CVD is very common among chronic haemodialysis patients who are at high risk of sudden cardiac death (SCD)(Reference Herzog, Mangrum and Passman1). Thus, SCD, most often caused by ventricular arrhythmias, accounts for approximately 30 % of total mortality in haemodialysis patients(Reference Herzog, Mangrum and Passman1). Not only traditional risk factors of CVD may contribute to the increased risk, but also non-traditional risk factors such as chronic uraemia, chronic fluid overload, electrolyte abnormalities and left ventricular hypertrophy may be of importance(Reference Herzog, Mangrum and Passman1) in haemodialysis patients. Owing to this high risk for SCD, it is of major interest to implement treatments that can reduce it(2, Reference Ritz and Bommer3). The QT interval may also be of importance in these patients since a prolonged QTc interval is associated with ventricular arrhythmias(Reference Bednar, Harrigan and Anziano4), and QT prolongation is an independent predictor of mortality in patients with end-stage renal disease(Reference Hage, de Mattos and Khamash5).
Haemodialysis patients also have a high prevalence of the arrhythmia atrial fibrillation (AF)(Reference Genovesi, Pogliani and Faini6, Reference Korantzopoulos, Kokkoris and Liu7), which is associated with increased mortality(Reference Genovesi, Vincenti and Rossi8) and SCD(Reference Genovesi, Valsecchi and Rossi9) in these patients.
An increased intake of n-3 PUFA and, in particular, the long-chained DHA and EPA has been associated with a reduced risk of SCD in healthy subjects(Reference Albert, Campos and Stampfer10, Reference Siscovick, Raghunathan and King11) and in patients with established CVD(Reference Marchioli, Barzi and Bomba12). Furthermore, an increased intake of n-3 PUFA might reduce the risk of AF(Reference Virtanen, Mursu and Voutilainen13, Reference Mozaffarian, Psaty and Rimm14). These possible beneficial effects of n-3 PUFA might be due to well-recognised antiarrhythmic effects demonstrated especially in animal studies(Reference Richardson, Iaizzo and Xiao15).
Levels of EPA and DHA are lower in plasma phospholipids in haemodialysis patients than those in subjects without kidney disease(Reference Madsen, Christensen and Svensson16), and a treatment strategy towards the serious supraventricular and ventricular arrhythmias in haemodialysis patients might be by increasing the n-3 PUFA intake. The aims of this study were to examine the association between n-3 PUFA and AF in haemodialysis patients as well as the effect of n-3 PUFA supplementation on the QTc interval.
Experimental methods
Study design
This substudy was based on data from a previously published trial regarding n-3 PUFA as secondary prevention against cardiovascular events and death in patients treated with chronic haemodialysis(Reference Svensson, Schmidt and Jorgensen17).
The study protocol has been described in detail previously(Reference Svensson, Schmidt and Jorgensen17). In brief, the inclusion criteria were known CVD (defined as previously reported myocardial infarction, angina pectoris, angiographically documented coronary atherosclerosis, stroke, transient ischaemic attack or peripheral vascular disease) and treatment of chronic haemodialysis for at least 6 months. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the regional ethics committee. Written informed consent was obtained from all the subjects/patients.
The study was a double-blinded randomised, placebo-controlled intervention trial with marine n-3 PUFA or control treatment. Randomisation was carried out using a computer-generated allocation sequence. All patients, investigators and monitors were masked to treatment allocation throughout the trial. The duration of this substudy was 3 months. Blood samples and electrocardiogram (ECG) evaluations were carried out at baseline and after 3 months.
Treatments
Supplementation with n-3 PUFA consisted of two capsules of Omacor (omega-3-ethyl esters 90) containing 37·5 % DHA (22 : 6n-3) and 45 % EPA (20 : 5n-3) (Pronova Biocare, Sandefjord, Norway). In total, 1·7 g n-3 PUFA was administered/d. The control capsules contained 77 % olive oil (18 : 1n-9). Active and control capsules were externally identical. Patients receiving n-3 PUFA before the study had a washout period of at least 4 weeks before inclusion.
Blood sampling and laboratory methods
Blood was drawn immediately before the patients' dialysis session. Owing to logistic reasons and time of dialysis session, only dialysis patients in the morning were assessed with fasting blood samples. Serum was stored at − 80°C in tubes filled with N2. Total lipids were extracted from serum and phospholipids were separated from other lipid classes, but the analysis has been described in detail previously(Reference Svensson, Schmidt and Jorgensen17). Levels of serum total-cholesterol, HDL and TAG were measured enzymatically by standard methods (Hitachi 991; Roche Diagnostics, New York, NY, USA).
The fatty acid composition of serum was analysed by GC using a Chrompack CP-9002 gas chromatograph (Varian, Middleburg, The Netherlands). With this quantification of fatty acids methyl esters with 14–24 carbon atoms and separation of several trans-fatty acids are possible. Inter-assay variation was 3·5 % for EPA and 2·8 % for DHA.
Electrocardiogram measures
A standardised 10-s twelve-lead surface ECG was obtained before each patient underwent dialysis at baseline and after 3 months of supplementation. If the patient reached an end point, an adverse event was observed or the patient was dropped out for other reasons, no ECG was obtained after 3 months. All interpretations of the ECG were carried out visually and all the measurements were performed manually using a standard ECG ruler. The criterion for having AF was that the 10-s baseline ECG showed AF. No attempts were made to further classify the AF, e.g. first detected, paroxysmal, persistent or permanent. For ECG with sinus rhythm (SR), electrocardiographic characteristics including heart rate (HR), PQ interval, QT interval, QRS duration (for QRS ≥ 120 ms) and RR (the interval between two R waves in the ECG) duration were measured.
To correct for sinus arrhythmia, the value of RR was the average of at least three measured RR intervals. The QT interval was an average of at least three measurements in three different leads, where onset and offset were best defined. Fridericia's formula (QTc = QT/(RR1/3))(Reference Fridericia18) was used to correct for HR for patients with a QRS < 120 ms. Bazett's formula (![]() )(Reference Bazett19) was also used (data not shown). In patients with a QRS ≥ 120 ms, the QT was corrected for HR and QRS duration as QTc = QT − 155 × (60/HR − 1) − 0.93 × (QRS − 139)+k, with k = − 22 ms for men and − 34 ms for women(Reference Surawicz, Childers and Deal20, Reference Rautaharju, Zhang and Prineas21). A QTc interval ≥ 450 ms for men and ≥ 460 ms for women was regarded as being prolonged(Reference Rautaharju, Surawicz and Gettes22).
)(Reference Bazett19) was also used (data not shown). In patients with a QRS ≥ 120 ms, the QT was corrected for HR and QRS duration as QTc = QT − 155 × (60/HR − 1) − 0.93 × (QRS − 139)+k, with k = − 22 ms for men and − 34 ms for women(Reference Surawicz, Childers and Deal20, Reference Rautaharju, Zhang and Prineas21). A QTc interval ≥ 450 ms for men and ≥ 460 ms for women was regarded as being prolonged(Reference Rautaharju, Surawicz and Gettes22).
Statistical analysis
Data were reported as mean values with their standard deviation. All P values were two sided and all confidence levels were computed to a 95 % level. P < 0·05 was considered as statistically significant. For comparisons of variables from baseline and after 3 months within the same group, paired t test was used. Comparisons of differences between the two groups were performed using a non-paired t test for continuous variables. The χ2 test was used for discrete variables and frequencies. To determine independent correlates of AF multivariate, we performed stepwise linear regression analysis.
The Statistical Package for the Social Sciences, version 11 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Patient data
In the present study, a total of 206 patients were randomised. Of these, thirty-eight patients discontinued intervention due to a cardiovascular event, adverse events, intercurrent disease or refusal. At baseline, twenty-seven patients had AF and 173 had SR, whereof 172 had a measurable QT interval.
At baseline average QTc interval for men was 411 (sd 24) ms (n 108) and for women it was 414 (sd 24) ms (n 64). In total, six men had a prolonged QTc (QTc ≥ 450 ms), while only one woman had a prolonged QTc (QTc ≥ 460 ms).
Before and after intervention one hundred and thirty-four patients had SR, and changes in QTc, PQ and HR could be analysed for these patients.
Atrial fibrillation and n-3 PUFA
At baseline twenty-seven (13 %) patients had AF. Baseline characteristics for the group with SR (n 173) and the group with AF (n 27) are given in Table 1. Level of DHA (but not EPA) was significantly lower in patients with AF compared with SR patients. Also patients with AF were significantly older and had a lower level of total cholesterol in serum and a lower systolic blood pressure.
Table 1 Baseline characteristics of the group with sinus rhythm (SR) and the group with atrial fibrillation (AF)
(Mean values, standard deviations, number of patients and percentages)

CABG, coronary artery bypass grafting; ACE, angiotensin-converting enzyme.
* Mean values were significantly different (P < 0·05).
In a multiple stepwise regression analysis AF was used as the dependent variable and the following parameters were included as independent variables: (1) DHA, (2) age, (3) serum HDL-cholesterol levels, (4) albumin, (5) duration of dialysis, (6) systolic blood pressure and (7) serum total cholesterol. All the patients were euthyroid. An echocardiogram was not performed in these patients, and therefore, left atrial dilatation (also a predictor of AF) could not be included in the model.
DHA, serum total cholesterol, systolic blood pressure, and age were independently associated with AF (Table 2).
Table 2 Multiple stepwise regression analysis with atrial fibrillation (present/not present) used as the dependent variable
(Standardised coefficients and t values)

* Independent variables included in the model: age, systolic blood pressure, DHA, total cholesterol, HDL-cholesterol, albumin and dialysis vintage.
QTc and n-3 PUFA
For the 137 patients with SR at baseline and after 3 months, sixty-seven were in the placebo group and seventy were in the n-3 PUFA group. Baseline characteristics were compared between the groups (Table 3). Only the use of aspirin was higher in the placebo group. The changes in QTc, HR and PQ over the 3 months are given in Table 4. QTc was not significantly affected by n-3 PUFA supplementation compared to controls (Table 4), although a trend towards a shorter QTc interval in the n-3 PUFA group was observed (P = 0·05). A subgroup analysis revealed that if the n-3 PUFA group was divided according to the upper median group (n 34) with the highest increase in DHA after supplementation (median increase 1·53 %), QTc decreased from 414 (sd 18) to 407 (sd 21) ms after n-3 PUFA supplementation (P = 0·01).
Table 3 Baseline characteristics for the 137 patients with sinus rhythm
(Mean values, standard deviations, number of patients and percentages)
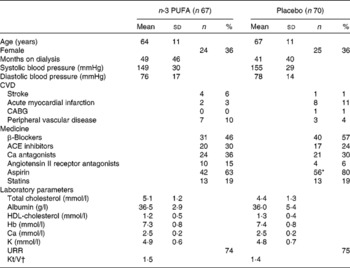
CABG, coronary artery bypass grafting; ACE, angiotensin-converting enzyme; URR, urea reduction rate.
* Values were significantly different (P < 0·05).
† Kt/V is a number used to quantify dialysis treatment adequacy.
Table 4 Electrocardiogram (ECG) values and compliance (n-3 PUFA levels) before and after supplementation
(Mean values, standard deviations and 95 % CI)
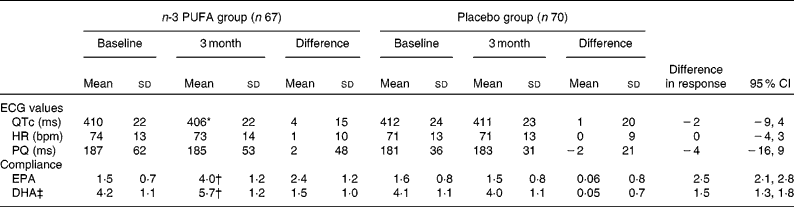
HR, heart rate; bpm, beats per minute.
* Mean values were significantly different from those of baseline (P = 0·05).
† Mean values were significantly different from those of baseline and placebo group (P < 0·0001).
‡ EPA and DHA are shown as % of total amount of fatty acids.
There was no effect of n-3 PUFA on the PQ interval or HR (Table 4). Upon using Bazett's formula, we found similar results (data not shown). Also, when controlling for the influence of β-blocking therapy on the ECG measures, the results were similar (data not shown).
During the supplementation period there was a significant increase in EPA and DHA in the n-3 PUFA group compared with the controls, indicating good compliance (Table 4).
Discussion
Atrial fibrillation and n-3 PUFA
The present study found an inverse association between the long-chained n-3 PUFA DHA and the prevalence of AF in chronic haemodialysis patients with CVD. This is, to the best of our knowledge, the first study to show this association in this population. This finding was further substantiated by multiple regression analysis including previously known risk factors for the development of AF in haemodialysis patients(Reference Korantzopoulos, Kokkoris and Liu7). Thus low DHA, low serum total cholesterol, increased systolic blood pressure and age all correlated independently with AF. This finding may suggest that marine n-3 PUFA could protect against AF, but clearly this hypothesis needs further testing in a large intervention study.
In the present study, the prevalence of total AF was 13 %, which is higher than that in the general population in which it is approximately 1 %, being 0·1 % below the age of 55 years and approximately 5·8 % in people aged 70–79 years(Reference Korantzopoulos, Kokkoris and Liu7, Reference Chen and Shen23). In line with the results of the present study, a recent large international study with 17 513 haemodialysis patients found a prevalence of 12·5 % and across all age groups the prevalence was higher than that in the general population(Reference Wizemann, Tong and Satayathum24). In the age group 65–74 years, equivalent to our study group, the average prevalence was 15 %. Also, other studies have indicated that AF is much more prevalent among end-stage renal disease patients(Reference Korantzopoulos, Kokkoris and Liu7). The occurrence of AF in haemodialysis patients is of importance, because AF has been associated with increased cardiovascular mortality in these patients(Reference Genovesi, Vincenti and Rossi8, Reference Wizemann, Tong and Satayathum24). In a study by Genovesi et al. (Reference Genovesi, Valsecchi and Rossi9) involving 476 haemodialysis patients, AF was also an independent risk factor for SCD.
Various studies have compared n-3 PUFA or fish intake with the incidence of AF in non-haemodialysis patients. In a prospective study 2174 men (aged 42–60 years) with a low prevalence of CVD were followed up for an average time of 17·7 years and a negative association between DHA (not EPA) and the incidence of AF was found(Reference Virtanen, Mursu and Voutilainen13). Similar results were found when comparing the amount of fish intake in an older population (aged >65 years) with the incidence of AF in 4815 participants during a 12-year follow-up(Reference Mozaffarian, Psaty and Rimm14). However, other epidemiological data have not confirmed the association between n-3 PUFA and AF(Reference Frost and Vestergaard25–Reference Brouwer, Heeringa and Geleijnse27).
The effect of n-3 PUFA supplementation has also been tested on postoperative AF in patients undergoing coronary artery bypass surgery. Two studies found a significant reduction in postoperative AF(Reference Heidt, Vician and Stracke28, Reference Calo, Bianconi and Colivicchi29), while two other studies found no effect on AF(Reference Saravanan, Bridgewater and West30, Reference Heidarsdottir, Arnar and Skuladottir31). Furthermore, n-3 PUFA supplementation was tested for the prevention of recurrent symptomatic AF in 663 patients with confirmed symptomatic paroxysmal or persistent AF(Reference Kowey, Reiffel and Ellenbogen32). n-3 PUFA could not prevent recurrent AF in these patients. However, it is possible that the mechanism behind postoperative AF may be different compared with AF in haemodialysis patients. Also, the duration of treatment with n-3 PUFA might affect outcome on AF.
A plausible explanation for the divergent findings is the heterogeneity across the studies(Reference Reiffel and McDonald33) and therefore the evidence for the effect of n-3 PUFA on AF is inconsistent. It is important to consider that conventional treatment of end-stage renal disease patients with AF is more complex and problematic than that in the general population due to the presence of comorbidities, contraindications and side effects of drugs(Reference Korantzopoulos, Kokkoris and Liu7). Given the large prevalence of AF in haemodialysis populations, it may be of relevance to conduct a larger intervention trial with n-3 PUFA in these patients also having in mind the relatively lack of side effect of these fatty acids(Reference Saravanan, Davidson and Schmidt34).
QTc, heart rate and n-3 PUFA
This study did not show a shortening of the QTc interval in patients with CVD receiving chronic haemodialysis after intake of n-3 PUFA compared with placebo.
In the present study only 4 % patients had a prolonged QTc. In a study by Hage et al. (Reference Hage, de Mattos and Khamash5) including 280 end-stage renal disease patients, 39 % patients had a prolonged QTc. Similar results were found among sixty-eight end-stage renal disease patients(Reference Voiculescu, Ionescu and Ismail35). Hage et al. also found that prolonged QTc was an independent risk factor of mortality in these patients. The definition for prolonged QTc interval used by Hage et al. was the same as used in our study, but the authors used Bazett's formula to correct the QTc for HR instead of Fridericia's. When using Bazett's formula in our study, the prevalence of prolonged QTc increased to 18 % (data not shown). Thus, the use of different methods cannot explain the difference in the prevalence of prolonged QTc. However, a major difference between our study population and the 280 end-stage renal disease patients examined by Hage et al. was the prevalence of diabetes mellitus being 24 % in our population and 60 % in the other population.
There are no previous data examining the effect of n-3 PUFA on QTc interval in haemodialysis patients. However, in line with the present study results, randomised studies in healthy individuals(Reference Geelen, Brouwer and Zock36) and individuals with frequent premature ventricular complexes(Reference Geelen, Zock and Brouwer37) showed no effect of n-3 PUFA on the QTc interval, though a non-significant decrease in the QTc interval was reported in the n-3 PUFA group among the healthy individuals compared with the placebo group. The duration of intervention for these studies was 12 and 14 weeks. Both studies had less than eighty-five participants and the daily supplementation was similar to the present study. In contrast, two cross-sectional studies found a significant association between a high intake of fish and a shorter QTc interval in 1822 healthy participants(Reference Chrysohoou, Panagiotakos and Pitsavos38) and 5096 patients with CVD(Reference Mozaffarian, Prineas and Stein39). Furthermore, one study in dogs has shown that n-3 PUFA shortens the QTc interval(Reference Billman, Kang and Leaf40). The consistent results in the randomised trials could be because the modifications caused by n-3 PUFA creating a change in the QTc interval are too small to detect by a standard surface ECG, as has been proposed previously(Reference Geelen, Brouwer and Zock36).
The amount of n-3 PUFA administered in our study was 1·7 g/d. Smaller doses of 1 g/d have been used(Reference Marchioli, Barzi and Bomba12), but it has been suggested that haemodialysis patients might have inadequate levels of marine fatty acids in tissue and blood because of increased breakdown of n-3 PUFA(Reference Friedman and Moe41). It is possible that the dose used was still too small to have an effect on the QTc interval and HR, but larger doses might not be clinically appropriate, because of the gastrointestinal discomforts associated with larger doses. The dosage issue is supported by the subgroup analysis, revealing a clearly significant decrease in QTc among 50 % of the patients with the highest increase in DHA after supplementation. The present study data on the subgroup analysis may support an antiarrhythmic effect of n-3 PUFA in haemodialysis patients but it should be emphasised that the intention-to-treat analysis did not show any effect on QTc when comparing the intervention group with the placebo group.
HR is associated with overall mortality and cardiovascular mortality. One of the most consistent findings is that n-3 PUFA slows HR(Reference Mozaffarian, Geelen and Brouwer42). In the present study, in haemodialysis patients HR in a resting ECG was not decreased after supplementation with n-3 PUFA. In a meta-analysis by Mozaffarian et al. (Reference Mozaffarian, Geelen and Brouwer42), including thirty randomised trials, it was documented that n-3 PUFA reduces HR. The reduction was most pronounced in trials lasting >12 weeks. The duration of the present study was 3 months and together with the above-mentioned medication including β-blockers it could be anticipated that any possible slowing effect of n-3 PUFA on HR might have been obscured by the medication the patient already received.
Strengths and limitations
One of the strengths of the present study was the randomised, double-blinded placebo-controlled design. Another strength was the use of serum n-3 PUFA measurements of phospholipids in serum to monitor compliance.
A limitation to AF data was the cross-sectional design when examining the association between AF and n-3 PUFA at baseline. DHA was independently negatively correlated with AF, but we could not conclude that there are causal interactions.
When comparing the QTc interval before and after intervention, data were available for only 137 of the 206 patients. It is therefore possible that we overlooked a significant difference in shortening of the QTc interval between the groups due to the small group examined. Also manual reading of the QTc interval and correcting it for HR(Reference Rautaharju, Surawicz and Gettes22) may have introduced bias. The onset of the QRS complex, the termination of the T wave as well as the proper lead to use can be difficult to determine. Also, it may be of importance that the patients were heavily medicated, which might have obscured any possible effects.
QTc is possibly a relatively crude predictor of outcome, and furthermore, as other risk markers for the development of cardiac arrhythmias, it has a low sensitivity and specificity for these arrhythmias.
Conclusion
AF in haemodialysis patients with CVD was associated with a low level of the n-3 PUFA DHA. The present study is the first to examine the effect of n-3 PUFA on the QTc interval in haemodialysis patients. n-3 PUFA did not significantly change the QTc in these patients when compared with placebo, but subgroup analysis pointed at a possible shortening effect of n-3 PUFA on QTc. Whether DHA may prevent AF in haemodialysis patients should be investigated in larger randomised trials in these patients without AF at baseline.
Acknowledgements
This work was supported by the Danish Heart Foundation, the Danish Kidney Foundation, the Research Foundation of the Country of Northern Jutland and Pronova Biocare. None of the authors has any conflict of interest to state. Contributors: E. K. and J. H. C. took part in organising the study, analysing and interpreting the data, and preparing the manuscript. C. S., E. B. S. and K. A. J. helped in preparing the manuscript. M. S. helped in preparing the manuscript and was responsible for all data collection and data management in the main study. E. K. was responsible for the operative organisation of this substudy and all ECG measurements and interpretation.






