eight studies that separately reported results for males and females, two showed a significant protective effect, two showed a harmful effect and the rest showed no association between Se and hypertension. In females, however, all of the studies found no relationship. This gender difference could be due to the antioxidant properties of oestrogen, which reduces the number of superoxide anions and results in less endothelial dysfunction in females( Reference Dantas, Tostes and Fortes 24 , Reference Salonen, Salonen and Penttila 25 ). Future studies will need to carefully examine a potential gender difference and plausible mechanisms for such difference.
Our review suggests that future studies investigating the relationship between Se and hypertension need to measure multiple antioxidants as well as Se in prospective designs and that the association between Se and blood pressure needs to be examined separately in hypertensive and normotensive subjects. It is also necessary to conduct more research including laboratory studies of animals focusing on the relationship between the antioxidant system and the blood pressure-regulating mechanism to provide better targets for epidemiological studies and randomized trials.
The current review has a number of strengths over previous literature reviews. Our review offered a comprehensive summary of up-to-date results on Se, hypertension and blood pressure. We also attempted to extract information on cohort characteristics, study design and other potentially relevant information from each study so that potential factors accounting for the diverse findings may be compared. There are also limitations to the review. The first is that our search terms were limited to ‘hypertension’ and ‘blood pressure’, thus studies that reported on other CVD without using blood pressure or hypertension as an outcome were not included. Second, our review excluded non-English articles and may bias the review results towards research publications in English. Since thirty-one non-English articles were identified after the initial search results out of 291 articles, assuming the same rate of usable information contained in the non-English articles as in the English articles, we anticipate missing approximately 10 % of articles (approx. three articles) with usable results on Se and hypertension.
Conclusion
The present systematic literature review does not offer conclusive evidence supporting an association between Se levels and blood pressure or hypertension. Future research focusing on the mechanism between the antioxidant system and blood pressure regulation would provide valuable input and better targets for RCT and prospective studies. These future studies should also be designed to address the role of Se in hypertension prevention separately from its role in hypertension treatment. In addition to measuring Se levels, future studies should also measure other antioxidants and GPx levels, if possible, to explore potential interactions with Se and to determine the mechanism underlying a potential relationship between Se and blood pressure.
Acknowledgements
Sources of funding: D.K. was supported by a Medical Student Training in Aging Research Award from the American Federation of Aging Research. The research was also supported by the National Institutes of Health (grant R01 AG019181) and P30 AG10133. Ethics: Ethical approval was not required as the study was a systematic review of published literature. Conflicts of interest: The authors report no conflict of interest. Authors’ contributions: literature search, D.K. and S.G.; literature review and summarization of results, D.K., S.G. and L.Y.; manuscript, D.K., S.G., H.C.H. and L.Y.







