Introduction
Epilepsy can have a profound impact on psychosocial function and quality of life (QOL). Cognitive, emotional, and behavioral conditions, ability to work, social functioning, family stability, self-esteem, stigma, and adjustment to seizures seem to be especially crucial to the QOL of patients with epilepsy. Reference Devinsky, Wyllie, Cascino, Gidal and Goodkin1,Reference Hermann2 Several studies indicate that complete seizure freedom is a strong positive predictor of psychosocial adjustment. Reference Lehrner, Kalchmayr and Serles3,Reference Gilliam, Kuzniecky and Meador4
Seizure freedom without deficits remains the prime goal for epilepsy surgery. However, patients with medically refractory epilepsy commonly suffer from a number of non-seizure co-morbidities, including psychiatric, cognitive, and sleep disturbances as well as various social problems and resultant stigma.
In addition to postoperative psychosis and mania, which may be transient, increases in anxiety, depression, and psychosocial adjustment difficulties have been reported. Reference Carran, Kohler, O'Connor, Bilker and Sperling5,Reference Wrench, Wilson and Bladin6 Some observers have found that there may not be a net increase in psychopathology, but rather a shift, with some preoperative disorders resolving and others developing, Reference Cankurtaran, Ulug, Saygi, Tiryaki and Akalan7,Reference Glosser, Zwil, Glosser, O'Connor and Sperling8,Reference Inoue and Mihara9 and yet other investigators have documented transient improvements and an overall stability of anxiety and depression. Reference Spencer, Berg and Vickrey10
While many recent studies on epilepsy surgery outcomes do mention associated QOL and neuropsychological outcomes, and many investigators have described the impact of epilepsy surgery on various non-seizure parameters such as cognitive functions, Reference Langfitt, Westerveld and Hamberger11–Reference Tang, Yu and Zhou13 social functioning, Reference Shih, Francis-Auton, Nikpour, Herkes, Bleasel and Rapport14 psychiatric morbidity, Reference Desai, Shukla and Goyal15,Reference Ramos-Perdigues, Bailles and Mane16 sleep quality Reference Zanzmera, Shukla and Gupta17 , and stigma and/or discrimination, Reference Fletcher, Sims-Williams, Wabulya and Boling18 results from comprehensive outcome assessments examining multiple common non-seizure outcomes of patients undergoing epilepsy surgery have scarcely been reported. The objective of this study was to comprehensively evaluate, through a structured format, the role of various non-seizure parameters on post-epilepsy surgery QOL.
Methods
This study was conducted prospectively at one center over a 2-year period between 2015 and 2017. Consecutive post-epilepsy surgery patients attending follow-up visits at a single clinical unit as part of the comprehensive epilepsy program of the All India Institute of Medical Sciences (AIIMS), New Delhi, India, who had provided consent for assessment, formed the study population.
Inclusion Criteria
Consecutive patients with medically refractory focal epilepsy who had undergone resective epilepsy surgery for the same at least 1 year prior to enrollment were included.
Exclusion Criteria
Patients who had undergone more than one surgery for epilepsy and those who had undergone disconnection (palliative) surgery were excluded. Those with serious non-neuropsychiatric co-morbidities such as chronic cardiac, renal, or other systemic diseases were also excluded. Neurostimulation techniques were not at the time being used for epilepsy treatment at this center; epilepsy patients who had received neurostimulation treatments or resective surgery at other centers were also excluded.
Epilepsy Surgery
All patients had undergone extensive presurgical evaluation as per the AIIMS protocol that included detailed clinical assessment by an epileptologist, continuous prolonged video-EEG monitoring with at least 2–3 habitual events recorded, epilepsy protocol 3 T magnetic resonance imaging (MRI), and ictal–interictal subtraction SPECT co-registered to MRI and/or FDG-PET when indicated (e.g., extratemporal lobe epilepsy or discordance between clinical, video-EEG, and MRI findings). Preoperative assessments had been conducted for all patients included in this study as a routine part of their presurgical evaluation. Selection for surgery and surgical procedure was made on a case-to-case basis through discussion at weekly multidisciplinary meetings of the epilepsy team, and surgery was offered with or without prior prolonged recordings using intracranial electrode placements or intraoperative electrocorticography.
Patient Categorization
Personal and demographic details along with a complete description of seizures and the epilepsy surgery were recorded for each of the participant patients. Epilepsy details including duration since first seizure, history of initial precipitating events, seizure semiology and type, epilepsy etiology, and seizure frequency, both before and after the surgery, were noted for all using a prestructured format. Seizure outcomes were documented according to the Modified Engel’s score Reference Pauli, Schwarzbold and Diaz12 and for the current study classified as seizure-free (group 1) or non-seizure-free (group 2).
Comprehensive Assessment of Non-Seizure Outcome Parameters
QOL was assessed using the QOLIE-31 instrument Reference Cramer, Perrine, Devinsky, Bryant-Comstock, Meador and Hermann19 , and the overall score was calculated as per the scoring manual where higher scores represent better QOL.
The overall score was then converted to obtain a T score as detailed in the QOLIE-31 scoring manual (https://www.rand.org/health-care/surveys_tools/qolie.html). Patients with a T score less than 40 (overall score = 46 points or lower) were categorized as having poor QOL in accordance with international standards of interpreting the results of neuropsychological tools and scales. Reference Heaton20,Reference Strauss and Spreen21
The non-seizure parameters assessed were cognition (language, memory, and executive function), psychiatric disturbances, social improvement (in education, employment, and marital prospects or harmony), presence or not of stigma, and sleep disturbances (sleeping too short or too long, excessive daytime sleepiness, non-refreshing sleep, SDB, or restless legs syndrome (RLS)). Simple categorization into good and poor outcome subgroups on each item was carried out by dichotomization of scores on each.
Cognitive Evaluation: Cognitive function assessment was carried out using tests used by us in previously published studies. A detailed assessment of memory and language was conducted using the Postgraduate Institute memory scale (PGI memory scale) Reference Pershad and Wig22 and Western Aphasia Battery, Reference Kertesz23,Reference Karanth24 respectively. Executive functions were assessed by using a battery of tests including Stroop Color Word Test, Trail Making Test A and B, and Digit Symbol tests. Reference Ivnik, Malec, Smith, Tangalos and Petersen25–Reference Tombaugh, Kozak and Rees27 Cognitive evaluation was completed by qualified clinical neuropsychologists.
Scores of the memory scale, Western Aphasia Battery, Stroop Word Task, Trail A, Trail B, and Digit Symbol substitution test were categorized into “Impaired” and “Normal” based on normative data.
Psychiatric Evaluation: The psychiatric evaluation focused on screening of three psychopathologies, namely anxiety, depression, and psychosis. Screening of anxiety and depression was done using the Hamilton Rating Scales for Anxiety (HAM-A) Reference Hamilton28 and Depression (HAM-D), Reference Hamilton29 respectively. Based on scores obtained on HAM-A and HAM-D, the patients were categorized as “Anxiety Present/No Anxiety” and “Depression Present/No Depression.” The cutoff provided by the authors were used for this categorization (Appendix 1). The screening of psychotic symptoms was done by noting if psychotic symptoms were reported or not as per the DSM V criteria.
Sleep Quality Evaluation: To assess sleep quality, seven questions were asked including the modified Indian version of the Epworth Sleepiness Scale. Reference Bajpai, Shukla and Pandey30 A sleep score was devised with one point given to each parameter (Appendix 2).
In addition, questions of the Berlin questionnaire Reference Netzer, Stoohs, Netzer, Clark and Strohl31 used for screening for sleep-disordered breathing (SDB) as well as questions based on the International RLS study group criteria for diagnosis of RLS Reference Allen, Picchietti and Garcia-Borreguero32 were also asked to look for the presence of either SDB or RLS, or both, as a possible cause for any sleep disturbance.
Social improvement: Patients were asked to report if they experienced improvement following epilepsy surgery, in either or all of:
-
Educational/employment status
-
Financial status
-
Marital status [prospects (for unmarried)/marital harmony (for married)]
A negative response to all three of the above questions would categorize the patient into the “no social improvement” category, while improvement reported in any of the above would be categorized as “social improvement present.”
In addition, social stigma was assessed using the Stigma Scale of Jacoby, Reference Jacoby33 which consists of three questions assessing perceived stigma. Patients giving a “Yes” response to any of the questions were considered to feel stigmatized.
As a protocol, anti-seizure medication (ASM) tapering was not initiated until 2 years of seizure freedom following epilepsy surgery. Since most of the common adverse effects of these medications were covered under the different categories of co-morbidities evaluated in detail (cognitive, psychiatric, and sleep) and overall QOL evaluation, and as it would be difficult for patients and assessors to determine whether or not to attribute certain symptoms to ASMs, this was not included as a separate category.
Statistical Analysis
Analysis was conducted using Stata 11.2 software. Mean and standard deviation were used to describe the continuous variables, whereas frequencies and percentages were used to describe the categorical variables.
As described above, the patients were divided into two groups based on complete seizure freedom (seizure-free/non-seizure-free). These groups were further subdivided based on QOL (good QOL/poor QOL). After categorization into groups, Student’s t-test or Wilcoxon rank sum test were applied to look for differences between the two groups. The Kruskal–Wallis test was applied in the case of more than two groups.
Additionally, binomial logistic regression analysis was applied to identify significant predictors of QOL status (i.e., good QOL/poor QOL). All p-values less than 0.05 were considered significant.
Results
Over the study period, a total of 37 patients (16 F) with mean age of 23.5 ± 5.6 years were evaluated.
Epilepsy details: The median age at onset of epilepsy was 7 years (range 1–15 years), and the median duration of epilepsy was 12 years (range 2–19 years). All patients were on two or more ASMs, the commonest prescribed ASM being oxcarbazepine (23 patients). As a regular policy of our program, continued use of more than two ASMs following epilepsy surgery had been proactively avoided, and at least one ASM was suggested to be continued lifelong. Anterior temporal lobectomy for mesial temporal lobe epilepsy was offered to 21 patients, 3 patients underwent lateral temporal lobe resection, 11 patients underwent extratemporal lobe resections (one received multilobar resection), and 2 patients underwent functional hemispherectomy. Resective surgery was performed on the left hemisphere in 17 patients and on the right hemisphere in 20 patients (Table 1).
Table 1: Epilepsy clinical and surgical details for patients undergoing comprehensive outcome assessment following surgical treatment (N = 37)
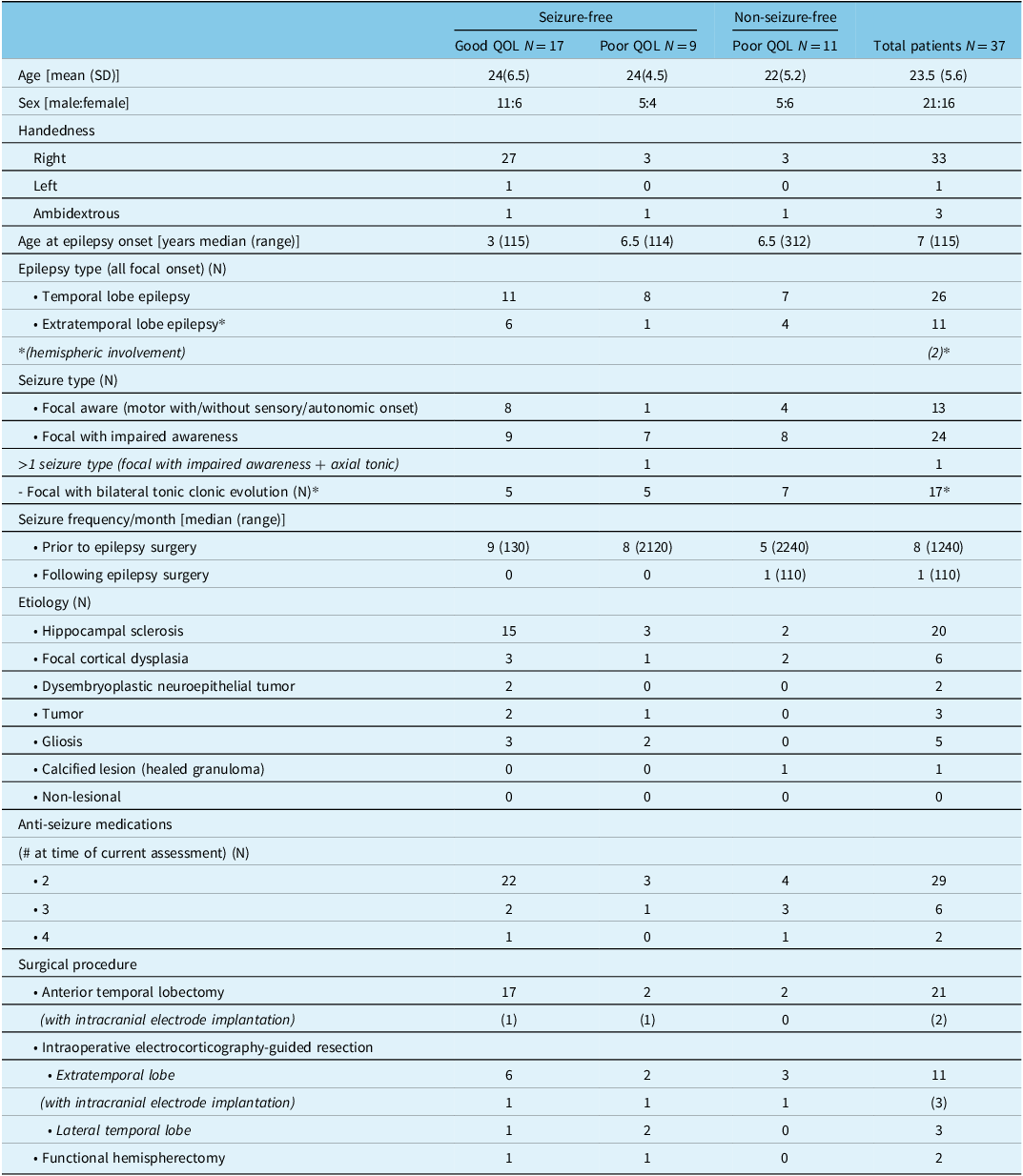
For the total group of 37 patients median, postsurgical follow-up was 19 months (range 14–53 months). Twenty-six patients were seizure-free (group 1), and the remaining 11 patients were categorized as non-seizure-free (group 2).
Seizure Outcomes and QOL
Among the 26 patients ingroup 1, 17 (65.4%) could be categorized in the “good” QOL group, whereas 9 (34.6%) had “poor” QOL scores. All patients in group 2 had poor QOL scores (Table 2). The distribution of various non-seizure outcomes parameters is presented in Table 2.
Table 2: Distribution of different non-seizure outcome parameters among seizure-free and non-seizure-free patients who underwent epilepsy surgery over a 2-year study period (N = 37)
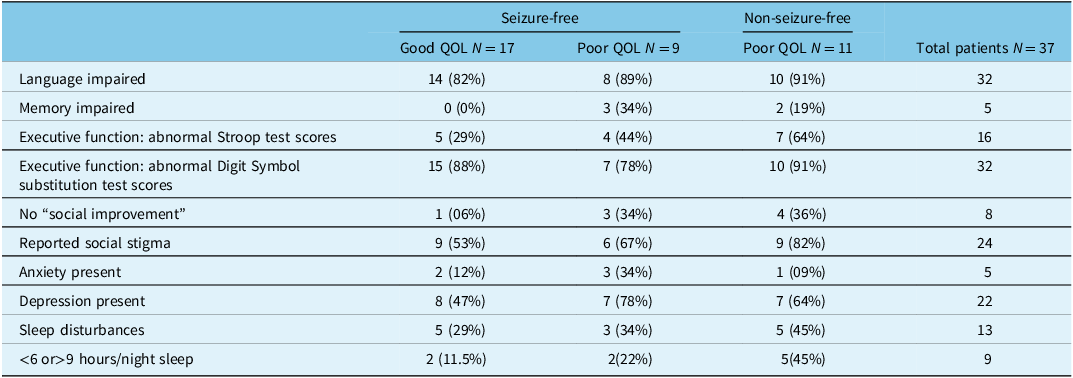
Interestingly, all patients in group 1 who had poor QOL scores had undergone temporal lobe resections, whereas > 50% (8/17) of those with good QOL scores had undergone extratemporal resection surgeries.
Non-Seizure Outcomes and QOL
More than a third of patients (35%) in group 1 reported a poor QOL post-epilepsy surgery, despite being seizure-free (Table 3). Among all group 1 patients, the subgroup with poor QOL contained a significantly higher proportion of patients with poor memory scores and depressive symptoms compared to the good QOL subgroup. In addition, although there was no significant difference in the proportion of group 1 patients with impaired language scores in the good versus poor QOL subgroups, mean aphasia quotient and cortical quotient subscores were significantly poorer among those with poor QOL. Additionally, patients who were not employed and/or actively engaged in educational activities appeared to experience poorer QOL, significantly so, if also experiencing (and reporting) social stigma (Table 3; Supplementary data appendix 1a,1b).
Table 3: Association of different parameters with post-epilepsy surgery QOL among seizure-free patients
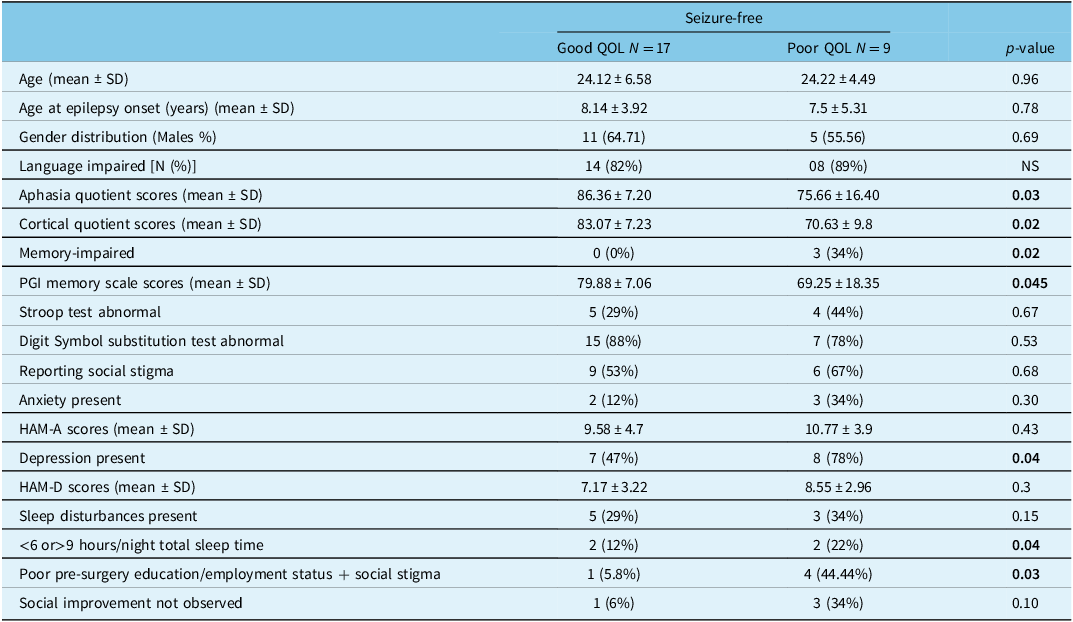
HAM-A = Hamilton Rating Scale for AnxietyReference Hamilton28; HAM-D = Hamilton Rating Scale for DepressionReference Hamilton29; PGI memory scale = Postgraduate Institute memory scaleReference Pershad and Wig22; QOL = quality of life; SD = standard deviation.
In group 2, all patients had poor QOL scores and 91% of patients had impaired language and executive functions, 82% reported social stigma, and 64% were depressed (Table 2).
Sleep disturbances were observed in 29–45% of patients among the different subgroups, but overall these were equally prevalent in group 1 and group 2 patients. However, the proportion of patients with fewer than 6 or more than 9 hours of sleep per night (on average, self-reported) was significantly higher in the poor QOL subgroup. None of the patients could be categorized as high risk on the Berlin questionnaire; one patient in group 2 fulfilled criteria for the diagnosis of RLS (Supplementary data appendix 2).
Binomial logistic regression analysis, conducted to identify predictors of QOL status (i.e., poor QOL vs. good QOL), showed none of the non-seizure parameters to be a significant predictor of QOL status (Table 4).
Table 4: Binomial logistic regression analysis for predictors of QOL status (good vs. poor)
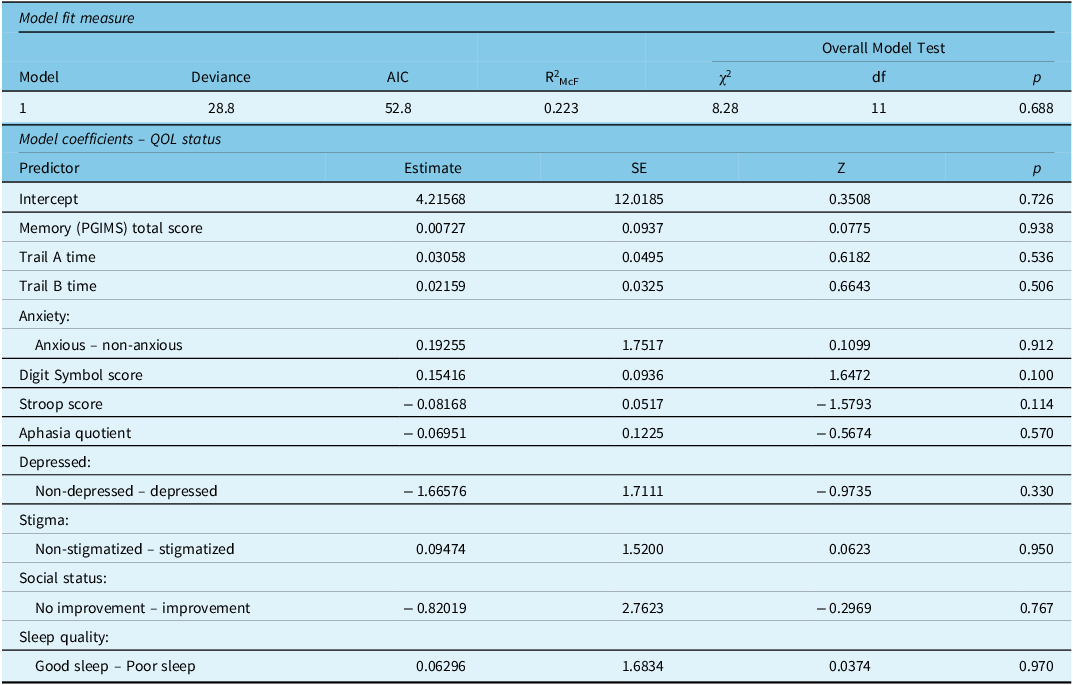
Estimates represent the log odds of “QOL Status = Poor QOL” versus “QOL Status = Good QOL.”
Discussion
This study involved a comprehensive outcome assessment of patients who underwent resective epilepsy surgery, including a detailed analysis of various non-seizure parameters potentially associated with QOL. We found that even among those who were rendered seizure-free following surgery, a significant proportion were experiencing poor QOL mainly due to the presence of cognitive deficits, depression, and lack of improvement in social parameters.
While many important non-seizure domains are part of the QOLIE-31 questionnaire, it is often difficult to objectively determine the associations of various different factors with perceived QOL, especially among patients who have undergone epilepsy surgery.
A systematic review was conducted by Seiam et al with the aim to review determinants of QOL after epilepsy surgery. Reference Seiam, Dhaliwal and Wiebe34 Of 39 eligible studies, 32 assessed the impact of surgery on QOL and 29 (90.6%) found a significant positive effect. The most prevalent preoperative determinant was psychological function. Seizure freedom was the most prevalent postoperative determinant (80% of studies), followed by ASM adverse events, employment status, and psychological function. Psychosocial (95%), physical (91%), and overall QOL (90%) domains improved most frequently, whereas role limitation (63%) and cognition (78%) improved least frequently. Dias et al compared patients who had undergone anterior temporal resections with a small control group of patients awaiting similar procedures. They evaluated all subjects with the QOLIE-31 and for depression, verbal memory and adverse drug effects along with seizure outcome evaluation. While regression analysis was carried out to identify determinants of QOL, seizure outcomes were also included in the model. Adverse drug effects, depression scores, and seizure outcomes were observed to be significant determinants. Reference Dias, de Angelis, Teixeira and Casulari35 In a long-term qualitative study on patient expectations from epilepsy surgery, Ozanne et al found a significant number of patients reporting a change in life for the worse due to adverse psychological as well as neurological effects, irrespective of whether or not they obtained seizure freedom. Reference Ozanne, Graneheim, Ekstedt and Malmgren36 Similarly, Taft et al reported that half of their patients who underwent epilepsy surgery and became seizure-free did not experience any improvement in health-related QOL after surgery. Reference Taft, Sager Magnusson, Ekstedt and Malmgren37 In many of these studies, certain QOL aspects such as mood and social functioning have been assessed; in the current study, we were able to fill gaps in the identification of non-seizure determinants of poor QOL following epilepsy surgery.
We observed a significant association of poor memory scores with poor QOL among seizure-free patients. Reporting observations from long-term follow-up of a large cohort of post-epilepsy surgery patients, Langfitt et al described poor QOL among non-seizure-free patients who also experienced memory decline, whereas they did not observe poor QOL among seizure-free patients, irrespective of memory scores. Reference Tang, Yu and Zhou13 Dias et al found a high prevalence of pre- and post-surgery verbal memory deficits and attributed their findings of no change in patients’ cognitive self-perception post-surgery to this observation. Reference Dias, de Angelis, Teixeira and Casulari35
While we did not find overall language scores to have a significant association with QOL, language subscores did correlate with QOL. A high percentage of patients demonstrating a decline in language scores is similar to other studies that prospectively assessed patients undergoing epilepsy surgery with either limited or extensive language testing. Reference Babajani-Feremi, Holder and Narayana38,Reference Ives-Deliperi and Butler39 Pauli et al described word finding difficulty (on the Boston naming test) as the only objective finding correlating with 12.5% of their patients reporting a perception of cognitive decline following surgery for mesial temporal lobe epilepsy. Reference Shih, Francis-Auton, Nikpour, Herkes, Bleasel and Rapport14
Our finding of depression as another significant factor associated with QOL is similar to other reports. Reference Taft, Sager Magnusson, Ekstedt and Malmgren37,Reference Hamid, Blackmon and Cong40 We have previously reported de novo psychiatric disorders (mainly depressive) in up to 20% of patients undergoing epilepsy surgery, despite overall QOL scores improving post-surgery. Reference Ramos-Perdigues, Bailles and Mane16
Another important determinant of QOL identified in this study was preoperative social status (employment or education status, financial independence, or marital status), especially if patients also noted stigma. While improvement on these parameters following surgery was seen more frequently among those with good QOL, the difference did not reach statistical significance. A recent study demonstrated both social functioning and QOL to improve among seizure-free patients following epilepsy surgery, with a significant positive correlation with preoperative baseline intelligence quotient and education levels. Reference Lin, Yu and Lu41 Similar reports from different parts of the world have demonstrated an effect of epilepsy surgery on stigma reduction; however, correlation with postsurgical QOL has been reported less clearly. Reference Fletcher, Sims-Williams, Wabulya and Boling18,Reference Boling, Means and Fletcher42
An important aspect of non-seizure parameters affecting QOL among people with epilepsy, comprehensively studied by us, is epilepsy-specific sleep quality. We found sleep-related problems to be quite common in all subgroups, with fewer patients with abnormal total sleep time in the good QOL group. In a previous study, we had reported significant improvement in self-reported as well as some polysomnographic sleep parameters among patients post-epilepsy surgery, Reference Zanzmera, Shukla and Gupta17 something that had not been studied by other investigators. The high prevalence of sleep disturbances and the multifactorial nature of potential causes (recurrent seizures prior to surgery, ASM effects, and long-standing mood disturbances) possibly underlies the lack of significant differences between the two QOL groups in this study.
The major strength of this study lies in the comprehensive evaluation of a wide variety of co-morbidities and social factors, in a structured format, and the assessment of their relationship with QOL in patients who had undergone resective epilepsy surgery. Study limitations include the relatively small sample size and the one-time post-surgery follow-up assessment. Our finding of nonsignificance for any of the studied non-seizure parameters in predicting QOL status on regression analysis may be attributable to the small sample size. Nonetheless, based on the observations described here, larger studies using the same comprehensive outcome assessment strategy can be planned in the future.
In conclusion, various non-seizure factors, including impaired cognitive functioning, psychiatric disturbances, poor sleep, presurgical education, and employment status are common contributors to poor QOL in patients who have become seizure-free following epilepsy surgery. Assessing each of these should be part of routine practice for identifying treatment targets in patients with medically refractory epilepsy.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2023.310.
Acknowledgments
The authors sincerely acknowledge the technical and administrative support received from Mrs. Jyoti Katoch.
Funding
None.
Competing interests
None.
Statement of authorship
Individual author contributions are listed below:
GS – Study conceptualization, epilepsy clinical data collection, video-EEG data interpretation and analysis, study implementation supervision, manuscript editing, and completion.
NN – Study implementation, data entry and analysis, and manuscript writing.
MA – Cognitive, mood evaluation, and cognitive +social data collation.
AG – Sleep data collection, entry, and tabulation.
PA – Epilepsy clinical data collection.
AS – Study design and implementation review.
MBS – Epilepsy clinical data collection, video-EEG data interpretation, and study design review.
AS’ – Epilepsy neurosurgical evaluation and treatment, study design, and implementation review.
Ethical approval
Ethics approval was obtained from the institutional ethics board prior to initiation of the study.






