Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder.Reference Tanner and Goldman 1 The prevalence of PD in industrialized countries has been estimated to be around 0.3% in the entire population and 3% in patients above 65 years of age.Reference Rajput 2 , Reference Gourie-Devi, Gururaj, Satishchandra and Subbakrishna 3 This disease tends to have a significant social and financial burden. Following the discovery of levodopa (LD) in the 1960s, it has remained the most widely used drug for the treatment of PD.Reference Hornykiewicz 4 Several other treatment options have become available, both medical and surgical, but pharmacotherapy remains the mainstay of treatment.
Several factors determine the choice of medications in patients with PD. The general principle of therapy is to keep the patient functionally independent for as long as possible.Reference Olanow, Stern and Sethi 5 The drugs employed depend on the age of the patient, the stage of the disease, affordability, the presence of motor fluctuations, and coexisting other neurological, psychiatric, and systemic disorders. Patients respond favorably to treatment in the early stage of disease. However, once the motor fluctuations and nonmotor symptoms become prominent, treatment needs to be individualized, with regular monitoring of efficacy and side effects.Reference Surathi, Jhunjhunwala, Yadav and Pal 6 It is also necessary to identify patients who may benefit from deep brain stimulation.
About 70% of the Indian population live in rural areas and have limited access to a movement-disorder specialist.Reference Bagcchi 7 General physicians or internists treat the majority of these patients.Reference Deo 8 The prescription pattern differs widely for several reasons: the experience of the treating physician, the cost of therapy, drug compliance, an awareness of the disease in the community, the patient’s education and understanding of the disease, a lack of medical insurance for most patients, and easy availability of indigenous and alternative therapies.Reference Bauer, Monz and Montejo 9 , Reference Tripathi, Avasthi and Desousa 10 Moreover, as our patients are free to consult any doctor, the prescription pattern also varies depending on the number of doctors consulted.
There is a need to assess the appropriateness and adequacy of the treatment of Parkinson’s disease in the community. We therefore undertook this study to review the pattern of medication prescription in the community prior to attending our tertiary-care center.
Methods
Our study was a chart review of 800 patients with PD attending for the first time the Department of Neurology of the National Institute of Mental Health and Neurosciences in Bangalore, India, which is a tertiary-care referral neurosciences center. The institutional ethics committee of the institute approved the study. PD patients presenting during the years 2001–2014 were included. The senior movement-disorder specialist (PKP) examined all the patients in detail. The diagnosis of PD was based on Calne’s criteria.Reference Calne, Snow and Lee 11
The demographic profile, referral diagnosis, and clinical details (including age at onset, duration of illness, and Hoehn and Yahr [H&Y] staging) were retrieved from the case records. A detailed history of all the medications used by the patients, including the dosage and duration of each drug, was recorded. Patients were subdivided into three groups: (1) group 1—patients who were on medications at the time of their first visit; (2) group 2—patients who initially received medications but later discontinued; and (3) group 3—patients who never took any medications for PD (drug-naïve). In all the groups, patients were subgrouped into tremor-predominant and akinetic-rigid PD.Reference Barbeau and Porcher 12 , Reference Jankovic, McDermott and Carter 13
The levodopa equivalent daily dosage (LEDD) was calculated for group 1 and 2 patients. These calculations were based on the standardized formulae proposed by Tomlinson et al.Reference Tomlinson, Stowe, Patel, Rick, Gray and Clarke 14 The conversion factors used were: ×1 for immediate-release LD, ×0.75 for controlled-release LD, ×0.33 for entacapone, ×100 for pramipexole, ×20 for ropinirole, ×10 for selegiline, ×100 for rasagiline, and ×1 for amantadine. The total LEDD (T-LEDD) was calculated by adding all dopaminergic medications including LD and a dopamine agonist (DA), while levodopa-LEDD (LD-LEDD) was calculated by adding only LD doses (both immediate-release and controlled-release).
The demographic and disease characteristics of the patients within the groups were compared using a one-way ANOVA test and post-hoc Bonferroni analysis (with a value of p<0.05 considered significant). The mean LEDD according to subtype of PD and presence of dyskinesia was calculated using the chi-square test. Pearson’s correlation was employed to analyze the relationship between T-LEDD and H&Y staging, duration of the disease, and the patient’s income.
Results
Demographics and General Characteristics (Table 1)
A total of 800 patients (23.8% women) were included in the study. The mean age of PD patients was 54.9±11.3 years, and the age at onset of motor symptoms was 51.1±11.8 years. The duration of motor symptoms at the time of their first visit to our center ranged from 1 month to 20 years, with a mean of 41.7±43.6 months (3.5±3.7 years). Approximately 42% patients were from rural areas. The average monthly income of the whole group was Rs 4903.5±5053.3 (USD 75.7±78.01). Approximately 84.5% had a monthly income of less than Rs 10,000 (~USD 154.4).
The initial diagnosis was made by general practitioners (with only an MBBS degree) in 29.1%, by internists (with an MD in medicine) in 53.8%, and by neurologists (a DM or DNB in neurology) in 16.8% of patients. Nearly three-quarters of patients (74.3%) consulted us for a second opinion regarding the diagnosis and treatment, and the rest were referred for better care.
The mean age at onset of motor symptoms in group 1 patients was 51.1±11.6 years, 52±13.1 years in group 2, and 50.4±12.5 years in group 3, the differences being statistically insignificant between groups. Men outnumbered women in all groups. The majority of patients in group 3 were from rural areas as compared to the other two groups. The duration of illness in groups 2 (28.7±25.4 months) and 3 (17.1±13.7 months) was shorter than in group 1 (46.6±46.5 months), which was statistically significant (p=0.001).
In the majority of patients (58.7%), the initial symptoms were noted on the left side. About 59.9% patients had tremor-predominant PD. Among the subgroups, group 1 (61.7%) and group 3 (58.9%) had tremor-predominant PD, whereas akinetic rigid-predominant PD was more common (53.8%) in group 2. The difference was significant between groups 1 and 2 (p<0.001) and between groups 2 and 3 (p<0.01). There was no significant difference in H&Y stage among the three groups. The details are presented in Table 1.
Table 1 General characteristics of the patients

* Indicates p value (ANOVA with Bonferroni post-hoc analysis).
LEDD=levodopa equivalent daily dose; SD=standard deviation.
Medications Used at the First Visit
At the first visit, 79.4% (group 1, n=635) of the patients were on medications for PD, 10% (group 2, n=80) of patients were on medications earlier but had discontinued, and 10.6% (group 3, n=85) were not on any antiparkinsonian drugs. The exact reasons for discontinuation of medications were not known in most of these patients, but in 44% the reasons for the visit to our hospital were either dissatisfaction with treatment or to obtain a second opinion about their illness.
A comparison of the patients in group 1 with those in group 2 (Table 1) showed that patients who discontinued medications (group 2) had a shorter duration of illness, lower socioeconomic status, and lesser incidence of a tremor-predominant phenotype of PD compared to those who continued medications (group 1). The various medications used by the patients at the time of the first consultation at our hospital are presented in Table 2.
Table 2 Profile of medications used by the patients
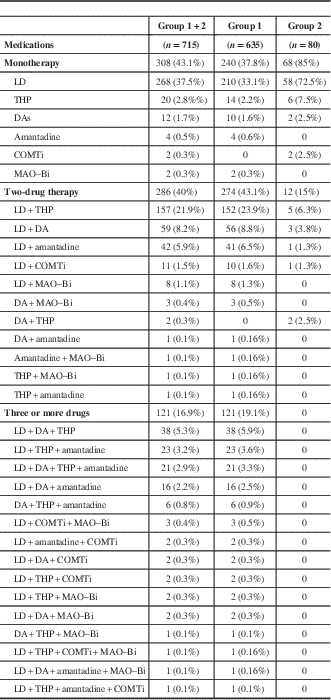
COMTi=catechol-O-methyl transferase inhibitor; DA=dopamine agonist; LD=levodopa; MAO–Bi=monoamine oxidase B inhibitor; THP=trihexyphenidyl.
LD was the most common medication prescribed in groups 1 (93.1%) and 2 (85%), either as monotherapy or in combination with other drugs. About 43.1% in group 1 were on two medications, 37.8% on monotherapy, and ~19.1% on three or more medications. LD monotherapy was most common (33.1%), followed by trihexyphenidyl (THP) (2.2%), DAs (1.6%), and amantadine (0.6%). Among the DAs, the ergot-derived piribedil was prescribed in 0.3% of patients. In those receiving polytherapy (62.2%), several drug combinations were employed (see Table 2), with LD plus THP being the most commonly used combination (23.9%). Other medications that were prescribed in a few of the PD patients were propranolol and clonazepam.
In group 2, LD monotherapy was also most common (72.5%), followed by THP monotherapy (7.5%) and the combination of LD plus THP (6.3%). Patients in this group were never prescribed three or more antiparkinsonian medications.
Further analysis to determine the frequency of individual drug prescriptions either as monotherapy or in combination with other drugs showed that THP was the next most commonly used drug after LD. The details are given in Table 3.
Table 3 Frequency of prescription of individual drugs in Parkinson’s disease
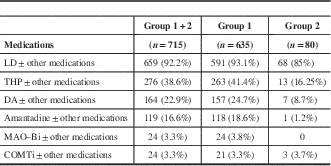
COMTi=catechol-O-methyl transferase inhibitor; DA=dopamine agonist; LD=levodopa; MAO–Bi=monoamine oxidase B inhibitor; THP=trihexyphenidyl.
A history of dyskinesia was more frequent in group 1 (20.3%) than in group 2 (10%), a difference that was statistically significant (p=0.02).
Analysis of LEDD Doses
The mean daily T-LEDD was 448.1±304.5 mg for the whole group, 469.5±308.9 mg for group 1, and 281.5±201.8 mg for group 2. The T-LEDD difference between groups 1 and 2 was significant (p ≤ 0.001). Similarly, the LD-LEDD for the whole group was 378.1±369.0 mg, 379.9±362.1 mg for group 1, and 376.3±106.1 mg for group 2. In group 1, patients with a history of dyskinesia (n=129) were on a higher T-LEDD compared to those without (n=506; 564.25±327.67 mg vs. 445.34± 299.56 mg; p<0.001).
Discussion
This study analyzed a cohort of 800 PD patients with different medication prescriptions before their first consultation with a movement-disorder specialist in a tertiary-care center in India. In comparison to other studies, the age at onset of our cohort was less by 10 to 20 years.Reference Gourie-Devi, Gururaj, Satishchandra and Subbakrishna 3 , Reference Das, Biswas and Roy 15 - Reference de Lau and Breteler 18 General practitioners and internists diagnosed most of the patients (82.9%) initially. Around 20.6% of patients (groups 2 and 3) were not on medications at the time of first attending our hospital. The factors more often associated with discontinuation of medications were probably shorter duration of illness, low socioeconomic status, and akinetic rigid phenotype. Poor medication compliance in PD has been reported to be around 15–67%.Reference Fleisher and Stern 19 This included skipping doses, taking lesser doses than prescribed, and taking medications at the wrong time of day. The reasons that have been attributed include depression, cognitive impairment, lack of social support, poor health education, the cost of medications, and patients’ unrealistic expectations about the beneficial effects of the medications.Reference Fleisher and Stern 19 - Reference Tarrants, Denarie, Castelli-Haley, Millard and Zhang 21
The most commonly prescribed medications in our study were LD alone (37.5%), followed by LD in combination with THP (21.9%) or with a DA (8.2%). This is in sharp contrast to the higher prevalence of a combination of LD plus a DA reported from other countries—such as Korea (61.2%), Japan (56%), Italy (41.3%), Europe (40%), and the United States (35%).Reference Lee, Kim and Lee 22 - Reference Leoni, Martignoni and Cosentino 24 Most of the patients in group 1 were on LD, either monotherapy or in combination with other drugs. A few patients (3.6%) were not on any dopaminergic drugs but were on such other drugs as anticholinergics and beta blockers only.
LD and THP were more often prescribed in India than DAs, which can be attributed to the increased cost of DAs, physicians’ lack of experience with DAs, or an awareness of the potential side effects of DAs (including nausea, vomiting, peripheral edema, hallucinations, delusions, orthostatic hypotension, and compulsive behavior).Reference Huse, Castelli-Haley, Orsini, Lenhart and Abdalla 25 In our study, THP was prescribed in all age groups equally, and it was the most common medication prescribed either alone or in combination (38.6%) after LD alone or in combination (92.2%). In addition, our study cohort was relatively younger (mean age of onset=54.9±11.3 years) than the PD patients in other countries, where the mean age of PD onset is much higher (65 years).Reference Hoehn and Yahr 16 - Reference de Lau and Breteler 18 It is also possible that most of our patients had tremor-predominant PD, which might have led to an increased number of prescriptions for THP. Ideally, THP needs to be avoided in the elderly, as it may precipitate cognitive dysfunction and can affect bladder function in older males. A U.S. studyReference Huse, Castelli-Haley, Orsini, Lenhart and Abdalla 25 found that the choice of initial therapy was determined by age, source of medical insurance, and comorbidities.
DAs alone or in combination with other drugs were prescribed in 22.9% of our 800 patients, which is much lower than that reported from other countries: Italy (42.3%), Germany (65%), Korea (68.1%), and Japan (71%).Reference Lee, Kim and Lee 22 - Reference Leoni, Martignoni and Cosentino 24 , Reference Schroder, Kuessner, Arnold, Zollner, Jones and Schaefer 26 In our study, those who discontinued medications had been prescribed DA monotherapy more often than those who continued treatment (2.5 vs. 1.6%, p=0.01). However, this was not observed when a DA was used along with other antiparkinsonian medications.
DA monotherapy was prescribed in 26.8% of PD patients in Singapore, and the preferred drug combination was LD plus benzhexol (13.7%).Reference Tan, Yeo, Tan, Pavanni and Wong 27 In a study from southern Italy,Reference Trifiro, Savica and Morgante 28 LD and anticholinergics were the most commonly prescribed medications.Reference Trifiro, Savica and Morgante 28 Anticholinergic monotherapy was used in 20.7%, ergot-derived DAs in 19.4%, LD in 14.8%, and non-ergot DAs in 6.5%. However, LD and other drug combinations were the most commonly prescribed medications in their patients. In our study, ergot-derived DAs (piribedil) were used only in 0.3% of patients. Piribedil is an expensive drug, and the lack of experience with this drug among our physicians and general practitioners may contribute to its reduced prescription rate. The side effects related to piribedil (an ergot alkaloid) and easy availability of other medications has also resulted in less use of this agent. In one study,Reference Tarrants, Denarie, Castelli-Haley, Millard and Zhang 21 maximum compliance was obtained with rasagiline.
Long-term affordability of medications in our patients is also an important factor, as most of them are not covered by medical insurance. Moreover, some patients may have preferred to take traditional forms of treatment, which are common for chronic diseases in our country. Finally, it is likely that our patients were not communicated with adequately about the nature of their illness, the need for drug compliance, the expected complications, and the importance of regular follow-ups.
The use of catechol-O-methyl transferase (COMT) and monoamine oxidase B (MAO–B) inhibitors were also very low in our study.
To determine the effects of different dopaminergic medications, we also calculated T-LEDD and LD-LEDD for all of our patients. The LEDD usually correlates with patient gender and age. We found a decrease in LEDD in women and older patients.Reference Lee, Kim and Lee 22 However, we did not find any effect of age and gender on LEDD.
Our patients who developed levodopa-induced dyskinesia (LID) were found to have a higher T-LEDD and LD-LEDD. Other studies have also found a higher LEDD in patients with LID compared to those without.Reference Suh, Pahwa and Mallya 29 The most common strategy to alleviate LID is to fractionate the LD dose. Other strategies include decreasing the LD dose and adding a DA with amantadine.Reference Muller, Woitalla, Russ, Hock and Haeger 30 Other important factors that determine LEDD are the stage and duration of the disease.Reference Hoehn and Yahr 16 Neither of these had any significant effect on LEDD in our patients. This suggests that adequate adjustment of medications according to the progression of the disease is lacking.
The strength of our study is that all the patients were evaluated by a single investigator, ensuring correct diagnosis of patients. The medication history was verified directly from the patients on their first visit to our hospital from their previous prescriptions and the medications they carried with them.
Limitations
One limitation of our study is that we did not assess the comorbidities of patients and the medications prescribed for other symptoms (including nonmotor symptoms) and other disorders, as these are very important factors in considering the type of treatment in PD patients. There may also be a small selection bias, as we reviewed only a fraction of the patients who attended our center.
Conclusions
The treatment of PD in our community, with most patients belonging to low and middle socioeconomic strata, may be inadequate in terms of using appropriate medications and adequate doses tailored to patients’ needs. Anticholinergic drugs are more often used, even in the elderly. Such other recommended medications as DAs as well as MAO–B and COMT inhibitors are infrequently prescribed, probably because of higher costs, the side-effect profile, and the need for long-term treatment. Many of our patients were also drug-naïve, and there was a high rate of discontinuation of medications.
The pattern of prescription in PD patients reported in our study cannot be generalized, and our practices may not be applicable to affluent PD patients. However, as most of our patients were of low socioeconomic status, the data presented here may truly reflect the prevalent prescription pattern in our country. Therefore, this trend of treatment may represent what is occurring in 70 to 80% of the Indian population.
The awareness among general practitioners and internists regarding the characteristics of the disease, the course of the illness, the available treatment options, and the associated long-term side effects that come with these medications needs to be increased. This would encourage them to treat patients confidently with adequate dosages, to use all the available pharmacological treatments appropriately, and to more diligently ensure drug compliance. They also need to be aware of the appropriate timepoint at which patients need to be referred to a tertiary-care center or to a movement-disorder specialist.
Disclosures
Pratibha Surathi, Nitish Kamble, Ketaki Swapnil Bhalsing, Ravi Yadav, and Pramod Kumar Pal do not have anything to disclose. None of the authors have any financial disclosure to make or have any conflicts of interest.





