Pontiac fever is a non-pneumonic illness caused by Legionella spp. leading to ‘flu-like’ symptoms such as headache, myalgia and fever. The first outbreak affected 144 people in a healthcare facility in Pontiac, Michigan in 1968 (retrospectively described in 1978) [Reference Glick1]. Although regarded as a milder illness than Legionnaires' disease, the pneumonic form of legionellosis, Pontiac fever, has been associated with the serious complication of acute disseminated encephalomyelitis [Reference Spieker2]. It is a self-limiting illness and is usually diagnosed in outbreak situations where cases present with similar symptoms over a very short time period following exposure to the same infective source. Several outbreaks of Pontiac fever have previously been described and all of these have been due to exposure to water droplet-borne Legionella spp. such as from defective air-conditioning units, cooling towers, fountains or whirlpool baths [Reference Huhn3]. In New Zealand the first suspected outbreak of Pontiac fever occurred during March 1998 in an office building in Hastings where the presumptive organism was Legionella pneumophila [Reference Maas, McElnay and Watson4].
We describe an outbreak of Pontiac fever in nine horticultural workers caused by L. longbeachae serogroup (sg) 2 present in potting mix and confirmed at the time of the investigation by the isolation of the same organism from sputum or the presence of species-specific antibodies in serum. To our knowledge, there have been no previous reports in the medical literature of an outbreak of Pontiac fever caused by L. longbeachae in potting mix.
Over 3 days in January 2007, three employees of a local nursery presented to Gisborne Hospital in the Tairawhiti district of the North Island of New Zealand. Tairawhiti is an agricultural region with a population of 45 000 people, of whom nearly half are Maori who retain strong whanau (family) cultural ties. As a result the three patients were well acquainted and volunteered that they knew of several other workmates who were unwell with similar symptoms. All of the workers had been using large quantities of potting mix for bagging Nikau palms and grape vine stock over the previous week and none had been wearing protective dust masks.
The three patients had become unwell on the afternoon of 12 January, 1, 2 and 3 days prior to their presentations, respectively. The first case was a 53-year-old male smoker with type II diabetes who complained of chest pain, fevers and night sweats. The second was a 31-year-old female smoker who complained of headaches, joint pains, neck pain and fever. The third was a 46-year-old female who presented with fever, upper abdominal tightness and back pain. Chest X-rays did not show any significant changes for any of the patients.
The comorbidities and vague symptoms lead initially to some diagnostic uncertainty but once the link and potential diagnosis had been made they were srarted on intravenous clarithromycin, 500 mg twice daily. Urine samples were tested for L. pneumophila sg 1 antigen, and blood for acute phase serology. Two of the three hospitalized patients were able to produce sputum after induction with hypertonic saline for culture and PCR. All three patients recovered from their fevers within 5 days of symptom onset and none developed evidence of pneumonia.
A case was defined as someone who had been working in the horticultural nursery potting Nikau palms and grape vine root stock, who developed one or more of the following symptoms; back pain, fever, headache, tight chest, lethargy, muscle pains and photophobia with an onset from 12 January 2007. The incubation period was calculated as <48 h from exposure.
Over the next week, all of the non-hospitalized workers were interviewed and six of the remaining seven complained of similar non-specific ‘flu-like’ symptoms and met the clinical case definition. All of those with symptoms were asked to visit their family doctor for Legionella antibody testing. None was sufficiently ill to justify admission and all spontaneously recovered.
Acute phase serum samples were received from all cases and convalescent serum samples from seven of the cases. An indirect immunofluorescent antibody test (IFAT) was performed to detect serum antibodies to heat-killed whole-cell antigens from L. pneumophila sg 1-15 and nine other species of Legionella including the two L. longbeachae serogroups. This panel was tailored to include types that have been isolated from New Zealand legionellosis cases over the last 20 years and therefore known to be circulating in the environment. Antibodies to Legionella spp. were detected with fluorescein isothiocyanate (FITC) conjugated sheep anti-human IgM, A and G antibody. Patient sera were pre-absorbed with a Campylobacter soluble antigen prior to testing for block cross-reacting antibodies to some Gram-negative bacteria [Reference Boswell, Marshall and Kudesia5]. A fourfold rise in titre to at least 256 was considered indicative of a recent infection.
DNA for the PCR tests was isolated from sputum and the gene targets were the Legionella 16S rRNA gene (using an in-house method based on methods described by Jonas et al. [Reference Jonas6] and van Der Zee et al. [Reference van Der Zee7]) or the Legionella mip gene [Reference Ratcliff8]. Two different PCR methods were used; one targeting the mip gene, the other targeting the Legionella 16S rRNA gene. PCR was performed with forward and reverse primers with amplification in a thermal cycler with the PCR product analysed by agarose gel electrophoresis and visualized with ethidium bromide staining. The Legionella 16S rRNA sequences were compared with those available through the EBI server (http://www.ebi.ac.uk/fasta33/nucleotide.html) using the Fasta3 alignment program. The mip gene sequences were compared with those available online at the UK Health Protection Agency website link (http://www.hpa-bioinfotools.org.uk/mip_ID.html).
Sputum samples were heat or acid treated prior to plating on buffered charcoal yeast extract (BCYE) and BCYE agar containing glycine, vancomycin HCl, polymixin B sulphate and cycloheximide supplement (GVPC) media. Plates were incubated for 10 days at 36°C in a humidified environment and regularly inspected for Legionella-like colonies.
Potting mix samples were collected from the workplace. The method used for the isolation and culture of legionellae from this material was based on the AS/NZS 5024(Int) 2005 standard [9]. A 25% w/v suspension of potting mix material was prepared in sterile, distilled water containing 0·3% w/w Tween-80. The suspension was shaken vigorously for 5 min and then held at room temperature for 30 min; it was shaken again and allowed to settle for 5 min before an aliquot of the cleared supernatant was removed. This was acid treated in a similar manner to the clinical samples and spread onto BCYE and GVPC agar plates. Aliquots of the untreated suspension were also spread onto GVPC agar plates. Water samples were treated according to International Organization for Standardization (ISO) 11731:2004 standard. Samples were filter-concentrated followed by acid or heat pre-treatment and cultured as above. Biofilm swab samples were tested for the presence of Legionella by transferring the swab and its transportation water to a sterile screw-capped container. The total volume was made up to 5 ml with 0·1% peptone water and the entire contents mixed vigorously by vortex before culturing treated and untreated aliquots as described.
Legionella-like colonies were subcultured on Columbia blood agar (CBA) and BCYE agar with and without l-cysteine. Colonies growing on BCYE agar containing l-cysteine and not on the other agars were considered to be Legionella spp. These were further identified to species and serogroup level using direct fluorescent antibody staining (m-Tech, USA) and by mip gene sequencing [Reference Ratcliff8]. Pulsed-field gel electrophoresis (PFGE) of SfiI chromosomal digests was performed on isolates from the clinical cases and the compost samples [Reference De Zoysa and Harrison10].
The potting process took place in a large horticultural warehouse ventilated by a large roller door in one corner and a large double door in an adjacent wall. Unopened 1-tonne bags of potting mix were brought in by fork-lift and hoisted over a large hopper potting mix machine. This bag was then tipped and potting mix spilled out into the hopper. Potting up was achieved by placing a potting bag under a chute from the hopper which was then partially filled with potting mix creating considerable dust very close to the worker's face. The bag was then manually moved to the potting table where the plant (grape stock or Nikau palm) was placed into the potting bag and more potting mix manually added to fill the bag. The plants were then watered and stored elsewhere in the warehouse. The potting mix involved was prepared off site by a manufacturing company to a specified mix and had been treated with methyl bromide to remove horticultural pathogens. It was delivered to Gisborne in 1-tonne bags which were then stored in the open. The potting was done over a period of 1 week.
No personal protective equipment was worn by the work group. Excess potting mix on the floor of the facility was swept up by broom and washed down at the end of the day using a low-pressure garden hose. The work group used a dedicated staff room and toilet facilities. There was no shower or air conditioning or other source of water droplets in the workplace. Since the entire bagging process created a lot of air-borne dust, and in the absence of a source of water droplets, we considered potting mix aerosolization as the likely mode of transmission. The nursery voluntarily closed to enable our investigation and immediately introduced face masks for personal protection.
The results of microbiological tests are shown in Table 1. Both of the sputum samples obtained from the hospitalized patients were culture-positive for L. longbeachae sg 2 which was considered confirmatory evidence of a Legionella infection. Sputa from these two cases were PCR positive for Legionella-specific 16S rRNA and the mip gene sequence showed 100% homology to L. longbeachae sg 2. None of the acute serum samples were positive by PCR, including samples from the two culture-positive cases.
Table 1. Epidemiological and microbiological findings of nine individuals meeting the case definition of legionellosis and one individual who did not meet the case definition
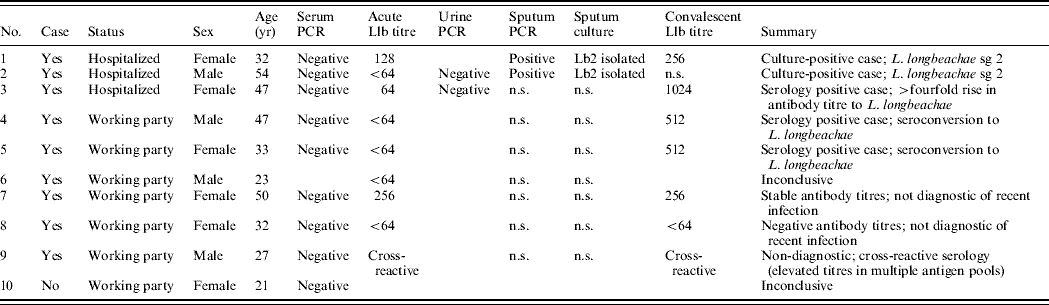
Lb2, Legionella longbeachae sg 2; Llb, Legionella longbeachae; n.s., no sample.
The Legionella IFAT is only diagnostically useful as a retrospective test for determining exposure to Legionella spp. Convalescent serum was obtained from seven of the nine cases. Serotyping with the monovalent antigens identified L. longbeachae sg 2 as the causative agent responsible for a ⩾fourfold elevation in antibody titres in three of the cases; this is considered confirmatory evidence of a Legionella infection. Of the nine suspected cases in the working party, five had microbiological findings compatible with a recent L. longbeachae infection. Two were culture-positive and three demonstrated a ⩾fourfold rise in antibody titre.
L. longbeachae sg 2 was the only strain of Legionella isolated from the two potting mix samples collected from the material to which the workers were exposed. No legionellae were isolated from any of the water samples collected, eliminating the water supply as a potential source.
The DNA profiles of the five isolates from the cases and the compost associated with this outbreak were indistinguishable from each other by PFGE while Legionella isolates from unrelated cases were distinct.
Nine of the ten exposed nursery workers were defined as having Pontiac fever; two of these were sputum culture-positive and three were serologically positive for L. longbeachae sg 2; none developed pneumonic features. The potting mix used by the exposed workers contained L. longbeachae sg 2 that was genetically indistinguishable from that isolated from the clinical samples. Three of the patients were admitted to hospital due to the severity of their symptoms but recovered without significant complications. This is the first recorded outbreak of Pontiac fever due to L. longbeachae sg 2 present in aerosolized potting mix. Although all ten individuals were in close contact with the contaminated potting mix and had similar exposure risks, in five, infection could not be proven. This was due to lack of seroconversion in one case, which is not uncommon in culture-positive cases [Reference Waterer, Baselski and Wunderink11], a raised titre to several Legionella serogroups due to cross-reaction in another, and a high stable titre in a further two cases which was suggestive of recent infection; the tenth member of the party did not fulfil the case definition.
The horticultural workers responsible for potting the plants operated in isolation and thus the outbreak was confined to their small group. Following closure of the nursery and subsequent introduction of face masks there were no further cases.
Currently in New Zealand large outbreaks (more than two cases) of legionellosis have been due to L. pneumophila sg 1. The first outbreak in 1991 was associated with a cooling tower [Reference Mitchell12] and the largest in 2005 was also associated with cooling towers [Reference Pink13]; both these outbreaks occurred in Christchurch in the South Island. In 2006 a further outbreak due to L. pneumophila sg 1 in a coastal community close to Auckland city occurred following contamination of rainwater tanks [Reference Simmons14].
Sporadic cases of Legionnaires' disease are caused by L. longbeachae accounting for as many as 50% of cases in New Zealand, and 30% of community-acquired sporadic cases in Australia [Reference Yu15]. The link with potting mix has, however, remained unproven although in 1994 a 79-year-old female, also from Gisborne, died from Legionnaires' disease shortly after inhaling debris from a dry bag of potting mix [Reference Kingston and Padwell16]. In New Zealand an outbreak is defined as two or more cases associated with a single site with dates of onset within 6 months of each other. In 2002 in the Northland region of New Zealand two people developed Legionnaires' disease due to L. longbeachae sg 1 following exposure believed to be to the same commercially prepared composted material [Reference Sneyd17].
The outbreak here led to the hospitalization of three patients with non-pneumonic legionellosis, but with Legionella bacteria cultured from induced sputum samples this is evidence of culture-proven legionellosis without pneumonia. There has been the suggestion in the past that Pontiac fever is caused by exposure to dead Legionella organisms because of the inability to isolate Legionella from Pontiac fever cases [Reference Edelstein18]. Our findings answer the question posed in this journal by O'Connor et al., ‘Does using potting mix make you sick?’ [Reference O'Connor19]. The outbreak was identified due to a unique set of circumstances in our region – a thriving horticultural industry and consequent widespread use of potting mix, a warm and temperate climate and a small community with a single referral hospital where strong family ties are retained. Furthermore, it would not have been apparent by legionellosis urine antigen testing which is often the diagnostic test undertaken, as it does not detect L. longbeachae. For these reasons we suspect that the number of people with Pontiac fever in areas with a high use of potting mix may be underestimated.
In August 2001 the UK Environment Agency issued a policy position statement on composting and health effects stating that commercial compostors must be maintained at least 250 m from residential property as estimates from previous studies indicate that bioaerosols associated with composting would disperse over this distance into the atmosphere and concentrations would be reduced to background levels [Reference Swan20]. In New Zealand there is currently no prescribed separation distance for risk assessment purposes. Similarly, in New Zealand there is no legislative requirement for the mandatory labelling of potting mix to alert users of the potential risk of exposure to legionellae; health warnings that are placed on the material by manufacturers are voluntary. Furthermore, the wording of these warnings under the Hazardous Substances and New Organisms Act 1996 for the use of composts, soil conditioners and mulches, is discretional. Consequently most warnings do not mention the necessity of wearing face masks to prevent the inhalation of airborne dust. Information put onto potting mix containers suggesting that it is free of pathogens because it has been treated with methyl bromide is misleading since this refers to horticultural pathogens and not human pathogens such as Legionella which can survive the methyl bromide treatment. This is despite the specific recognition by the Department of Labour that exposure to organic dust when using potting mix is an occupational hazard that requires elimination or control. Exposure to Legionella can be eliminated or significantly reduced by wearing face masks as part of appropriate personal protective equipment, using good ventilation of the workplace and storing potting mix in cool areas [Reference Lee21, Reference Epstein22]
As a result of this outbreak, we advocate the introduction of an industry standard ensuring the use of masks when handling potting mix and the attachment of masks to potting mix bags when sold to the public. Given the inconsistent and sometimes misleading messages on potting mix bags suggesting that they are pathogen free, we also advocate mandatory labelling warning consumers of the risk of Legionella infection.
DECLARATION OF INTEREST
None.



