INTRODUCTION
Hepatitis B virus (HBV) is a major cause of liver disease, cirrhosis and hepatocellular carcinoma with an estimated 400 millions person infected worldwide [Reference Maddrey1]. It is classified into eight genotypes (A–H) based on an intergroup divergence of ⩾8% in nucleotide sequence over the entire genome [Reference Schaefer2, Reference Bartholomeusz and Schaefer3]. There is increasing evidence that HBV genotypes have clinical and therapeutic relevance. The eight HBV genotypes show a distinct geographic distribution with a high prevalence of genotype D in the Mediterranean countries [Reference Wai and Fontana4, Reference Fung and Lok5].
On the other hand, the natural history of HBV infection is now better understood with an HBe antigen (HBeAg) seroconversion resulting in a chronic carrier state or HBeAg-negative chronic hepatitis. The prevalence of the latter has recently increased worldwide, becoming more prevalent than the wild HBeAg-positive HBV chronic hepatitis B in many countries [Reference Zarski6].
Currently, there is no published study on the HBV genotype distribution in Syria, a country of intermediate endemicity of HBV with a prevalence of 5–7% in a population of 20 millions inhabitants [Reference Antaki7]. The aims of our study were to determine the HBV genotype distribution in Syria, the prevalence of HBeAg-positive and HBeAg-negative chronic hepatitis and to compare the clinical characteristics of both groups.
METHODS
Subjects
All patients naive to treatment and referred to nine medical centres in Syria between 1 January 2008 and 31 December 2008, for evaluation of a positive hepatitis B surface antigen (HBsAg) were included in the study. Patients with acute HBV hepatitis were excluded as well as paediatric patients aged <14 years. A complete history was obtained with an emphasis on epidemiological and risk factors such as a family history, blood transfusion, haemodialysis and intravenous drug use.
Blood testing for HBsAg, HBeAg, antibody to hepatitis B antigen (anti-HBe), liver function tests, HBV DNA level and HBV genotyping were performed. A liver biopsy was offered to all patients. The patients were divided into four diagnosis groups based on clinical, biochemical, virological and histological parameters:
(1) Group A included patients in the immune tolerance phase, defined by HBeAg positivity, high levels of serum HBV DNA, normal or low levels of aminotransferase (ALT), no or mild liver necroinflammation and fibrosis.
(2) Group B included patients with immune active (or clearance) phase characterized by HBeAg positivity, moderate levels of serum HBV DNA, increased ALT and moderate to severe liver necroinflammation and fibrosis.
(3) Group C included patients in ‘inactive HBV carrier state’ defined by anti-HBe positivity, low or undetectable serum HBV DNA levels and normal or very low ALT.
(4) Group D included patients with HBeAg-negative chronic hepatitis B with anti-HBe positivity, low and fluctuating patterns of serum HBV DNA and ALT.
Virological tests
Virological tests were performed in five laboratories using the same methods. HBsAg, HBeAg and anti-HBe were performed by Elecsys assay (Roche Diagnostics, Switzerland). HBV viral load was measured by real-time PCR (Roboscreen GmbH, Germany). The HBV genotyping was obtained by INNO-LiPA (Innogenetics, Belgium). The liver biopsies were evaluated using the Metavir scoring system for activity and fibrosis [Reference Bedossa and Poynard8].
Statistics
Statistical analysis was performed using SPSS for Windows version 11.0 (SPSS Inc., USA). Continuous variables are reported as mean and standard deviation (s.d.), while categorical variables are shown as count and proportion Mann–Whitney U tests, χ2 tests were used to compare between groups as appropriate. For all tests, two-sided P values were calculated and the results were considered statistically significant if P<0·05.
Ethical statement
The study was conducted in accordance with the Helsinki Declaration for the protection of human subjects. Informed consent was obtained from all patients that allowed a review of patients' medical records for research purposes.
RESULTS
Demographic and clinical data
During the study period, 220 HBsAg-positive patients were evaluated. The age of the patients varied between 14 and 85 years with a mean (±s.d.) age of 37±12·9 years. A total of 181 (82%) patients were male. Seven patients were in group A, 56 patients in group B, 43 patients in group C and 114 patients in group D. Sixty-three (28·6%) patients were HBeAg positive and 157 (71·4%) patients were HBeAg negative. For the mode of transmission, 59 patients (26·8%) reported a family history of HBV infection while four (1·8%) patients received blood transfusion. Two (0·9%) patients were on haemodialysis and there were no intravenous drug users. The mode of transmission could not be determined in the other 155 patients. Liver biopsy was discussed with all patients and was performed on 106 (48%) patients.
Genotype distribution
Genotype D was found in 213/220 (97%) patients and genotypes A, C, F and D/H in one patient each. Three patients had undetermined genotype.
Comparison between groups
When we compared group B (patients with HBeAg-positive chronic hepatitis B) to group D (patients with HBeAg-negative chronic hepatitis B), we found that in the former group, the mean age was lower (31±12·6 vs. 40±12·3 years, P<0·001), more were female (19·6% vs. 13·2%, P=0·27), and viral replication was higher (mean HBV DNA 3·2×107 IU/ml vs. 8731984 IU/ml, P<0·001). Liver biopsy demonstrated more cirrhosis in group D patients (36·6% vs. 10·3%) while none or mild fibrosis was more prevalent in group B patients (38% vs. 22·5%). The number of patients with moderate fibrosis (F2 in the Metavir scoring system) was similar in both groups (38% vs. 31%) (Table 1). The ALT level was also similar in both groups.
Table 1. Comparison between diagnostics groups
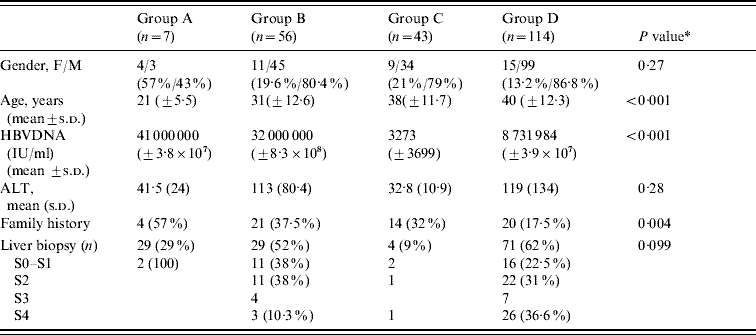
ALT, Aminotransferase; s.d., Standard deviation.
* For comparisons between groups B and D.
When comparing the four diagnostic groups, we found that the mean age increased from group A to group D reflecting the natural history curve of the infection (group A, 21 years; group B, 31 years; group C, 38 years; group D, 40 years). The viral load was higher in groups A and B (4·1×107 IU/ml and 3·2×107 IU/ml, respectively) compared to groups C and D (3273 IU/ml and 8731984 IU/ml, respectively). Concerning the mode of transmission, 57%, 37·5%, 32% and 17% of patients in groups A, B, C and D, respectively, had a family history of HBV infection. If we compared HBeAg-positive patients (groups A and B) to HBeAg-negative patients (groups C and D), we observed that in the former group, the mean age was lower (30±12·46 vs. 40±12·19 years, P<0·001) and with regard to the mode of contamination, we found that the mode of transmission was known in 41·2% and 24·8%, respectively (Table 2).
Table 2. Comparison between HBeAg-positive patients (groups A and B) and HBeAg-negative patients (groups C and D)
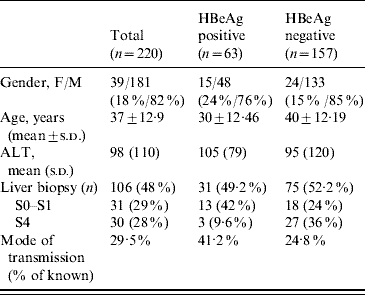
ALT, Aminotransferase; s.d., Standard deviation.
DISCUSSION
The eight HBV genotypes show a distinct geographic distribution. Genotype A is most frequently seen in Northern Europe, North America, India and sub-Saharan Africa. Genotypes B and C are prevalent in East Asia and the Pacific. Genotype D is most often found in Southern and Central Europe and the Middle East. Genotype E has been detected in Africa, while genotypes F and H have been detected in Central and South America. Genotype D was found in 100% of HBV-infected patients in Lebanon, Egypt, Turkey, Jordan and Iran [Reference Ramia9–Reference Aghakhani13] and in 82% of patients in Saudi Arabia [Reference Abdo14]. Our study confirms that similar to other countries in the Middle East, genotype D is almost exclusively found in HBV-infected Syrian patients. Determining the HBV genotype has clinical and therapeutic significance; studies have demonstrated that genotype B is associated with less active liver disease, a slower rate of disease progression, a higher rate of early spontaneous HBeAg seroconversion compared to genotype C [Reference Kobayashi15, Reference Sakugawa16]. The latter was shown to be an independent risk factor for the development of hepatocellular carcinoma [Reference Orito17, Reference Chan18]. It was also demonstrated that genotype D is associated with a lower rate of sustained remission and HBsAg clearance and a more severe disease compared to genotype A [Reference Sanchez-Tapias19, Reference Thakur20]. Patients with genotype D may develop fulminant hepatitis with higher frequency than other genotypes [Reference Wai21]. On the therapeutic level, studies have reported lower response rates to interferon-α and pegylated interferon in patients infected with genotypes C and D than in those infected with genotypes B and A, respectively [Reference Wai22, Reference Janssen23]. Finally, Lamivudine resistance is less frequent in patients with genotype D than in patients with genotype A [Reference Chien24, Reference Zollner25].
Our study shows that, similar to other Mediterranean countries, HBeAg-negative status is most prevalent in HBV-infected Syrian patients: 71·5% of all HBV-infected patients. This number includes patients in inactive HBV carrier state and patients with HBeAg-negative chronic hepatitis B. It confirms that loss of HBeAg and the presence of anti-HBe are not necessarily evidence of better prognosis, less necrotic activity or less replication and infectivity. In fact, 24% of our HBeAg-negative patients had no or mild fibrosis on liver biopsy. However, 30·6% had moderate fibrosis, 9% had severe fibrosis and 36% had cirrhosis. The prevalence of HBeAg-negative chronic hepatitis B is increasing worldwide, e.g. in France where over the last 10 years it has increased from 25% to 72% [Reference Zarski6]. This fact has important therapeutic implications as the response to treatment and the treatment options are different [Reference Lock and Mc Mahon26].
The main causes of contamination in Syrian patients are vertical transmission from mother to newborn and intra-familial transmission in infancy [Reference Antaki7]. The natural history of HBV infection when acquired in infancy is now well established. It starts with an immune tolerance phase with high viral replication and no necrotic activity which may last for two or three decades, followed by an immune clearance phase characterized by an HBeAg-positive chronic hepatitis in which an HBeAg seroconversion may happen with the loss of HBeAg and the appearance of anti-HBe. This phase is followed by a low or no replicative phase also called chronic carrier state. Finally, HBeAg-negative chronic hepatitis B may follow seroconversion and the patients harbour HBV mutants with nucleoside substitutions in the precore and/or the basal core promoter regions unable to express HBeAg [Reference Fattovich, Bortolotti and Donato27–29]. The results of our study confirmed this natural evolution of the infection when it is longstanding, since acute hepatitis following recently acquired infection in adulthood (by blood transfusion, intravenous drug use or homosexuality) is very rare in Syria.
Our study has some limitations. The least important is a referral bias since the patients were referred to major centres from their physicians and may not be representative of the general population of HBsAg carriers in Syria. The most important limitation is that only half of the patients included in the study agreed to have a liver biopsy. However, these patients were divided into the four diagnosis groups based not only on histology but also on clinical, biochemical and virological parameters.
CONCLUSION
HBV genotype D is almost exclusively found in HBV-infected Syrian patients (97%). This may affect the choice of treatment regimen. Most patients (71·5%) are HBeAg negative. These patients are older, have a lower viral load and have more cirrhosis than HBeAg-positive chronic hepatitis patients. Since vertical and intra-familial transmissions are the main modes of contamination in Syrian patients, the high prevalence of HBeAg-negative in active hepatitis, inactive carriers and cirrhosis demonstrate the longstanding evolution of the infection.
ACKNOWLEDGEMENTS
The authors of this study are members of the Syrian Working Group for the Study of Viral Hepatitis (SWGSVH) whose objectives are to conduct research and studies on viral hepatitis and to publish Syrian national guidelines for the management of viral hepatitis.
DECLARATION OF INTEREST
None.




