Introduction
Mild traumatic brain injuries (MTBI) are very common in developed countries and approximately half of those who sustain them experience post-concussion symptoms (PCSs) (Jennett & Frankowski, Reference Jennett, Frankowski and Brinkman1990; Kraus & Nourjah, Reference Kraus and Nourjah1988; Mandel, Reference Mandel1989). PCSs are a cluster of cognitive, somatic and emotional symptoms caused by neuropathological and/or psychological mechanisms which are poorly understood (Lishman, Reference Lishman1988). They include headache, dizziness, fatigue, irritability, reduced concentration, sleep disturbance, memory dysfunction, sensitivity to light or noise, double or blurred vision, frustration, restlessness, anxiety, depression and sensitivity to alcohol (Evans, Reference Evans1994). These symptoms completely resolve over the first few days or weeks for the vast majority (Alves, Colohan, O'Leary, Rimel, & Jane, Reference Alves, Colohan, O'Leary, Rimel and Jane1986; Leninger, Gramling & Farrel, Reference Leninger, Gramling and Farrel1990) but a minority have persisting symptoms (e.g., up to 6 months post injury) (King, Reference King1997). A smaller minority have prolonged symptoms (e.g., up to 1 year) (Middelboe, Anderson, Birket-Smith, & Friis, Reference Middelboe, Anderson, Birket-Smith and Friis1992; Rutherford, Merrett, & McDonald, Reference Rutherford, Merrett and McDonald1979) and a very small minority have long-term or permanent symptoms (Binder, Reference Binder1986; Englander, Hall, Simpson, & Chaffin, Reference Englander, Hall, Simpson and Chaffin1992). The latter may, however, represent up to 5–10% of those admitted to hospital with MTBI (Binder, Rohling, & Larrabee, Reference Binder, Rohling and Larrabee1997).
Unfortunately the vast majority of studies examining MTBI and PCSs have focused on early, persisting or prolonged symptoms but not permanent ones (King, Reference King2003). This is a very large gap in the literature as it is this group who often pose the greatest challenges for assessment and treatment, in both clinical and medico-legal contexts (Binder & Rohling, Reference Binder and Rohling1996; Fee & Rutherford, Reference Fee and Rutherford1988). While some more recent studies have investigated factors affecting the likelihood of experiencing permanent PCSs (Andersson, Bedics, & Falkmer, Reference Andersson, Bedics and Falkmer2011; Nicholas & Zasler, Reference Nicholas and Zasler2010) none have described the types of symptoms typically reported. It is therefore unclear what the most and least common symptoms are in this group. This dearth poses substantial problems for those treating such patients, as their types of disability may be very similar to those of patients with much more severe traumatic brain imjury (TBI) but without any measurable evidence of any significant neuropathological involvement (King, Reference King2003).
After more severe TBI the vast majority of cerebral neurological repair occurs within the first 18 months (King & Tyerman, Reference King, Tyerman, Tyerman and King2008) and therefore those with MTBI who have significant PCSs beyond a year and a half are likely to be at a very high risk of developing permanent symptoms (King & Kirwilliam, Reference King and Kirwilliam2011). The first study, to the authors’ knowledge, to use quantitative methods to examine a wide range of demographic, cognitive and psychosocial variables in a representative sample of working-age patients with permanent PCSs (7 years post injury) was published recently (King & Kirwilliam, Reference King and Kirwilliam2010, Reference King and Kirwilliam2011). Patients in this sample: (i) were older than those typically presenting with MTBI; (ii) often had pre- or post-morbid concurrent factors which might exacerbate PCSs; (iii) reported very high levels of PCSs; (iv) experienced elevated levels of anxiety and depression; and (v) demonstrated mildly reduced scoring on tests of short-term memory and speed of information processing. Important data on the nature and frequency of the PCSs experienced by this representative sample, however, were not reported. The primary aim of the present paper is therefore to describe, for the first time, the different types of symptoms typically experienced by patients with permanent PCSs.
A secondary aim is to provide some extremely tentative data regarding the nature of PCSs over time, to hopefully stimulate more robust research in this area. PCSs are thought to be caused by neurogenic factors and/or psychological factors (and the interaction between the two) (King, Reference King1997; Lishman, Reference Lishman1988). While the relative contribution of these variables across individuals varies enormously (Binder et al., Reference Binder, Rohling and Larrabee1997) there is broad agreement that psychological factors are likely to increase with time and neurogenic ones decrease (Potter & Brown, Reference Potter and Brown2012). Indeed, at least one model (Windows of Vulnerability Model (King, Reference King2003)) describes how different psychological factors may operate at different times post injury and how they may have a cumulative effect. Within such a context, one area of significant clinical importance that remains unclear is whether persisting PCSs are a dynamic entity (changing over time) or a more static one (with a reasonably consistent presentation regardless of time post injury). If the contribution of psychological factors increases over time, then both the type and aetiology of permanent symptoms may differ from those presented at earlier stages (King, Reference King2003). Although this type of question has very occasionally been investigated in longitudinal studies (Lundin, De Boussard, Edma, & Borg, Reference Lundin, De Boussard, Edma and Borg2006; Meares et al., Reference Meares, Shores, Taylor, Batchelor, Bryant, Baguley and Marosszeky2011), these have focused solely on early and persisting PCSs (i.e., up to 6 months post injury) and consequently have not addressed long-term PCSs at all.
To start to stimulate robust longitudinal research to answer this secondary question, this paper also presents the frequencies of PCSs experienced by representative groups of MTBI patients in early (7–10 days post injury) and persisting (6 months post injury) cohorts. These are taken from a neighbouring county to the permanent PCS group, with very similar demographics. It is hoped that this cross-sectional, retrospective and opportunistic comparison will at least provide some very tentative early data to begin to explore this area empirically.
Methods
Participants
Institutional ethics approval was obtained and all patients referred to the Community Head Injury Service (CHIS) in Buckinghamshire, UK, for the treatment of persisting PCSs following a MTBI between 1997 and 2008 were identified from clinical records. CHIS provides community-based neurorehabilitation to all referred working-aged, head-injured patients of all severities (mild, moderate, severe and very severe) in Buckinghamshire. It serves a mixed rural and urban population of approximately 725,000 and its referrals come from a wide range of community sources – predominantly from GPs and brain injury clinical nurse specialists, but also from self- or family referrals, neurologists, social services or other clinicians. Referrals are therefore likely to be as representative of this group of long-term PCS patients as possible. Participants were required to have sustained a MTBI at least 18 months prior to the study. The Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine definition of MTBI was used, i.e., a head injury resulting in post-traumatic amnesia (PTA) of less than 24 h, Glasgow Coma Scale (GCS) of between 13 and 15 and no inter- or intracerebral complications. They were also required to have at least three current PCSs, as assessed by the Rivermead Post Concussion Symptoms Questionnaire (RPQ) (King, Crawford, Wenden, Moss, & Wade, Reference King, Crawford, Wenden, Moss and Wade1995) so as to fulfil the minimum criteria for post-concussion syndrome.
Individuals were excluded from the study if they had any history prior to, or following, MTBI of a severe head injury, substance/alcohol dependence or other neurological condition. They were also excluded if they had any pre or post history of other medical or psychological conditions likely to substantially account for post-concussional-type symptoms, e.g., severe and chronic pain, severe depression.
Procedure
As part of a more-extensive assessment of the characteristics of the above sample, all participants who were identified, from the clinical records, as fulfilling the inclusion criteria were posted an RPQ to complete. This is a 16-item questionnaire about commonly experienced PCSs after a MTBI. The participant is asked to rate any PCS-type problems experienced over the previous week on a five-point scale in accordance with how severely they experienced the symptom compared to before their injury. Scores of 0 or 1 indicate that a symptom is not experienced at all or experienced to the same degree as before the head injury. Scores of 2, 3 or 4 indicate that the symptom is a mild, moderate or severe problem, respectively. The RPQ has good reliability and validity as a measure of PCSs in this type of population (King et al., Reference King, Crawford, Wenden, Moss and Wade1995; Potter, Leigh, Wade, & Fleminger, Reference Potter, Leigh, Wade and Fleminger2006). These data provided the symptom frequencies of permanent PCSs to answer the first research question.
Symptom frequencies were then obtained from representative samples of MTBI patients at earlier stages post injury for comparison, in order to begin to answer tentatively the secondary question regarding the extent to which PCSs change over time. They were obtained from two previous studies conducted in the neighbouring county of Oxfordshire at 7–10 days post injury (King et al., Reference King, Crawford, Wenden, Moss and Wade1995) and 6 months post injury (Wade, Crawford, Wenden, King, & Moss, Reference Wade, Crawford, Wenden, King and Moss1997). Oxfordshire (Oxon) has a similar mixed rural and urban population to Buckinghamshire and is of a similar size (population approximately 600,000). Both the Oxon samples were consecutive referrals to the Oxford Head Injury Service which, during its existence, followed up every MTBI in the county. The former study administered RPQs to a random half of MTBI patients aged 16–65 years who were seen consecutively in the two admitting hospitals in the county, as part of a large-scale randomised controlled trial (RCT) investigating early intervention for PCSs. Patients were seen at 7–10 days post injury, had a mean age of 31 years, had not received treatment for their PCSs and constituted 22 men and 19 women. The latter study administered RPQs to a random half of untreated MTBI patients aged 16–65 years who were seen consecutively in the same two hospitals at 6 months post injury as part of the same RCT. They constituted 143 men and 83 women with a mean age of 32 years. These samples were therefore representative of MTBI patients in Oxfordshire. The definition of MTBI was similar across these two studies and the present study. The first-named author was involved in both of the studies, and data published in King et al. (Reference King, Crawford, Wenden, Moss and Wade1995) and Wade et al. (Reference Wade, Crawford, Wenden, King and Moss1997) were sufficient to provide comparison data.
Results
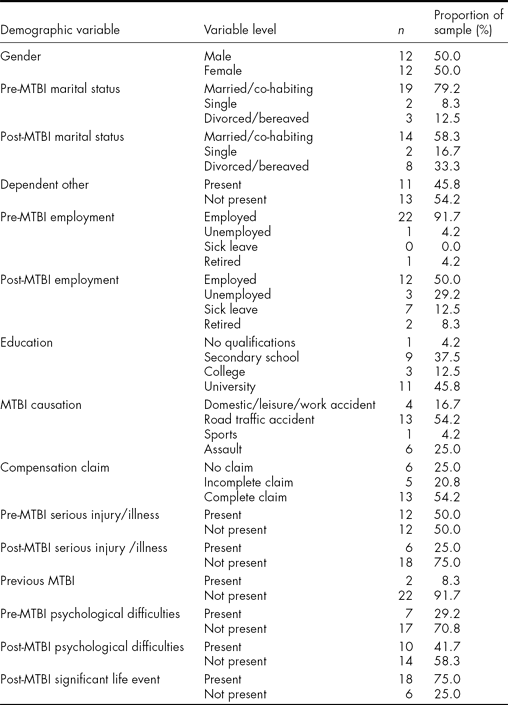
One hundred individuals were identified as eligible and invited to take part by letter, which included an RPQ to complete. Thirty individuals consented (all of whom had at least three current PCSs). Six withdrew and the sample was therefore 24. The mean time post injury was 83.7 months (SD = 47.6, range = 24–202 months) (i.e., 6.9 years), there were an equal number of men and women and the mean age was 44.7 years (SD = 8.8, range = 30–64 years). Mean PTA was 157 min (SD = 340.1, range = 0–1440 min). The participant group did not differ significantly from those not participating in terms of gender or length of PTA, but the latter may have been slightly younger than the former (38.6 years – significantly different at p < .05 but not < .01). The demographics of the sample are presented in Table 1.
TABLE 1 Demographic Characteristics of the Sample

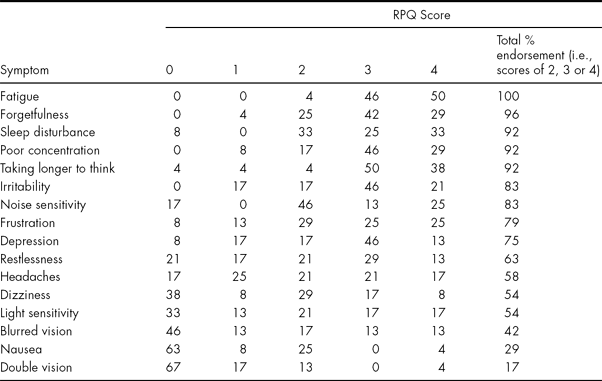
To answer the primary question regarding the nature of permanent PCSs, Table 2 displays the RPQ scores of the long-term sample.
TABLE 2 Percentage Endorsement of RPQ Scores at 7 years post injury

Where the sum of individual RPQ percentages does not tally with ‘Total %’ or add up to 100% this is due to rounding up/down of the data.
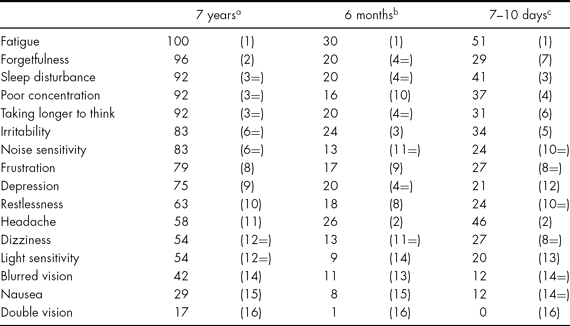
Data relating to the relationship between PCSs and psychosocial factors in the long-term sample are published in King & Kirwilliam (Reference King and Kirwilliam2011). To answer tentatively the secondary question regarding the nature of PCSs over time, Table 3 displays the frequencies of the different symptoms reported by the long-term sample alongside those reported in the samples from 7–10 days and 6 months post injury. A symptom was deemed to be present if a score of 2, 3 or 4 was endorsed.
TABLE 3 Percentage of PCSs Reported at 7 years, 6 months and 7–10 days Post Injury (Ranked Frequency)

a Present study (n = 24); b Wade et al. (Reference Wade, Crawford, Wenden, King and Moss1997) (n = 226); c King et al. (Reference King, Crawford, Wenden, Moss and Wade1995) (n = 41).
Table 2 indicates that those with permanent PCSs all had very high levels of symptoms and all reported fatigue as part of their symptom picture. Forgetfulness, sleep disturbance, poor concentration, taking longer to think and irritability were also extremely common symptoms. The least common ones were headache, dizziness, light sensitivity, blurred vision, nausea and double vision. Table 3 indicates that the most and least commonly experienced PCSs were also those endorsed as being the most and least ‘severe’, respectively (with the exception of ‘headache’).
Table 3 also shows that there was a cluster of PCSs very frequently reported across all time frames post injury, i.e., ranked in the top six symptoms in all samples. These were fatigue (ranked first in all samples), sleep disturbance (ranked third, third and fourth), taking longer to think (ranked third, fourth and sixth) and irritability (ranked third, fifth and sixth). Similarly, the least frequently reported symptoms were consistently endorsed over time, i.e., ranked in the bottom four – sensitivity to light, blurred vision, nausea and double vision.
Discussion
The vast majority of the most commonly reported permanent PCSs (i.e., forgetfulness, poor concentration, etc.) have been conceptualised as being primarily cognitive–affective in nature (Cicerone & Kalmar, Reference Cicerone and Kalmar1995; Levin, Mattis, & Ruff, Reference Levin, Mattis and Ruff1987). The least common ones (i.e., nausea, blurred vision, etc.) have been conceptualised as somatic–sensory (Cicerone & Kalmar, Reference Cicerone and Kalmar1995; Levin et al., Reference Levin, Mattis and Ruff1987). It has been speculated that cognitive–affective symptoms may reflect predominantly limbic dysfunction, and somatic–sensory ones, vestibular and visual disturbances (Cicerone & Kalmar, Reference Cicerone and Kalmar1995). Should the current findings be replicated from larger samples, it is possible that permanent PCSs will be shown to adhere generally to a particular grouping of symptoms and be associated with particular kinds of neuropathological dysfunction. It should be acknowledged, however, that this neurogenic explanation is highly speculative and it could be strongly argued that the very high levels of symptoms overall may reflect the accumulation of psychological factors over a long period of time. Indeed, the most frequently reported permanent PCSs are very similar to the PCS-type symptoms which are most often reported in non-head-injured populations, e.g., in pain (sleep disturbance, fatigue and irritability) (Iverson & McCracken, Reference Iverson and McCracken1997), depression (fatigue, sleep disturbance, poor concentration, forgetfulness and irritability) (Iverson, Reference Iverson2006) and healthy controls (fatigue, irritability, poor concentration, poor sleep, tension and sadness) (Iverson & Lange, Reference Iverson and Lange2003). The present findings, therefore, clearly do not allow for the testing of any aetiological hypotheses but might stimulate studies that could shed further light on this area.
The very high frequencies of symptoms in the permanent PCS group were almost certainly due the self-selected nature of this symptomatic and treatment-seeking population. The lowish response rate of 24% also means that the sample has additional inherent biases. This, alongside the small sample size, means that all conclusions regarding the nature of permanent PCSs should be considered as very tentative, pending replication with larger and more representative samples. It should also be acknowledged, though, that the response rate is not substantially lower than many other studies following up MTBI patients late-on post injury (e.g., the very well resourced, large-scale study used as a comparator in the present paper applied considerable effort and resources to maximise their response rate and achieved a take-up of 41% at 6 months (Wade et al., Reference Wade, Crawford, Wenden, King and Moss1997)). Finally to access representative samples of symptomatic MTBI patients at 7 years post injury, where the vast majority will be asymptomatic and may not even remember having received an injury, is likely to be prohibitively difficult without very high resource levels. Pragmatically therefore, exploring treatment-seeking patients with known ongoing symptoms represents a very useful start in describing the nature of permanent PCSs and furthering the understanding of this condition.
The study's finding that the most and least common PCSs were very similar over time also provides some extremely tentative initial evidence that there may be some consistency in the nature of PCSs regardless of whether they are early, persisting or permanent symptoms. It might, therefore, weakly suggest that the nature of PCSs may be more fixed than dynamic, and that any increasing psychological factors over time may add to the severity of a patient's presentation rather than the type of it. Unfortunately, the types of commonly reported PCSs do not discriminate well from the kinds of symptoms reported by non-head-injured populations, and the results do not clarify how to discern in any given case the relative contribution of neurogenic, psychological or mixed factors in a patient's presentation. Individualised and thoughtful clinical judgements will therefore continue to be essential in all cases of permanent PCSs (King, Reference King2003).
Conclusions regarding the nature of PCSs over time should be made very tentatively due to two reasons (in addition to the limitations already described). First, the comparison groups in the present study were not equivalent in a number of important areas – treatment seeking vs non-treatment seeking, selected for endorsement of PCSs vs non-selected, mean age (45 vs 32 vs 31 years), gender mix (50 vs 63 vs 54% men) and sample size (24 vs 226 vs 41). The significant strengths of the comparison groups, however, were the strong similarities in the sources of the samples (mixed rural and urban populations of a similar size from an adjacent county) and the consistency of the definitions and methodologies used. Second, because this part of the study was mainly cross-sectional, opportunistic, descriptive and retrospective.
Longitudinal studies with better control of the variables and larger sample sizes will clearly be required for many of the conclusions to be confirmed and asserted. The study does, nevertheless, provide the first empirical attempt to answer some key clinical and theoretical questions regarding the nature of permanent PCSs. As no data in this area have been reported previously, even a small empirical sample may be a helpful start in beginning to examine the type of symptoms typically reported by this group and the degree to which PCSs may change or remain the same over time.