Introduction
Butyric acid is one of the short short-chain fatty acids (SCFAs) generated at millimolar levels in the bird cecum, which is the major site for microbial fermentation of unabsorbed starch (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017). Butyric acid in its unprotected form is rapidly absorbed in the upper gastrointestinal tract (GIT), suggesting that protection is needed to positively affect the small intestine (Kaczmarek et al., Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016; Elnesr et al., Reference Elnesr, Alagawany, Elwan, Fathi and Farag2020; Silva et al., Reference Silva, Milbradt, Zame, Padovani, de Lima Almeida Paz, Hataka, Okamoto, Gross and Filho2020).
Due to bans on using in-feed antibiotic growth promoters, the poultry industry has been focused on finding new ways to improve performance through improving nutrient and energy utilization while maintaining and potentially improving the health of poultry without using antibiotic growth promoters. The uses of organic acids for improving performance parameters in both layers and broilers have been increasingly explored. Organic acids, including butyric acid and its salts, have shown positive effects on growth performance, egg production, and egg quality due to the source of the acid, diet composition and environment (Soltan, Reference Soltan2008; Elnesr et al., Reference Elnesr, Ropy and Abdel-Razik2019; Maty and Hassan, Reference Maty and Hassan2020).
Organic acids, such as butyric acid, improve gut health by providing carbon sources for villi growth, promoting the growth of beneficial bacteria (lactobacilli and Bifidobacteria), and decreasing harmful bacteria (Salmonella, Clostridium, and Escherichia coli) by decreasing luminal pH. Improved gut health is theorized to allow increased absorption, resulting in increased nutrient and energy utilization in poultry, thereby improving performance (Qaisrani et al., Reference Qaisrani, Van Krimpen, Kwakkel, Verstegen and Hendriks2015; Maty and Hassan, Reference Maty and Hassan2020).
In layer farming, egg production and egg quality are of great economic concern. Improved egg quality can be identified as improving eggshell strength while maintaining a good egg size. Calcium is a major component of the layer diet and is incorporated into both eggshell and bone (Clunies et al., Reference Clunies, Parks and Leeson1992; Makled et al., Reference Makled, Abouelezz, Gad-Elkareem and Sayed2019) by absorption from the small intestine (Saunders-Blades et al., Reference Saunders-Blades, MacIsaac, Korver and Anderson2009). As the hen ages, its ability to absorb nutrients, including calcium, declines, decreasing eggshell thickness and thus breaking strength. This leads to increased economic losses due to broken eggs (Molnár et al., Reference Molnár, Maertens, Ampe, Buyse, Zoons and Delezie2018; Maty and Hassan, Reference Maty and Hassan2020).
Organic acids improve the mineral absorption from the intestine by lowering the pH of digesta and inhibiting the formation of calcium-phytate complexes (Boling et al., Reference Boling, Webel, Mavromichalis, Parsons and Baker2000; Rafacz-Livingston et al., Reference Rafacz-Livingston, Parsons and Jungk2005). It has been found that supplementation of butyric acid and its salts (sodium butyrate) increases serum calcium, phosphorus (Mahdavi and Torki, Reference Mahdavi and Torki2009; Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010; Kamal and Ragaa, Reference Kamal and Ragaa2014), and magnesium levels (Kamal and Ragaa, Reference Kamal and Ragaa2014). This review aims to provide current knowledge about the effects of butyric acid on gut health, performance, nutrient utilization, immunity, osteoporosis, and egg quality in poultry.
Different formulations of butyric acid
The efficacy of butyric acid was improved when fed in a coated form, such as encapsulation, suggesting that such protection positively affected the GIT (Kaczmarek et al., Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016; Elnesr et al., Reference Elnesr, Alagawany, Elwan, Fathi and Farag2020). Previous studies showed variable results, perhaps due to factors such as age, nutrition, diet structure, experimental conditions, flock health, source of butyric acid, and inclusion rate (Taherpour et al., Reference Taherpour, Moravej, Shivazad, Adibmoradi and Yakhchali2009; Levy et al., Reference Levy, Kessler, Fuller, Williams, Mathis, Lumpkins and Valdez2015; Qaisrani et al., Reference Qaisrani, Van Krimpen, Kwakkel, Verstegen and Hendriks2015; Kaczmarek et al., Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016).
Kaczmarek et al. (Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016) found that protected butyrate at various doses significantly improved villi height and apparent metabolizable energy (AME) in broilers, while Levy et al. (Reference Levy, Kessler, Fuller, Williams, Mathis, Lumpkins and Valdez2015) found no significant effect of encapsulated butyric acid on villi height compared to controls in broilers. These variations in findings suggest a need for further research to determine the optimal source and inclusion rate of butyric acid, to overcome the variation seen due to other potential factors.
Currently, poultry feed manufacturers tend to produce coated types of butyric acid to overcome its odor and rapid volatility. However, the problem of traditional coated products is the low concentration of butyric acid, as coated salts usually include about 25–30% butyric acid, which is very low; therefore, future research should involve means to coat butyric acid while increasing its concentration to maximize its benefit.
Butyric acid as an alternative to antibiotics
In areas of the world such as the European Union and the United States, antibiotics/antibiotic growth promoters are no longer being added to poultry diets (Opinion of the Economic and Social Committee, 1998; Ricke, Reference Ricke2003; Deepa et al., Reference Deepa, Purushothaman, VasanthaKumar and Sivakumar2018), due to the high concentrations of antibiotic residues found in meat and meat products, undesired changes in the microbial communities of the GIT (Kulshreshtha et al., Reference Kulshreshtha, Rathgeber, Stratton, Thomas, Evans, Critchley, Hafting and Prithiviraj2014), and increase in antibiotic resistance in pathogenic bacteria (Ricke, Reference Ricke2003; Raza et al., Reference Raza, Biswas, Mir and Mandal2019).
Ideally, alternative supplements would improve growth performance by acting to improve feed efficiency and nutrient absorption and utilization, as well as to regulate microbial populations in such a way to promote the growth of beneficial microbes and reduce pathogenic microbes in the GIT (Biggs and Parsons, Reference Biggs and Parsons2008; Deepa et al., Reference Deepa, Purushothaman, VasanthaKumar and Sivakumar2018). Organic acids, specifically SCFAs, are considered as alternatives. Detailed interactions of butyric acid to enhance the growth performance of poultry through different systems are presented in Fig. 1. Most European Union member states generally regard organic acids and their salts as safe, and they have approved them for use as feed additives for livestock and poultry production (Adil et al., Reference Adil, Banday, Bhat, Qureshi and Wani2011).
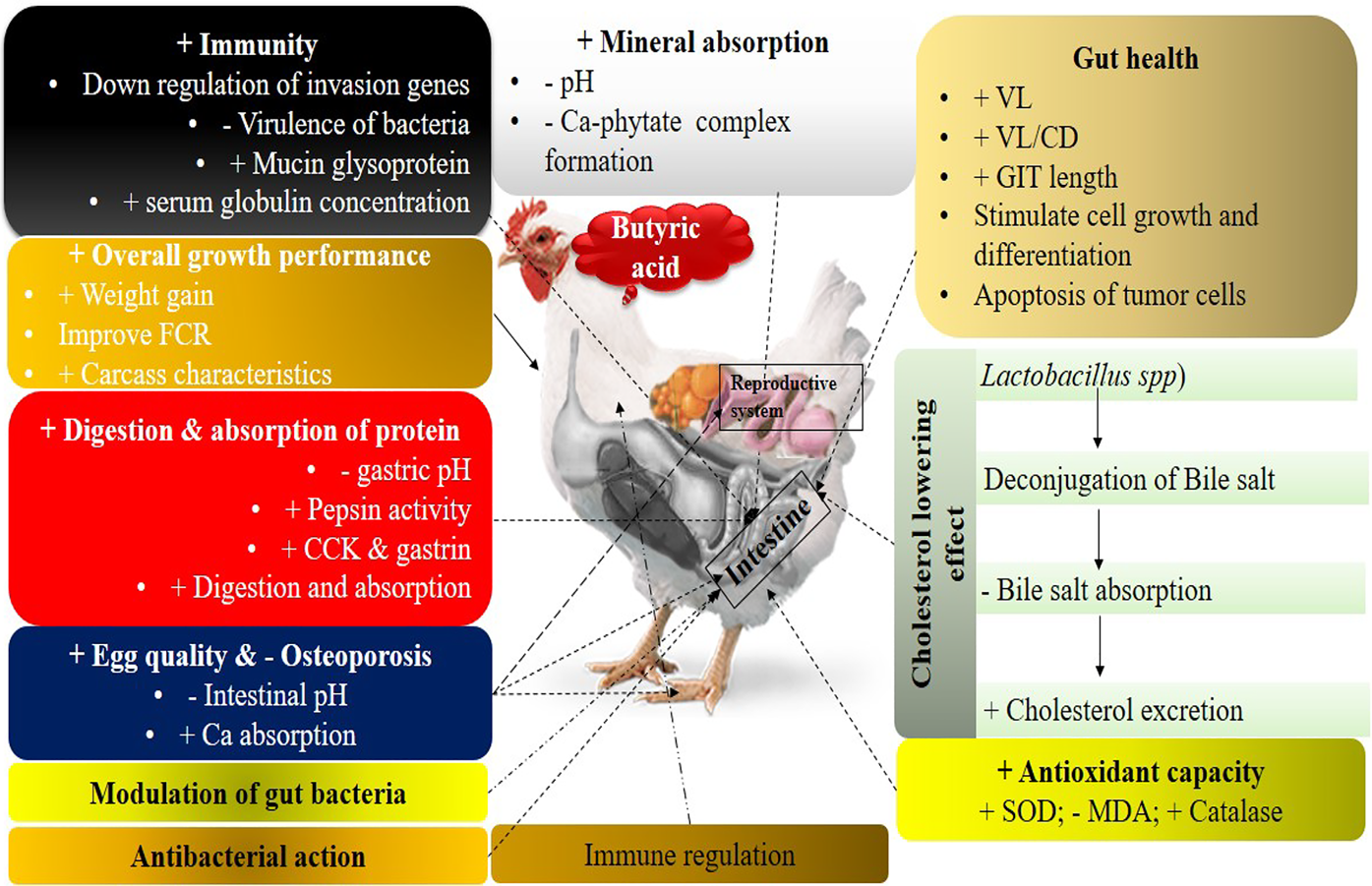
Fig. 1. Effect of butyric acid and its salts to enhance the growth performance of poultry. +, improve or enhance; −, lower or decrease. Butyric acid has a pronounced effect on gut health through different ways. It decreases the pH of the gut and digesta, which is generally good for beneficial bacteria (Lactobacillus and Bifidobacterium spp.) and toxic for pathogenic bacteria like Salmonella and Escherichia coli. Reduction in the population of pathogenic bacteria increases the availability of nutrients to beneficial bacteria and the host.
SCFAs can also be described as saturated straight-chain monocarboxylic acids, fatty acids, volatile fatty acids, and weak or carboxylic acids. Originally, SCFAs were added to animal feed to prevent fungal growth (Dixon and Hamilton, Reference Dixon and Hamilton1981). Propionate and formic acid (and various combinations) have bactericidal activity in feeds contaminated with foodborne pathogens such as Salmonella spp. (Ricke, Reference Ricke2003; Raza et al., Reference Raza, Biswas, Mir and Mandal2019), and butyric acid is thought to reduce intestinal populations of pathogenic bacteria in different ways (Figs 2 and 3). Butyric acid is now increasingly researched as a feed additive for poultry due to its proposed effectiveness to improve feed conversion efficiency, gut health, and growth performance (Deepa et al., Reference Deepa, Purushothaman, VasanthaKumar and Sivakumar2018; Imran et al., Reference Imran, Ahmed, Ditta, Mehmood, Khan, Gillani, Rasool, Sohail, Mushtaq and Umar2018).
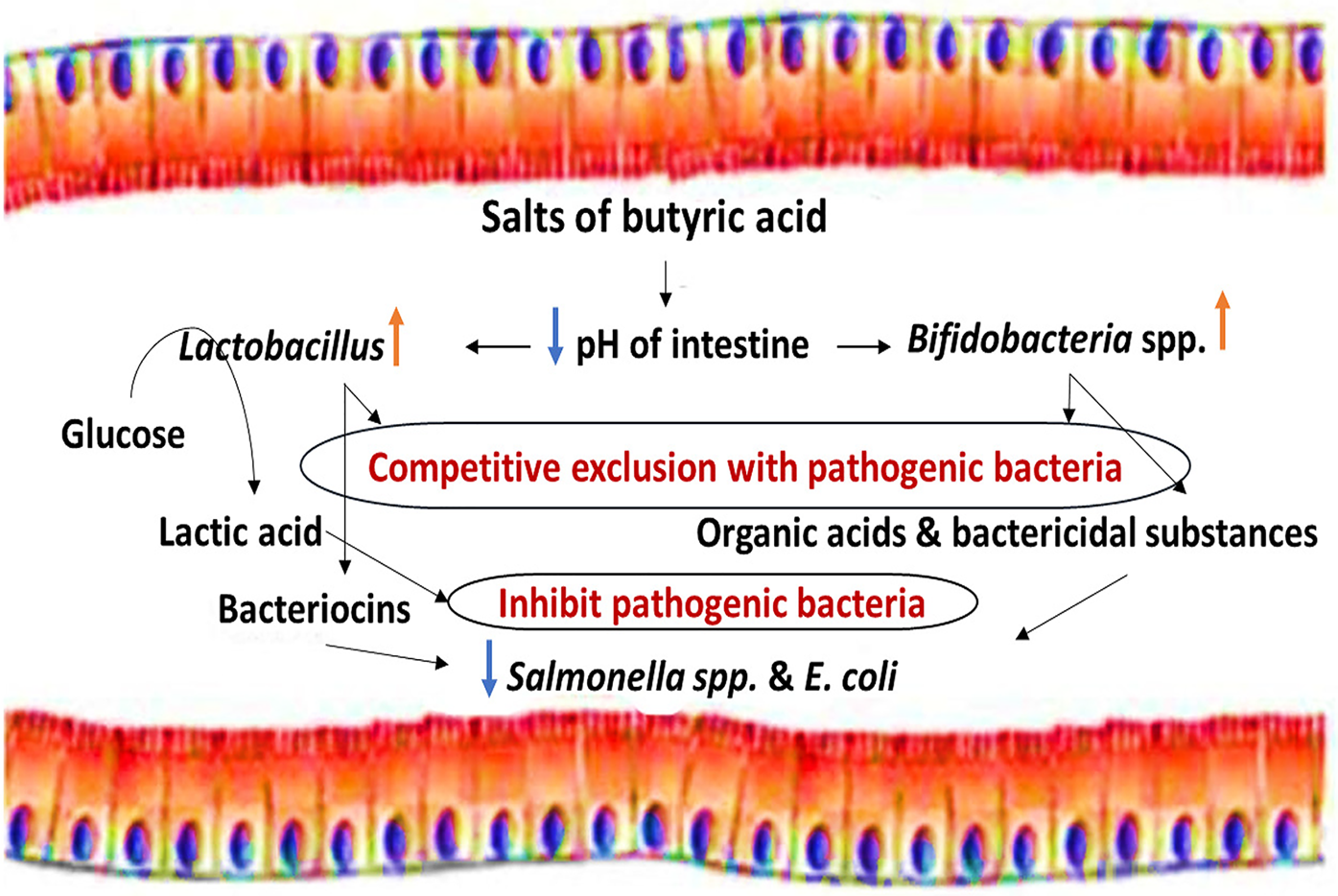
Fig. 2. Indirect bactericidal effects of butyric acid.
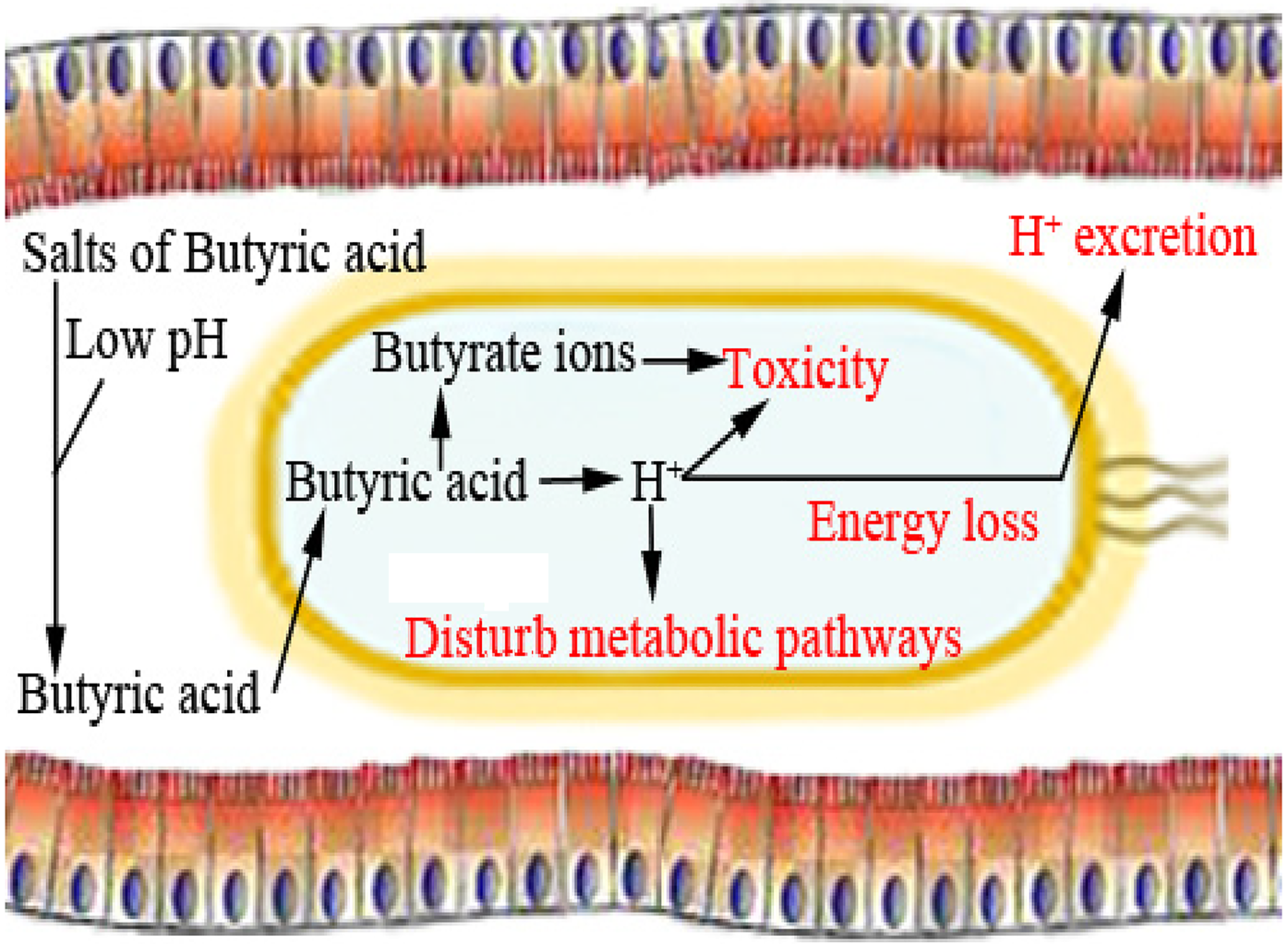
Fig. 3. Direct bactericidal effects of butyric acid.
Sources of butyric acid
Butyric acid, along with other SCFAs, is produced in millimolar amounts in the GITs of people and animals, within locations that predominantly contain strictly anaerobic microflora (Ricke, Reference Ricke2003). For poultry, this area is the cecum, which is the major site of microbial fermentation of unabsorbed starch (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017), non-starch polysaccharides (Levy et al., Reference Levy, Kessler, Fuller, Williams, Mathis, Lumpkins and Valdez2015), and proteins (Kulshreshtha et al., Reference Kulshreshtha, Rathgeber, Stratton, Thomas, Evans, Critchley, Hafting and Prithiviraj2014). Butyric acid, propionic acid, and acetic acid are the major byproducts of these processes (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017).
Butyric acid is most effective in its undissociated (non-ionized, more lipophilic) form (Leeson et al., Reference Leeson, Namkung, Antongiovanni and Lee2005; Wu et al., Reference Wu, Xiao, An, Dong and Zhang2018), but is often supplemented as butyrate in the diet because of its volatile nature (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017) and pungent smell (Kaczmarek et al., Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016). Adil et al. (Reference Adil, Banday, Bhat, Qureshi and Wani2011) suggested that reduced feed intake can be observed due to reduced palatability of the feed when SCFAs are supplemented in their acid form (properties of butyric acid are presented in Fig. 4).
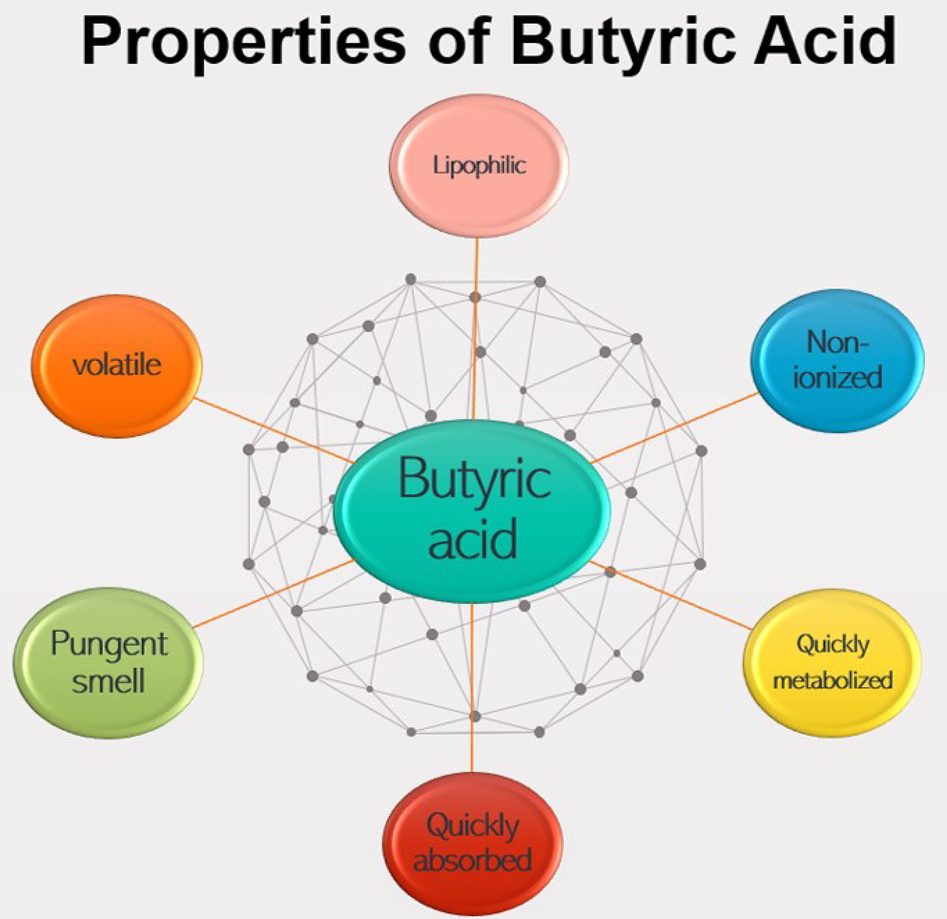
Fig. 4. Properties of butyric acid.
Another advantage of butyric acid supplemented in salt form is that it is less corrosive and more water-soluble (Khan and Iqbal, Reference Khan and Iqbal2016; Silva et al., Reference Silva, Milbradt, Zame, Padovani, de Lima Almeida Paz, Hataka, Okamoto, Gross and Filho2020). Butyric acid is quickly absorbed and metabolized by mucosal cells. Absorption and metabolism of butyric acid begin in the mucosa of the crop, and this process continues throughout the GIT. This rapid absorption limits the amount of butyric acid that will arrive in and affect the small intestine. Butyrate can be microencapsulated to reduce rapid absorption, thus helping improve its efficiency by allowing it to stay intact until it arrives in the small intestine. A common method of encapsulation is stearin or vegetable fat, and it has been found that this method has had positive effects on gut morphology and reduction of pathogen colonization in the intestine (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017; Makled et al., Reference Makled, Abouelezz, Gad-Elkareem and Sayed2019).
In a study by Liu et al. (Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017), researchers created an assay to determine the optimal time for butyric acid release from the GIT for broilers. It was found that encapsulated butyric acid aiming to stimulate epithelial cell development and improve digestibility should release at 30 min to 2.5 h post-ingestion; to focus on hind gut control, and the release should be at 2.5–4 h post-ingestion (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017; Makled et al., Reference Makled, Abouelezz, Gad-Elkareem and Sayed2019). Butyric acid needs to be in its undissociated form before it arrives at the hindgut to utilize its antimicrobial effect. Meanwhile, release in the small intestine should affect villi development and nutrient digestibility (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017).
Butyrate in its free form is used mostly as a feed sanitizer rather than as a supplement because it is quickly absorbed in the crop (Leeson et al., Reference Leeson, Namkung, Antongiovanni and Lee2005; Maty and Hassan, Reference Maty and Hassan2020). Unprotected butyrate is active in the crop, proventriculus, and gizzard. Tributyrin (a triglyceride of butyrate) is active in the small intestine and fat-coated/encapsulated butyrate is active in the ceca and colon (Moquet et al., Reference Moquet, Salami, Onrust, Hendriks and Kwakkel2018; Deepa et al., Reference Deepa, Purushothaman, VasanthaKumar and Sivakumar2018). Butyrate facilitates passage to the lower GIT where butyrate is released by lipase activity (Moquet et al., Reference Moquet, Salami, Onrust, Hendriks and Kwakkel2018). Monobutyrin has been used to potentially improve growth performance in broilers (Ahsan et al., Reference Ahsan, Cengiz, Raza, Kuter, Chacher, Iqbal, Umar and Çakir2016; Bedford et al., Reference Bedford, Yu, Squires, Leeson and Gong2017).
A recent study by Bedford et al. (Reference Bedford, Yu, Squires, Leeson and Gong2017) found that supplementing tributyrin alone had no significant effect on growth performance in broilers, whereas mixtures of mainly monobutyrin and tributyrin, with some dibutyltin, had positive effects on growth performance. Sodium butyrate promotes water absorption and proliferation of epithelial cells, provides energy, stimulates the synthesis of gastrointestinal hormones, and stimulates intestinal blood flow in broiler chicks (Hu and Guo, Reference Hu and Guo2007).
In a study by Moquet et al. (Reference Moquet, Salami, Onrust, Hendriks and Kwakkel2018), three forms of butyrate were tested in a diet with a poorly digestible protein source, to investigate the effect of butyrate on various parts of the GIT. It was reported that the presence of butyrate beyond the gizzard had an anorexic (appetite-reducing) effect, which was considered unusual for 1 g kg−1 of supplemented butyrate (Ahsan et al., Reference Ahsan, Cengiz, Raza, Kuter, Chacher, Iqbal, Umar and Çakir2016; Moquet et al., Reference Moquet, Salami, Onrust, Hendriks and Kwakkel2018). Studies have found that this anorexic effect caused by butyrate (and other SCFAs) is modulated by colonic L-cells that produce glucagon-like peptide 1 (GLP-1) and peptide YY (PYY). GLP-1 is released in the presence of digested protein as well as free fatty acids. PYY has an orexigenic (appetite-stimulating) effect in chickens, whereas it has an anorexic effect in rodents. PYY acts directly on the hypothalamus and triggers cholecystokinin (CCK), which promotes the satiety effect via the vagus nerve, reducing rodent appetite. The mechanism by which an orexigenic effect occurs in poultry is unclear (Furness et al., Reference Furness, Rivera, Cho, Bravo and Callaghan2013; Maty and Hassan, Reference Maty and Hassan2020).
In poultry, L-cells are located all along the distal small intestine, but the colon is the main site of anorexic effects (Moquet et al., Reference Moquet, Salami, Onrust, Hendriks and Kwakkel2018). L-cells are enteroendocrine cells that function by stimulating carbohydrate uptake, releasing insulin, and slowing intestine transit (Furness et al., Reference Furness, Rivera, Cho, Bravo and Callaghan2013; Wu et al., Reference Wu, Xiao, An, Dong and Zhang2018). Moquet et al. (Reference Moquet, Salami, Onrust, Hendriks and Kwakkel2018) reported that anorexic effects were reduced when butyrate was delivered to the crop, gizzard, and proventriculus in the unprotected form. It was also found that butyrate in the colon and ceca, in the protected form, increased total tract retention times, allowing more time for absorption and thus improved feed efficiency. Very few studies have demonstrated a link between colon motility and the butyrate effects (Moquet et al., Reference Moquet, Salami, Onrust, Hendriks and Kwakkel2018; Makled et al., Reference Makled, Abouelezz, Gad-Elkareem and Sayed2019).
SCFAs have also been found to increase ileal proglucagon mRNA, protein, and glucose transporter (GLUT-2) expression, potentially improving gut epithelial cell proliferation (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010; Ahsan et al., Reference Ahsan, Cengiz, Raza, Kuter, Chacher, Iqbal, Umar and Çakir2016). In addition to finding which butyrate source is most effective, researchers have begun to examine which diet composition and form is best suited to maximize the effects of butyric acid and its salts when added to different diets. A portion of studies carried out by Zhou et al. (Reference Zhou, Packialakshmi, Makkar, Dridi and Rath2014) and Qaisrani et al. (Reference Qaisrani, Van Krimpen, Kwakkel, Verstegen and Hendriks2015) looked at how the relationship between diet structure (coarse or fine) and butyric acid supplementation (with or without) affected growth performance and gut morphology in broilers. It was found that feeding a course diet supplemented with butyric acid positively affected performance, decreased crypt depth, and increased villus height to crypt depth ratio when added to a poorly digestible protein source (Qaisrani et al., Reference Qaisrani, Van Krimpen, Kwakkel, Verstegen and Hendriks2015).
Gut health
The impact of butyric acid on gut health is presented in Fig. 5. Gut health can be affected by nutrition, environment, or infectious disease agents. There is a direct relationship between gut health and animal performance, and many researchers have attempted to create a gut health scoring index that can be applied to poultry diets (Kraieski et al., Reference Kraieski, Hayashi, Sanches, Almeida and Santin2017). Researchers spend more time studying gut health because it is a major factor in the performance of both broilers and layers (Grashorn et al., Reference Grashorn, Gruzauskas, Dauksiene, Raceviciute-Stupeliene, Zdunczyk, Juśkiewicz, Bliznikas, Švirmickas and Slausgalvis2013; Silva et al., Reference Silva, Milbradt, Zame, Padovani, de Lima Almeida Paz, Hataka, Okamoto, Gross and Filho2020). Optimal gut health is characterized in several ways. One of them is the villi height to crypt depth ratio. A high ratio indicates mature and well-functioning villi with a shallow crypt that constantly provides cell renewal (Kaczmarek et al., Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016). Improved gut health has also been attributed to the increased length of the GIT, allowing increased absorption (Adil et al., Reference Adil, Banday, Bhat, Qureshi and Wani2011).
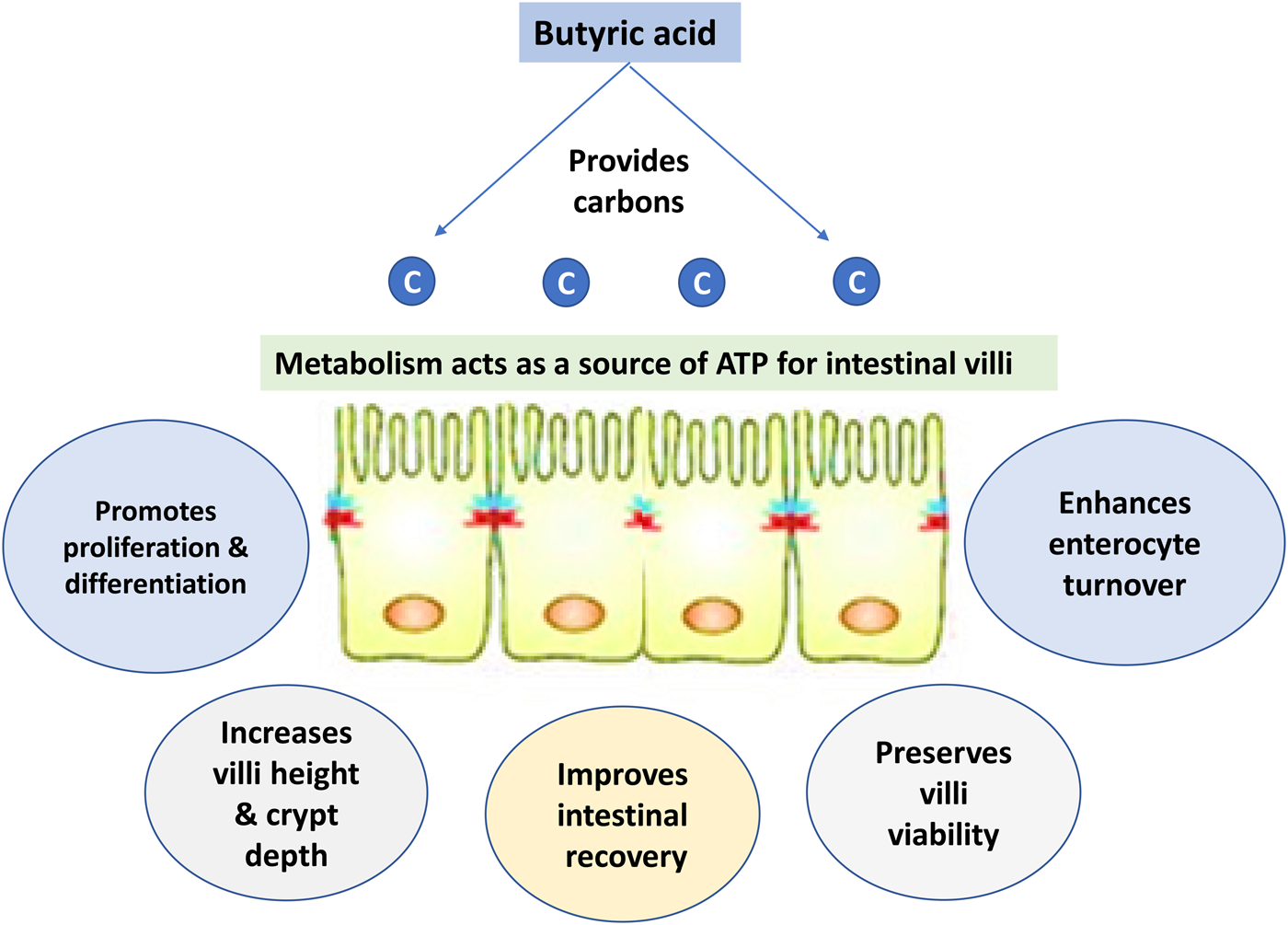
Fig. 5. Impacts of butyric acid on gut health.
Dietary supplementation with organic acid supports the gut health of poultry species (Alagawany et al., Reference Alagawany, Elnesr, Farag, Abd El-Hack, Barkat, Gabr, Foda, Noreldin, Khafaga, El-Sabrout, Elwan, Tiwari, Yatoo, Michalak, Di Cerbo and Dhama2021). Butyric acid can improve epithelial cell development (Levy et al., Reference Levy, Kessler, Fuller, Williams, Mathis, Lumpkins and Valdez2015). Butyric acid is also thought to effectively preserve cell viability and enhance enterocyte turnover, which may improve intestinal recovery. It has been observed that butyrate supplementation can increase villi height and decline crypt depth in poultry and other non-ruminant animals, thereby increasing the absorptive surface (Qaisrani et al., Reference Qaisrani, Van Krimpen, Kwakkel, Verstegen and Hendriks2015; Wu et al., Reference Wu, Xiao, An, Dong and Zhang2018; Elnesr et al., Reference Elnesr, Ropy and Abdel-Razik2019). SCFAs are theorized to have several mechanisms of antimicrobial activity. One of the most widely accepted mechanisms by which butyric acid can destroy pathogenic bacteria and express its antibacterial activity is that the acid changes the internal pH of the microbe (depolarization) and therefore disrupts nutrient synthesis and transport as well as energy metabolism of that microbe (Figs 2 and 6) (Adil et al., Reference Adil, Banday, Bhat, Qureshi and Wani2011).
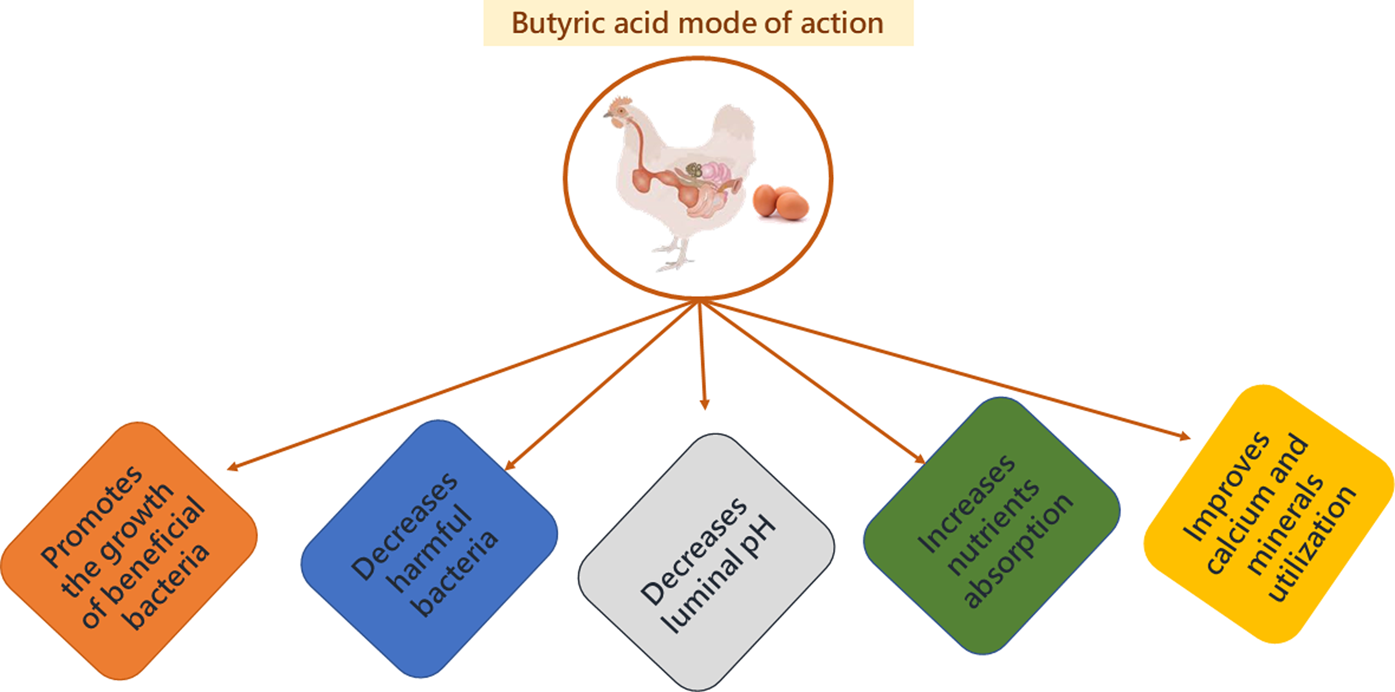
Fig. 6. Mode of action of butyric acid.
Organic acids can penetrate the surrounding membranes of bacteria. Once inside the membrane, they will dissociate, forming H+ ions as a result of the neutral pH, releasing excess protons that will lower the pH. The microbe will then attempt to maintain a neutral pH by transporting excess protons outside of cells via ATP synthase, depleting its cellular energy (Fig. 3) (Biggs and Parsons, Reference Biggs and Parsons2008; Zhou et al., Reference Zhou, Packialakshmi, Makkar, Dridi and Rath2014). The microbe is then no longer able to multiply efficiently (Adil et al., Reference Adil, Banday, Bhat, Qureshi and Wani2011). Butyric acid can have antimicrobial effects by decreasing the luminal pH and reducing bacterial colonization in the intestinal wall (Panda et al., Reference Panda, Rao, Raju and Sunder2009; Elnesr et al., Reference Elnesr, Alagawany, Elwan, Fathi and Farag2020), resulting in less damage to epithelial cells (Qaisrani et al., Reference Qaisrani, Van Krimpen, Kwakkel, Verstegen and Hendriks2015; Wu et al., Reference Wu, Xiao, An, Dong and Zhang2018).
Damaging epithelial cells can disrupt the barrier between the internal and external environment of the lumen, allowing toxins to enter the circulation and increasing the susceptibility of the intestine to colonization by pathogenic bacteria (Abdelqader and Al-Fataftah, Reference Abdelqader and Al-Fataftah2016). Butyric acid functions by inhibiting Salmonella colonization in the ceca due to the improvement of intestinal barrier function (Abdelqader and Al-Fataftah, Reference Abdelqader and Al-Fataftah2016) and downregulates Salmonella gene expression (Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017).
Decreasing luminal pH is also beneficial because it stimulates the growth of beneficial bacteria and hampers the growth of pathogenic bacteria (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010). Commonly, pathogen growth is likely to occur in the GIT when the lumen of the small intestine and ceca exceed a pH of 5.8–6.0 and the large intestine exceeds pH 6.2 (Brzóska et al., Reference Brzóska, Śliwiński and Michalik-Rutkowska2013). In a study by Adil et al. (Reference Adil, Banday, Bhat, Mir and Rehman2010), villus height was significantly different in the duodenum and jejunum when chicks were fed organic acids, with the highest height in chicks consuming the 3% butyric acid diet. It was suggested that the significant growth of the villi was due to a reduction in the growth of pathogenic and non-pathogenic bacteria, decreasing colonization and inflammatory responses of the intestinal mucosa (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010; Silva et al., Reference Silva, Milbradt, Zame, Padovani, de Lima Almeida Paz, Hataka, Okamoto, Gross and Filho2020).
Inflammation of the intestinal mucosa due to increased pathogenic activity can lead to necrosis of the intestinal epithelium (Brzóska et al., Reference Brzóska, Śliwiński and Michalik-Rutkowska2013). Crypt depth was not affected compared to broilers fed the control diet (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010). A study by Hu and Guo (Reference Hu and Guo2007) found that supplemented sodium butyrate at 2000 mg kg−1 in broilers had no effect on jejunal villi height and crypt depth and significantly increased the villi height to crypt depth ratio when compared to the control. Table 1 shows the effect of different sources of butyric acid, at varying levels, on different parameters related to gut health.
Table 1. Effect of various forms and levels of butyric acid supplementation on gut morphology and bacterial count in broiler compared to control group

VL, villus length; CD, crypt depth; VL/CD, villus length/crypt depth; GIT, gastrointestinal tract; NA, not affected; *significant effect (P < 0.05); +, enhanced/improve; −, reduced/lower.
Immunity
Butyric acid has a positive impact on bird immunity through the improvement of gut eubiosis, increasing number of beneficial bacteria, limiting the colonization of pathogens, and improving gut pH, and all these factors positively reflected on the birds’ immune responses (Sikandar et al., Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017). It was found that the inclusion of butyric acid in poultry ration was associated with better cell-mediated immune responses in chickens 48 h after-phytohemagglutinin-P inoculation, improved humoral immunity, and better antibody production after Newcastle disease vaccine and injection of sheep red blood cells. This resulted in better thymus and spleen weight with better thymus medulla and germinal spleen centers. It also improved the intestinal villi length and depth, and increased goblet cells containing mucins of acidic nature (Sikandar et al., Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017).
Performance parameters
Increased absorption efficiency due to improved gut health has led to various effects on performance parameters in broilers and laying hens, depending on the forms of butyric acid used and the inclusion rate. Researchers have found that butyric acid supplementation had positive effects on body weight gain (BWG) and feed conversion rate (FCR) in broilers (Leonel and Alvarez-Leite, Reference Leonel and Alvarez-Leite2012; Liu et al., Reference Liu, Bayir, Cosby, Cox, Williams and Fowler2017). Detailed data regarding the effects of butyric acid on different growth performances are presented in Table 2.
Table 2. Effect of various forms and levels of butyric acid supplementation on growth performance of broiler compared to control group

FCR, feed conversion ratio.
a Experimental bird was Japanese quail.
*Significant difference (P < 0.05).
Several studies have shown that butyric acid does not significantly affect feed consumption. A significant difference in FCR and BWG was seen in favor of organic acids, suggesting better absorption and nutrient utilization than birds on the control diet (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010). It was also found in this study that there were higher serum calcium and phosphorus concentrations when compared to the control (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010). When researching butyric acid, there are often contradiction due to the type of diet and the forms of butyrate (calcium salt, sodium salt, glyceride, etc.) (Leonel and Alvarez-Leite, Reference Leonel and Alvarez-Leite2012; Kaczmarek et al., Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016).
In a study by Kaczmarek et al. (Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016) with broilers, researchers attempted to find a ‘matrix value’ for butyrate in poultry diets to maximize its efficacy. The experiment showed that butyric acid positively affected FCR and BWG, and the addition of 0.2 g kg−1 of butyrate improved FCR, 0.3 g kg−1 improved FCR regardless of the age of birds. In comparison, 0.4 g kg−1 decreased feed intake (FI) and significantly increased FCR. This study indicated that the 0.3 g kg−1 provided produced the most positive effects compared to the control and other butyrate doses. Leeson et al. (Reference Leeson, Namkung, Antongiovanni and Lee2005), found contradictory results, when 0.2 g kg−1 butyrate was supplemented as a glyceride. This addition maintained the performance and carcass quality in vaccinated broilers challenged with coccidia. It was also found that butyrate in the glyceride form caused FI depression similar to that of 0.4 g kg−1 in the Kaczmarek et al. (Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016) experiment. Abdelqader and Al-Fataftah (Reference Abdelqader and Al-Fataftah2016) found that 0.5 g of butyric acid kg−1 diet recovered intestinal epithelia and improved integrity in heat-stressed broilers compared to controls.
A study by Taherpour et al. (Reference Taherpour, Moravej, Shivazad, Adibmoradi and Yakhchali2009) showed that broilers supplemented with butyric acid glyceride showed improved BWG compared to the control. In this study, the contradiction of these results compared to other studies was attributed to differences in preparation of the diet composition or particle size and experimental conditions. Like the studies above, FCR improved while FI was increased in favor of butyric acid glyceride. There was no significant difference in mortality in this study. Brzóska et al. (Reference Brzóska, Śliwiński and Michalik-Rutkowska2013) suggested that the use of organic acids in the diet promotes the production of prebiotics and probiotic lactic acid bacteria in young birds.
Brzóska et al. (Reference Brzóska, Śliwiński and Michalik-Rutkowska2013) and Zhou et al. (Reference Zhou, Packialakshmi, Makkar, Dridi and Rath2014) noted that, in several studies, organic acids, including butyric acid, significantly reduced mortality when compared to controls. Finally, the performance improvement may be attributed to the role of butyric acid in the control of the intestinal barrier, supplying energy to the colonocytes, augmenting the differentiation and maturation of the intestinal cells, thus nutrient utilization, feed efficiency, and the positive immune response of birds. The impacts of adding butyric acid in poultry feed are illustrated in Fig. 7.
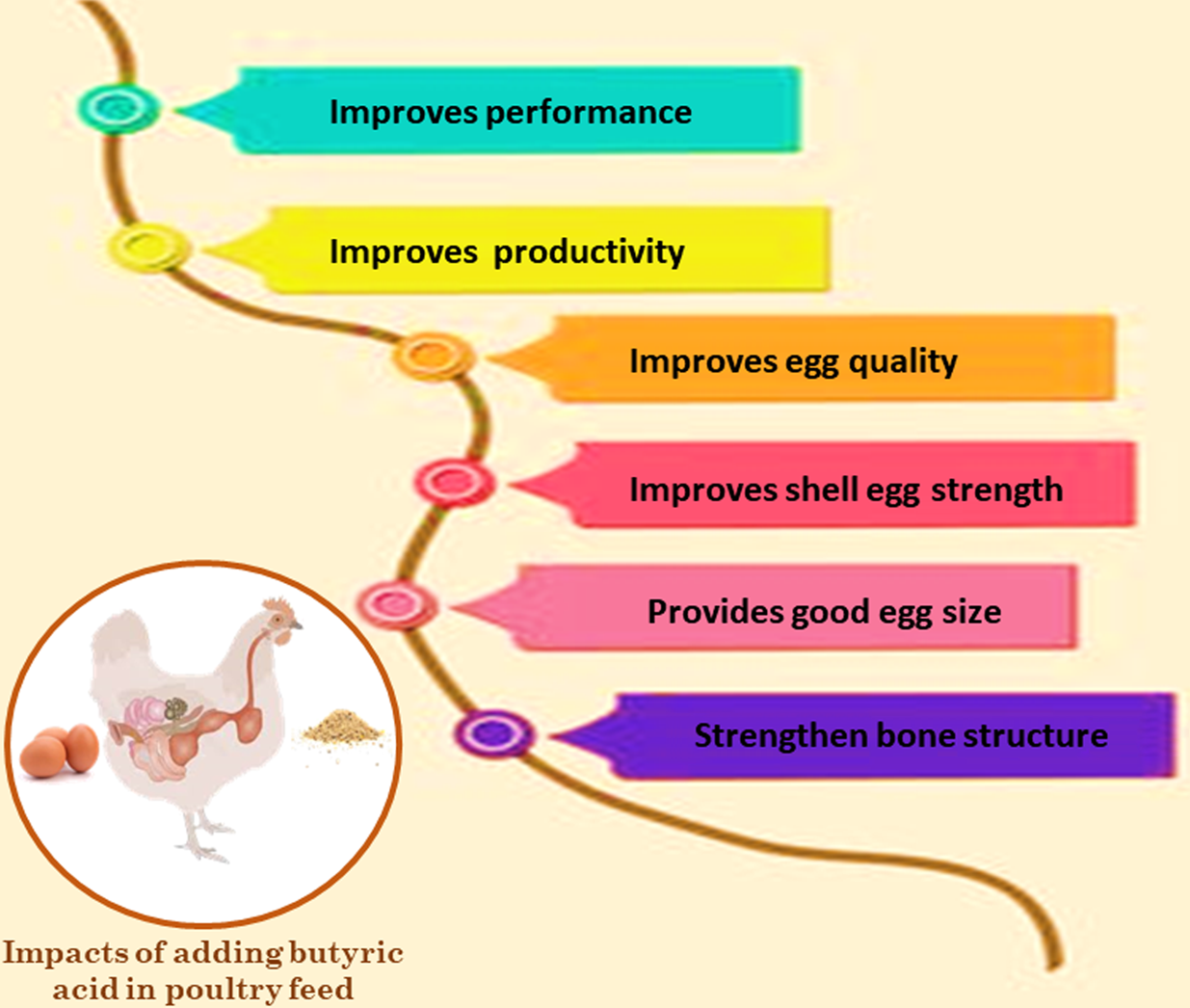
Fig. 7. Impacts of adding butyric acid in poultry feed.
Metabolizable energy and nutrient utilization
Organic acids have been found to increase the digestibility of calcium, phosphorus, magnesium, zinc, and protein (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010). Supplementation of organic acids in broiler diets enhanced serum calcium and phosphorus concentrations. These results were credited with the notion that acidic ions form a complex with minerals such as calcium and phosphorus, thereby increasing their digestibility (Table 3). Organic acids also act as substrates for intermediary metabolism (Adil et al., Reference Adil, Banday, Bhat, Qureshi and Wani2011). Butyric acid can increase the feed solubility, digestion, and nutrient absorption (Rahman et al., Reference Rahman, Howlider, Mahiuddin and Rahman2008; Leonel and Alvarez-Leite, Reference Leonel and Alvarez-Leite2012).
Table 3. Effect of various forms and levels of butyric acid supplementation on nutrient digestibility of broiler compared to control group
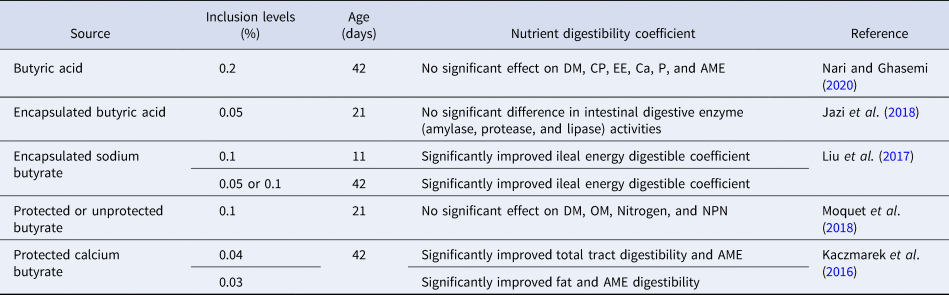
DM, dry matter; OM, organic matter; CP, crude protein; EE, ether extract; Ca, calcium; P, phosphorus; AME, apparent metabolizable energy; NPN, non-protein nitrogen.
With fewer pathogenic bacteria, due to organic acids, there is a reduced microbial metabolic need, thereby allowing more nutrients to be available for absorption by the host. The decrease in the toxins produced by harmful bacteria can also cause an increase in energy availability and protein digestibility (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010; Silva et al., Reference Silva, Milbradt, Zame, Padovani, de Lima Almeida Paz, Hataka, Okamoto, Gross and Filho2020). Adil et al. (Reference Adil, Banday, Bhat, Qureshi and Wani2011) suggested that increased protein digestibility because feeding organic acids in the diet reduces gastric pH resulting in increased pepsin activity. Pepsin proteolysis of proteins releases peptides that trigger hormones such as CCK and gastrin to be released. These hormones play a significant role in the digestion and absorption of proteins (Adil et al., Reference Adil, Banday, Bhat, Qureshi and Wani2011).
Goodarzi Boroojeni et al. (Reference Goodarzi Boroojeni, Mader, Knorr, Ruhnke, Röhe, Hafeez and Zentek2014) noted that the effects of organic acids on digestibility were debatable due to multiple factors affecting the results. There was no significant effect found for nutrient digestibility in this study compared to the control for broilers. Kaczmarek et al. (Reference Kaczmarek, Barri, Hejdysz and Rutkowski2016) found that, 0.2, 0.3, and 0.4 g kg−1 of butyrate increased the AME compared to the control diet for broilers, due to the significant increase in villi height and numerically increased mucosal thickness observed in this study.
Egg quality
Improved egg quality can be identified as improving eggshell strength while maintaining a good egg size. Laying hens must consume the correct ratio of manganese, vitamin D, calcium, and phosphorus to produce strong eggshells. As the hen ages, mucosal cells in the duodenum have weakened villi, which begin to shorten, and absorption in the small intestine decreases, resulting in reduced eggshell quality (Sengor et al., Reference Sengor, Yardimci, Cetingul, Bayram, Sahin and Dogan2007). Sengor et al. (Reference Sengor, Yardimci, Cetingul, Bayram, Sahin and Dogan2007) suggested that butyrate can function in maintaining the mucosa and epithelial cells. In this study, improvements in eggshell strength and increased egg production were observed and attributed to the healing of damaged epithelial cells in addition to increased villi growth (Sengor et al., Reference Sengor, Yardimci, Cetingul, Bayram, Sahin and Dogan2007; Sikandar et al., Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017).
Maintaining a high eggshell breaking strength is needed to protect the egg from penetration by pathogenic bacteria. Broken shells are a significant source of economic losses for producers (Świątkiewicz et al., Reference Świątkiewicz, Koreleski and Arczewska2010). The formation of a normal (not misshapen) eggshell requires minerals to be released from the shell gland in the right proportions, at the right time, to ensure good eggshell quality. There must be adequate absorption and metabolism of nutrients to achieve this physiologic state (Sengor et al., Reference Sengor, Yardimci, Cetingul, Bayram, Sahin and Dogan2007). Butyrate has improved calcium metabolism and absorption by increasing villi growth (Rahman et al., Reference Rahman, Howlider, Mahiuddin and Rahman2008).
Sengor et al. (Reference Sengor, Yardimci, Cetingul, Bayram, Sahin and Dogan2007) suggested that weakness in the eggshell in older hens can be altered with butyrate, if supplemented at 285 mg kg, resulting in increased eggshell strength and decreased malformed eggs. Świątkiewicz et al. (Reference Świątkiewicz, Koreleski and Arczewska2010) reported that one of the main concerns is a decrease in eggshell quality as the hen ages due to an increase in egg weight without an increase in the amount of calcium carbonate deposited in the shells. They found a positive effect of the organic acids on some eggshell quality parameters in older hens, probably due to their beneficial effect on calcium absorption.
It was also determined that lowering the pH of the diet can benefit eggshell quality (Świątkiewicz et al., Reference Świątkiewicz, Koreleski and Arczewska2010). Butyric acid and its salts have shown different results for egg quality and egg production. This difference has been attributed to the source of butyric acid, the inclusion rate, and the environmental conditions and diet composition (Soltan, Reference Soltan2008; Sikandar et al., Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017). Rahman et al. (Reference Rahman, Howlider, Mahiuddin and Rahman2008) reported a significant increase in egg production in 67–74 weeks old hens when fed on a diet supplemented with various concentrations of organic acids, including calcium butyrate, compared to the control diet. They illustrated that the mixture of fumaric acid, salts of butyric, propionic, and lactic acids, did not affect egg weight and eggshell%. In contrast, the egg size and albumen% were increased and yolk% was decreased with dietary supplementation of organic acids.
Also, organic acids significantly increased the eggshell thickness (Rahman et al., Reference Rahman, Howlider, Mahiuddin and Rahman2008; Sunkara et al., Reference Sunkara, Achanta, Schreiber, Bommineni, Dai, Jiang, Lamont, Lillehoj, Beker, Teeter and Zhang2011). These findings are in agreement with Soltan (Reference Soltan2008) but contrary to the study performed by Yesilbag and Colpan (Reference Yesilbag and Colpan2006), using an organic acid mixture that did not include butyric acid or its salt (Rahman et al., Reference Rahman, Howlider, Mahiuddin and Rahman2008). Work is needed to improve the perception of the effects of butyric acid on egg quality and what can be done in the future to better utilize butyric acid as an antibiotic alternative.
Osteoporosis
Osteoporosis can be described as an increased porosity and reduced bone thickness, which is a major bone-related disease that can occur when there is an increased demand for calcium from the medullary bone for the eggshell formation and maintenance of eggshell quality. This reduced thickness can result in bone breakage. In hens, osteoporosis manifests as cage layer fatigue (Webster, Reference Webster2004; Khan and Iqbal, Reference Khan and Iqbal2016). Cage layer fatigue can be identified by the inability of hen to stand or walk. These hens tend to have a willingness to eat or drink. The hen will die if cage layer fatigue is not treated. Cage layer fatigue can be classified as peracute and acute. Peracute fatigue occurs when the hen dies suddenly with no visible symptoms, and acute fatigue is when the hen experiences leg paralysis and can potentially recover with assistance (Bell and Siller, Reference Bell and Siller1962). Young hens that are in peak production are most likely to develop osteoporosis.
Bell and Siller (Reference Bell and Siller1962) also concluded that some genetic lines of layers were more susceptible to osteoporosis than others. Medullary bone development and the end of structural bone remodeling occur simultaneously with the beginning of hen sexual maturity. The medullary bone stores large amounts of calcium, which is released later in the formation of the eggshell when calcium is absent or cannot be readily absorbed from the digestive tract. Osteoporosis will occur if not enough calcium is absorbed from the intestine to remodel the structural bone after it provides calcium to the medullary bone (Webster, Reference Webster2004; Khan and Iqbal, Reference Khan and Iqbal2016).
Studies done in ovariectomized rats suggest that organic acids can prevent osteoporosis by reducing the amount of bone turnover due to lowering gut pH and improving calcium absorption, solubility, and utilization, and could further improve osteoporosis and egg quality (Kamal and Ragaa, Reference Kamal and Ragaa2014). It has also been suggested that osteoporosis cannot be avoided in the caged modern hybrid laying hen due to confinement and its high egg production (Webster, Reference Webster2004). This situation necessitates the use of additives to mitigate adverse effects on birth, health, and production.
Conclusion
Butyric acid has enhanced nutrient/energy utilization, gut health, and production performance in poultry, by improving mineral absorption, immunity, and reducing the populations and products of pathogenic bacteria. Our findings suggest that the high stability of tributyrin in the feed and stomach should increase the efficacy of butyric acid, thereby improving the efficiency of gut health and absorption of nutrients, leading to improved performance. However, further investigations are required to explore the effect of butyric acid and its salts on poultry immunity.
Conflict of interest
None.













