Introduction
Older adults with dementia spend several years in severe stages of the disease (Brodaty et al. Reference Brodaty, Seeher and Gibson2012) experiencing considerable symptoms (Schulz et al. Reference Schulz, McGinnis and Zhang2008). In the context of severe dementia, previous studies have shown that symptoms encompass functional limitations, behavioral problems, lack of social interaction, and emotional symptoms (Cipher and Clifford Reference Cipher and Clifford2004; Cipriani et al. Reference Cipriani, Danti and Picchi2020; Malhotra et al. Reference Malhotra, Hazirah and Tan2021a; Mitchell and Solomon Reference Mitchell and Solomon2015). However, no study has yet identified distinct profiles of older adults with severe dementia based on the unique combination of the various symptoms they experience. Determining these distinct profiles is important to guide the development and scope of interventions aimed at managing symptoms among older adults at the end of life. For example, if a profile represents older adults with eating problems, lack of social interactions, and lack of ability to communicate verbally or through eye contact, then a holistic person-centered intervention targeting all of these multiple symptoms will likely be more beneficial rather than one focused only on eating problems.
Literature shows that older adults’ symptoms may lead them to use potentially burdensome health-care interventions such as hospitalization, tube feeding, and restraints and influences their caregivers’ satisfaction with health care, distress, and burden (Epstein-Lubow et al. Reference Epstein-Lubow, Gaudiano and Darling2012; Evans and Cotter Reference Evans and Cotter2008; Hoffmann et al. Reference Hoffmann, Strautmann and Allers2019; Regier and Gitlin Reference Regier and Gitlin2018; Zekry et al. Reference Zekry, Herrmann and Grandjean2009). Studies have shown that hospitalization among older adults with dementia is distressful and results in inadequate pain relief, potentially high use of harmful medication, and high societal costs (Shepherd et al. Reference Shepherd, Livingston and Chan2019). Tube feeding older adults with severe dementia does not prolong survival or improve nutrition and increases the likelihood of them being restrained (Lee et al. Reference Lee, Hsu and Liang2021). Therefore, use of such health-care interventions to manage symptoms of older adults with severe dementia is unlikely to improve their quality of life or end-of-life experience.
However, it is not known whether certain symptom profiles among older adults are associated with greater use of such interventions, and if caregivers of these older adults are at greater risk of adverse outcomes.
Our first aim was to delineate distinct symptom profiles of older adults with severe dementia. We used latent class analysis (LCA) to identify distinct profiles of older adults based on the symptoms they experienced. LCA is a statistical method that splits seemingly heterogeneous data into 2 or more homogeneous classes (Williams and Kibowski Reference Williams, Kibowski, Jason and Glenwick2016). This technique identifies unobserved classes of individuals similar in specified key traits, such as the symptoms experienced (Ferrat et al. Reference Ferrat, Audureau and Paillaud2016). Past studies have used LCA to delineate, characterize, and validate profiles of patients or caregivers based on their health status or care experiences (Ferrat et al. Reference Ferrat, Audureau and Paillaud2016; Grant et al. Reference Grant, McCloskey and Hatfield2020).
Our second aim was to validate the delineated profiles by associating them with key older adults’ and caregiver characteristics. We hypothesized that profiles of older adults representing multiple symptoms are more likely to have advanced dementia and history of using potentially burdensome interventions to manage symptoms, and their caregivers report greater burden, distress, grief, and lower satisfaction with health care.
Lastly, we aimed to assess whether the delineated profiles were prospectively associated with 1-year mortality. We hypothesized that profiles of older adults representing greater functional deficits will be at greater risk of 1-year mortality.
Methods
Study design and participants
We used data from “Panel study Investigating Status of Cognitively impaired Elderly in Singapore (PISCES)” study, a prospective cohort of 215 primary informal caregivers of community-dwelling older adults with severe dementia in Singapore. The sample size for this cohort study was considered based on estimating main effects over all follow-up times. Details of the study (trial registration: NCT03382223) and sample size calculation are published (Malhotra et al. Reference Malhotra, Vishwanath and Yong2020). Between May 2018 and March 2021, we recruited eligible participants from 7 major public restructured hospitals, 6 home care foundations, and 2 hospices. Eligibility criteria for older adults included those with diagnosis of dementia and Functional Assessment Staging Test (FAST) criteria 6C or higher (Sclan and Reisberg Reference Sclan and Reisberg1992). Eligibility criteria for caregivers included age ≥21 years, being a family member and primary decision-maker for older adult’s treatment or responsible for ensuring their well-being, meet the older adult at least 1 day per week, and intact cognition as determined through Abbreviated Mental Test for those aged ≥65 years. Institutional Review Boards at SingHealth and the National University of Singapore approved the study.
Study measures
Indicators for LCA
Based on previous literature (Hendriks et al. Reference Hendriks, Smalbrugge and Hertogh2014; Mitchell and Solomon Reference Mitchell and Solomon2015; Mitchell et al. Reference Mitchell, Teno and Kiely2009; Morrison and Siu Reference Morrison and Siu2000; Schmidt et al. Reference Schmidt, Eisenmann and Golla2018; Yuan et al. Reference Yuan, Wang and Tan2021), we assessed symptoms and medical problems among older adults, as reported by their caregiver, using data from the first wave of PISCES. These included physical, emotional, and functional symptoms and responsive behaviors among older adults. When symptoms were highly correlated (e.g. eating and malnutrition and aggressive and nonaggressive responsive behaviors), we combined them to form a single indicator. The following 10 indicators were used for LCA:
Recent acute medical problem (yes/no): Any of the following in the past 4 months – pneumonia, urinary tract infection, fever, stroke, hip fracture or any other medical problem.
Pain (yes/no or not sure): Presence of pain in the past week adapted from Mini-Suffering State Examination (Aminoff et al. Reference Aminoff, Purits and Noy2004). Response options were categorized as “yes” or “no/not sure.”
Eating difficulty (yes/no): Response of “yes” to either or both of the following 2 questions in the Mini-Suffering State Examination(Aminoff et al. Reference Aminoff, Purits and Noy2004), in the context of the past week: (i) refusal to eat, difficulty or discomfort with swallowing, loss of appetite, or need of feeding tube and (ii) appearing malnourished including weight loss and sunken eyes or cheeks.
Appearance of discomfort (yes/no): Response of “nearly half the day” or “most of the day” (vs. “rarely or never,” “less than once a day,” or “at least once a day”) for 1 item of the Quality of Life in Late-Stage Dementia (QUALID) (Weiner et al. Reference Weiner, Martin-Cook and Svetlik2000) asking whether the older adult appeared physically uncomfortable in the past week.
Loss of spontaneous smile (yes/no): Using 1 item of the QUALID (Weiner et al. Reference Weiner, Martin-Cook and Svetlik2000) assessing whether older adult could smile spontaneously, we considered the older adult to have lost the ability to spontaneously smile if the older adult was reported to smile only in response to external stimuli less than once a day or rarely at all.
Signs of unhappiness (yes/no): The older adult was considered to be unhappy if he/she appeared to display any of the following 3 signs without any reason or cause, as assessed using the QUALID (Weiner et al. Reference Weiner, Martin-Cook and Svetlik2000) – appears sad; makes statements or sounds suggesting discontent, unhappiness, or discomfort; and cries.
Disturbance in sleep–wake cycle and/or muscle rigidity/contracture (pathological impairment): We used 2 questions from the Bedford Alzheimer Nursing Severity Scale (BANS-S) (Bellelli et al. Reference Bellelli, Frisoni and Bianchetti1997) to assess occurrence of pathological impairment (sleep disruption and muscle rigidity/contraction). The score on each item (4-point Likert scale) was summed; total score ranged from 2 to 8, a higher score indicated more pathological impairment.
Loss of speech and eye contact (cognitive impairment): We used the cognitive impairment subscale of the BANS-S to assess loss of speech and eye contact (Bellelli et al. Reference Bellelli, Frisoni and Bianchetti1997). Each item was rated on a 4-point Likert scale; the score from both items were summed for a total score ranging from 2 to 8, a higher score indicating greater cognitive impairment.
Functional deficits: We assessed difficulties in dressing, eating, and ambulation using 3 items from the BANS-S (Bellelli et al. Reference Bellelli, Frisoni and Bianchetti1997). Each item was rated on a 4-point Likert scale indicating progressively greater levels of dependence in that activity. Total score was the sum of all 3 items ranging between 3 and 12, a higher score indicating greater functional deficits.
Responsive behaviors: These were assessed using 14 items from the Cohen-Mansfield Agitation Inventory(Cohen-Mansfield Reference Cohen-Mansfield1991). Each item was rated on a 4-point Likert scale; the total score was the sum of all items, ranging from 14 to 70. A higher score indicating a higher extent of responsive behaviors.
Other characteristics of older adults with severe dementia
Older adult sociodemographics
Sociodemographic factors included age, gender, number of comorbidities (heart failure, any other heart disease, cancer, cerebrovascular disease, diabetes, chronic obstructive lung disease, renal failure, Parkinson’s disease, or any other disease), and FAST stage (Sclan and Reisberg Reference Sclan and Reisberg1992).
Potentially burdensome interventions
Potentially burdensome interventions included any of the following in the past 4 months – admission to emergency room, hospitalization, cardiopulmonary resuscitation, tracheal intubation, admission to intensive care unit, mechanical ventilation, intravenous fluids, chemotherapy, radiotherapy, intravenous antibiotics, blood transfusion, dialysis, pacemaker or surgery, current tube feeding, and use of restraints.
Mortality
Information on date of death of older adults was collected from caregivers’ reports during follow-up.
Caregiver characteristics
Psychological distress
Psychological distress was assessed using the 14-item Hospital Anxiety and Depression Scale (Zigmond and Snaith Reference Zigmond and Snaith1983). Total score ranged between 0 and 42, a higher score indicating greater distress.
Burden
Burden was assessed using the modified Caregiver Reaction Assessment (Malhotra et al. Reference Malhotra, Chan and Malhotra2012) scale to determine the impact of caregiving on 3 domains, schedule and health (8 items), finances (2 items), and lack of family support (5 items). Each item was scored on a 5-point Likert scale ranging from strongly disagree (1) to strongly agree (5). The items in each domain were averaged to generate a subscale score ranging from 1 to 5. A higher score indicated greater burden in that domain.
Anticipatory grief
Anticipatory grief was assessed with the 18-item Singapore version of the Marwit Meuser Caregiver Grief Inventory-Short Form (Liew Reference Liew2016). Total score ranged between 1 and 90, a higher score indicated greater grief.
Satisfaction with care at the end of life in dementia
Satisfaction with care at the end of life in dementia (Volicer et al. Reference Volicer, Hurley and Blasi2001) included 10 items. The total score ranged from 1 to 40; a higher score indicated greater satisfaction.
Coping
We assessed adaptive (16 items: score range 1–64) and maladaptive (10 items: score range 1–40) coping using the Brief-COPE (Moore et al. Reference Moore, Biegel and McMahon2011).
The face validity of all scales used in this study was assessed through cognitive interviews during pilot testing. Further, scales used were checked for internal reliability using the participant data and showed reliability with a Cronbach’s alpha score ≥0.7 across all scales (details provided in Supplementary Table S1). These scales have been previously used in older adults with dementia (Hum et al. Reference Hum, Tay and Wong2020; Ng et al. Reference Ng, Niti and Chiam2007; Wong and Zelman Reference Wong and Zelman2020) and their caregivers in Singapore (Lim et al. Reference Lim, Griva and Goh2011; Liew Reference Liew2016; Malhotra et al. Reference Malhotra, Chan and Malhotra2012; Tay et al. Reference Tay, Hum and Ali2020; Yuan et al. Reference Yuan, Wang and Tan2021) and other Asian countries (Kang et al. Reference Kang, Song and Kim2020; Liang et al. Reference Liang, Guo and Luo2016), exhibiting similar patient characteristics.
Statistical analysis
We used Stata version 16 to perform all the analyses. First, we systematically tested a series of models with increasing number of latent classes (i.e. symptom profiles of severe dementia) including the covariates (age, gender, and number of comorbidities) to identify the best fitting model. We sequentially tested models with increasing number of classes until the models failed to converge. We considered Akaike’s Information Criteria, Bayesian Information Criteria (BIC), percentage change in BIC, and entropy to choose the optimal number of classes. Entropy is a standardized index of model-based classification accuracy, with higher values indicating more precise assignment of individuals to latent classes (Wang et al. Reference Wang, Deng and Bi2017). Entropy value of ≥0.80 is considered as high. We assessed the marginal predicted means for continuous indicator variables and predicted probabilities for categorical indicator variables, within each latent class (Park et al. Reference Park, Jang and Lee2018). The correlation between symptoms included in the LCA was low (Pearson’s correlation coefficient <0.5).
Validation of the delineated profiles
We assessed whether older adult and caregiver characteristics varied between the delineated profiles of older adults. We assessed the association of delieneated symptom profiles with FAST stage and use of potentially burdensome interventions (including and not including physical restraints) using chi-square test.
We used separate linear regressions to assess whether caregiver characteristics (dependent variables: psychological distress, burden, anticipatory grief, satisfaction with care, and coping) varied by the delineated profiles (independent variable). Each regression model was adjusted for older adults’ age, gender, and number of comorbidities.
Lastly, we assessed the unadjusted (using the log-rank test) and adjusted (using the Cox-proportional Hazard survival model, adjusting for potential confounding due to older adult’s age, gender, and number of comorbidities) association of the delineated profiles with 1-year mortality of the older adults from the time of study enrollment. We tested proportionality assumption using Schoenfeld residuals for the overall model and for each predictor (p>0.10 for all).
Results
We approached 293 eligible caregivers to participate in the study; 215 (73%) consented and were interviewed. A total of 42 older adults with severe dementia (20%) died within 1 year of study enrollment.
Table 1 shows the sample characteristics. Older adults were, on average, aged 83.2 (SD: 8.1) years and over three-quarter were females (77%). Mean number of comorbidities among older adults was 1.8 (SD: 1.2). Nearly two-thirds of the older adults were having FAST stage 7 dementia. Majority of the caregivers were children of older adults (83%). Nearly two-third (63%) of the older adults had received at least one potentially burdensome intervention to manage symptoms in the past 4 months. Among these, nearly half (47%) had experienced physical restraints, the interventions included hospitalization (30%), intravenous antibiotics (13%), intravenous fluids (12%), tube feeding (10%), emergency room visit (2%), blood transfusion (2%), surgery (1%), and others (3%).
Table 1. Sample characteristics, N = 215

Notes:
a Functional Assessment Staging Test.
b Includes pneumonia, urinary tract infection, fever, stroke, hip fracture, or any other medical problem in the past 4 months.
c Includes refusal to eat, difficulty or discomfort with swallowing, loss of appetite or need of feeding tube, or weight loss, sunken eyes or cheeks.
d Appears physically uncomfortable – squirms, writhes, or frequently changes position nearly half the day or most of the day.
e Smile in response to external stimuli less than once a day or rarely at all.
f Appears sad, makes statements or sounds suggesting discontent, unhappiness or discomfort, and/or cries.
g Based on 14 items of Cohen-Mansfield Agitation Inventory (CMAI).
h Dressing, eating, and walking impairment.
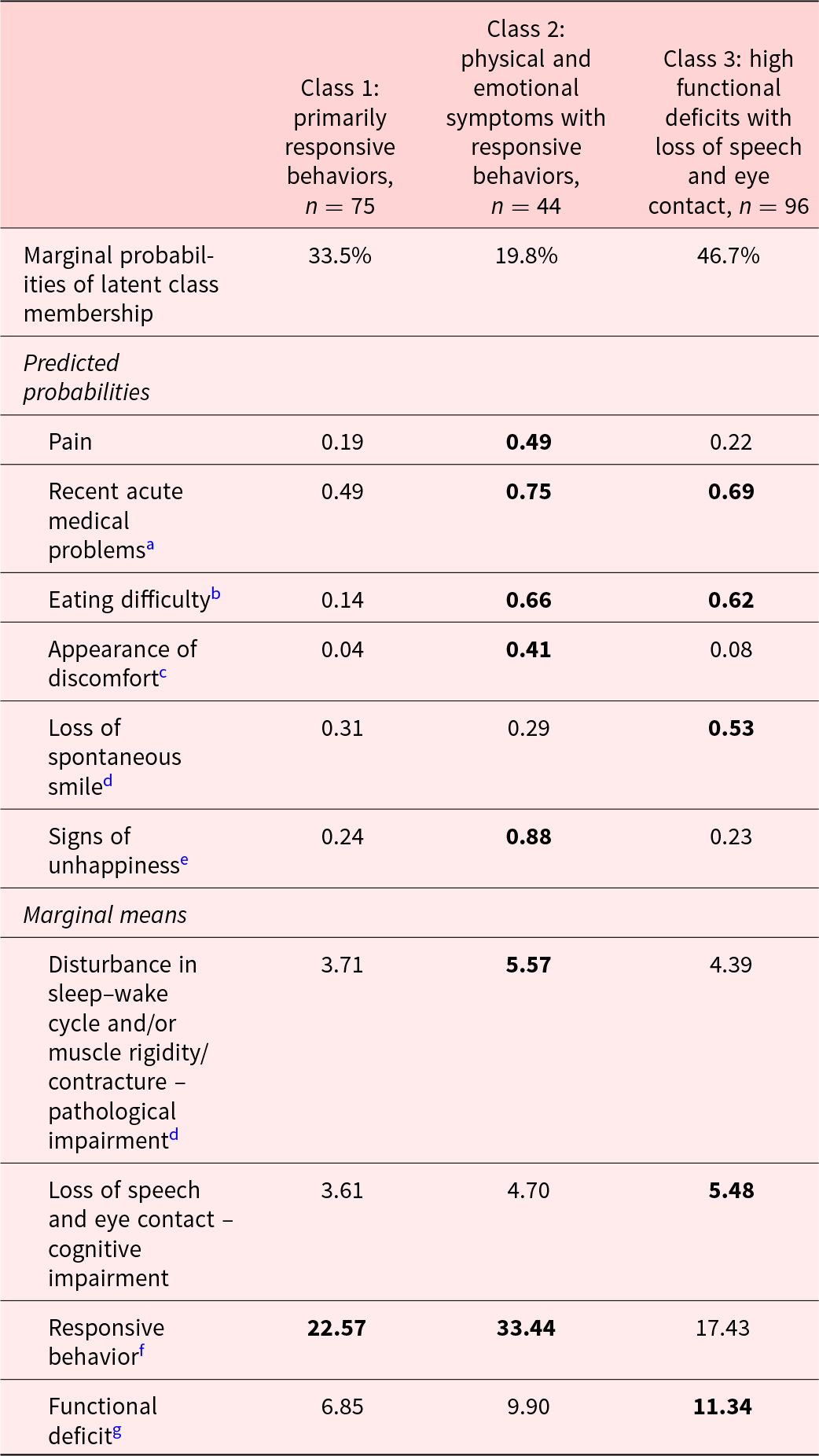
We fitted 4 models for determining the optimal number of latent classes. The model with 4 classes failed to converge; therefore, we selected the 3-class model with high entropy (0.95) (Supplementary Table S1). The 3 latent classes representing 3 distinct symptom profiles were as follows: Class 1 – primarily responsive behaviors (33%); Class 2 – physical and emotional symptoms with responsive behaviors (20%); Class 3 – high functional deficits with loss of speech and eye contact (47%). The average posterior probability of being in each class was >0.8, supporting that all 3 classes were well defined and distinct from each other. The latent classes did not vary by older adults’ age, gender, and number of comorbidities.
Relationship of delineated profiles with FAST stage and use of potentially burdensome interventions (Table 2; Figures 1 and 2)
Older adults with “primarily responsive behaviors” had a high score for responsive behaviors. The largest proportion (36%) of older adults with severe dementia in this profile were in FAST stage 6C.
Table 2. Predicted probabilities and marginal means of indicators within each of the 3 delineated symptom profiles among older adults with severe dementia, N = 215
Notes:
a Includes pneumonia, urinary tract infection, fever, stroke, hip fracture, or any other medical problem in the past 4 months.
b Includes refusal to eat, difficulty or discomfort with swallowing, loss of appetite or need of feeding tube, or weight loss, sunken eyes or cheeks.
c Appears physically uncomfortable – squirms, writhes, or frequently changing position nearly half the day or most of the day.
d Smile in response to external stimuli less than once a day or rarely at all.
e Appears sad, makes statements or sounds suggesting discontent, unhappiness, or discomfort, and/or cries.
f Based on 14 items of Cohen-Mansfield Agitation Inventory (CMAI).
g Dressing, eating, and walking impairment.
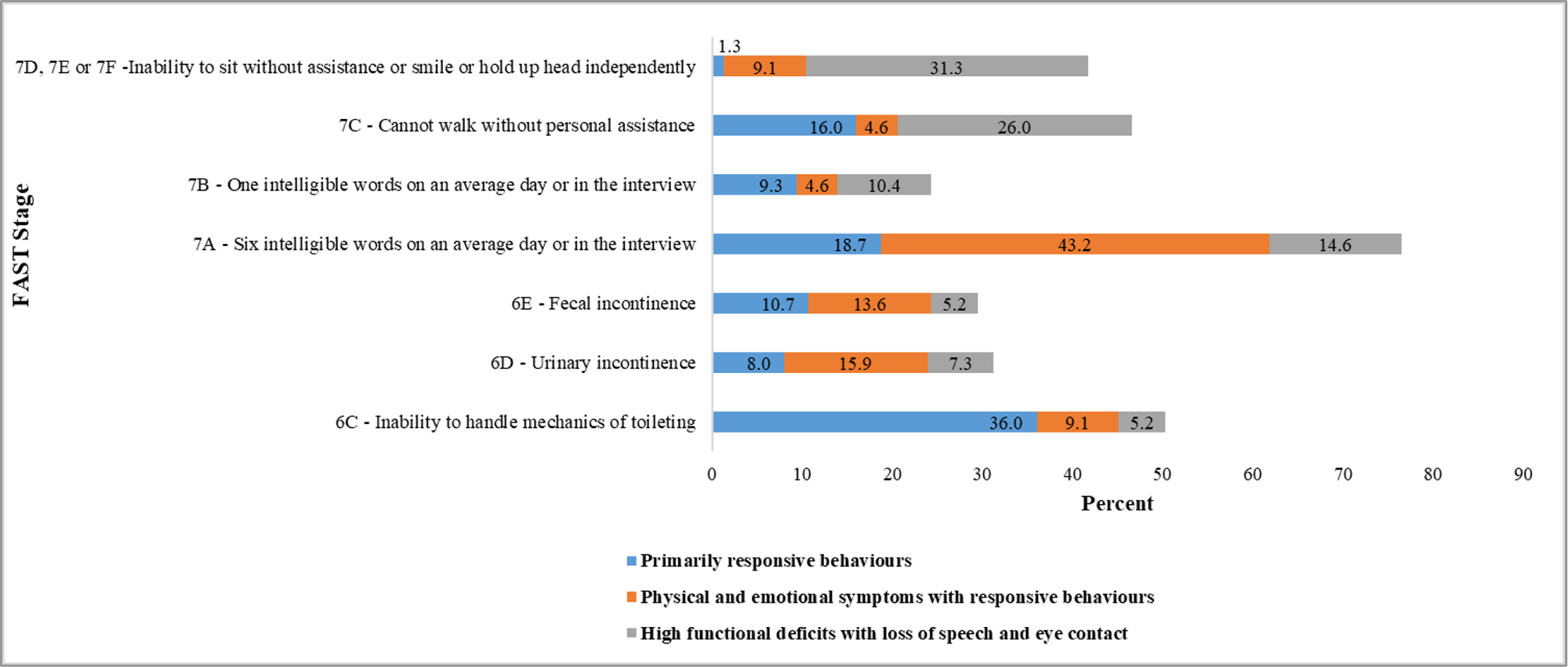
Fig. 1. Functional Assessment Staging Test (FAST) classification by symptom profiles.

Fig. 2. Potentially burdensome interventions by symptom profiles.
Older adults with “physical and emotional symptoms with responsive behaviors” had the highest level of pain, acute medical problems in past 4 months, eating problems, signs of physical discomfort, and pathological impairment, all of which indicated high levels of physical symptoms. They also showed signs of unhappiness (indicating emotional symptoms) and responsive behaviors. The largest proportion (43%) of older adults with severe dementia in this profile were in FAST 7A.
Older adults with “high functional deficits with loss of speech and eye contact” had the highest level of functional deficits. Despite having high levels of acute medical problems in past 4 months, eating problems, loss of smile, and highest cognitive impairment score, these older adults showed low levels of physical discomfort signs, pain, and unhappiness, suggesting their loss of speech and eye contact as their physical and emotional symptoms. The largest proportion of older adults with severe dementia in this profile belonged to FAST stages 7B–7F (68%).
More than 68% of the older adults with “physical and emotional symptoms with responsive behaviors” and “high functional deficits with loss of speech and eye contact” had received a potentially burdensome intervention to manage symptoms in the past 4 months compared to 53% of older adults with “primarily responsive behaviors.” More than 40% of older adults in “physical and emotional symptoms with responsive behaviors” and “high functional deficits with loss of speech and eye contact” (vs. 28% with “primarily responsive behaviors”) had received a potentially burdensome intervention (not including a physical restraint) (Figure 2).
Variation in caregiver characteristics between delineated profiles
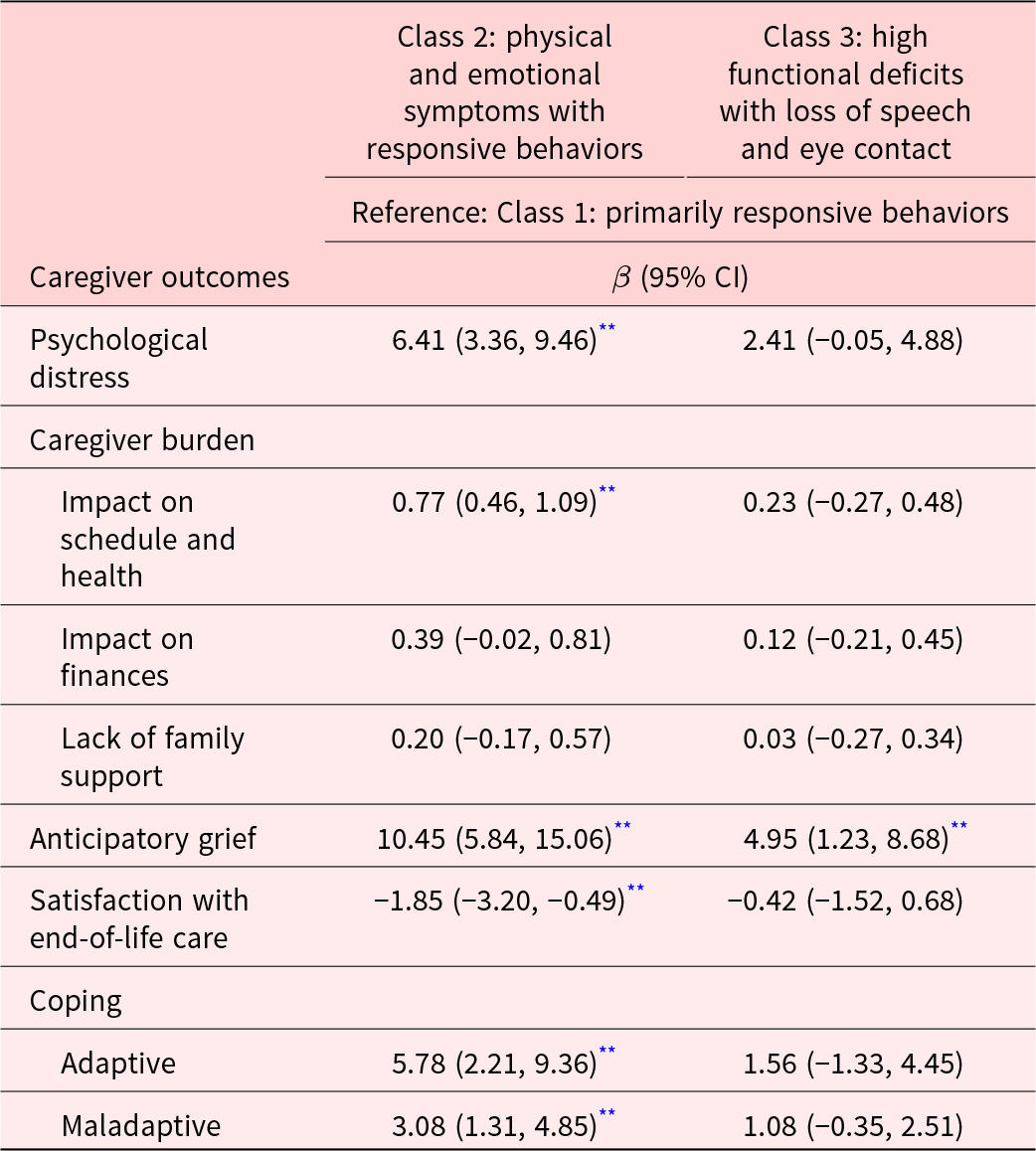
Compared to caregivers of older adults with “primarily responsive behaviors,” caregivers of older adults with “physical and emotional symptoms with responsive behaviors” reported significantly higher psychological distress, greater impact of caregiving on their schedule and health, higher use of adaptive and maladaptive coping, and lower satisfaction with care (Table 3). Caregivers of older adults with “physical and emotional symptoms with responsive behaviors” and “high functional deficits with loss of speech and eye contact” had higher anticipatory grief compared to those caring for older adults with “primarily responsive behaviors.”
Table 3. Association of caregiver outcomes with symptom profiles among older adults with severe dementia, N = 215
** p-value <0.01;
Relationship between delineated profiles and mortality
Median survival was 5.5 months for older adults with “primarily responsive behaviors,” 6.3 months for older adults with “physical and emotional symptoms with responsive behaviors,” and 4.9 months for older adults with “high functional deficits with loss of speech and eye contact” (log-rank p = 0.02). After controlling for potential confounders, the latter 2 profiles had a higher hazard for 1-year mortality (hazard ratio (95% confidence interval [CI]): Class 2: 3.62 (1.37, 9.54) and Class 3: 3.22 (1.37, 7.56)).
Discussion
To our knowledge, this is the first study to empirically characterize symptom profiles of older adults with severe dementia. We delineated three symptom profiles of older adults.
While older adults with “primarily responsive behaviors” mostly experienced only responsive behaviors, those with “physical and emotional symptoms with responsive behaviors” additionally had other multiple physical symptoms. Caregivers of older adults with “physical and emotional symptoms with responsive behaviors” experienced the highest distress, burden, and coping behaviors, which may be a result of high functional caregiving needs and responsive behaviors among older adults. Previous studies also support that caregivers of older adults with responsive behaviors experience very high caregiving burden and distress (Huang et al. Reference Huang, Lee and Liao2012; Matsumoto et al. Reference Matsumoto, Ikeda and Fukuhara2007), which may have also triggered the use of both adaptive and maladaptive coping (Huang et al. Reference Huang, Lee and Liao2012; Matsumoto et al. Reference Matsumoto, Ikeda and Fukuhara2007; Yuan et al. Reference Yuan, Wang and Tan2021). All of this suggests that these caregivers require high levels of support from health and social care systems for both older adults and themselves. Yet, this support was likely inadequate as shown by caregivers’ low levels of satisfaction with older adults’ care.
The profile with “high functional deficits with loss of speech and eye contact” had the highest number of older adults with the most advanced stage of dementia and included those with the greatest level of functional incapacity. Although responsive behaviors were less common, these older adults experienced loss of speech, eye contact, and smile – limiting their verbal and nonverbal communication with the caregivers. As a result, these older adults may have been less able to communicate their physical discomfort, pain, and emotions to their caregivers, thus explaining low probability of caregivers reporting symptoms such as pain and signs of physical discomfort and unhappiness. This is consistent with observations that in very severe stages of dementia, the ability to effectively communicate discomforts and physical needs is reduced (Schmidt et al. Reference Schmidt, Eisenmann and Golla2018). Burden and distress for caregivers of these older adults were not significantly greater than caregivers of older adults with “primarily responsive behaviors,” likely due to the low prevalence of responsive behaviors among older adults and the inability of caregivers to discern older adults’ symptoms.
Not surprisingly, we found that older adults with “physical and emotional symptoms with responsive behaviors” and “high functional deficits with loss of speech and eye contact” had a higher likelihood of dying within 1 year compared to those with “primarily responsive behaviors.” This is consistent with studies showing that older adults with greater functional incapacity have the highest risk of mortality (Connors et al. Reference Connors, Ames and Boundy2016; Mitchell et al. Reference Mitchell, Miller and Teno2010). In many countries, older adults with FAST 7C and above staging are eligible for hospice admission and are cared for using a palliative approach (Mitchell et al. Reference Mitchell, Miller and Teno2010). Despite this, more than two-thirds of the older adults with these 2 profiles had received a potentially burdensome intervention in the past 4 months to manage their symptoms. Many of these interventions were likely to have been administered to manage older adults’ acute medical problems, such as infections and eating difficulties (Lee et al. Reference Lee, Hsu and Liang2021; Morrison and Siu Reference Morrison and Siu2000). Decisions for the management of acute medical problems and eating difficulties among older adults pose an ethical dilemma for caregivers (Zain et al. Reference Zain, Mohamad and Seow2020). Although the literature and guidelines suggest that these potentially burdensome interventions to manage symptoms increase discomfort for older adults while only marginally increasing their length of life (Givens et al. Reference Givens, Jones and Shaffer2010), our previous work has found that many caregivers prefer to use them even when their overall goal of care for older adults is provision of comfort (Malhotra et al. Reference Malhotra, Mohamad and Østbye2021b). Caregiver–provider discussions related to older adults’ goals of care can enable health-care providers to clarify the pros and cons of using these interventions, thus enabling provision of care consistent with older adults’ and caregivers’ goals and effectively managing older adults’ symptoms.
Our findings have clinical implications regarding managing older adults’ care holistically. The 3 delineated profiles only had some association with FAST stages and provided additional information to health-care providers regarding older adults’ symptoms not available through screening based on FAST staging. For instance, for older adults with “physical and emotional symptoms with responsive behaviors,” rather than addressing only specific acute medical problems or eating difficulties, health-care providers can optimize care plans using a holistic dyad-centered approach. This would include a detailed assessment and management of older adults’ symptoms and caregivers’ burden, distress, anticipatory grief, and coping. Caregivers of older adults with “high functional deficits with loss of speech and eye contact” can be taught to recognize and interpret nonverbal and physical cues to effectively manage older adults’ symptoms.
Recognizing that older adults with multiple symptoms have poor prognosis, communication between providers and informal caregivers can help to establish goals of care for older adults to guide treatment and care decisions. Caregivers can also be counseled on benefits and discomforts associated with the use of potentially burdensome health-care interventions to manage symptoms and provided alternative ways to manage older adults’ condition.
Our study had several strengths. This is the first study examining symptom profiles among older adults with severe dementia. We validated the delineated symptom profiles using a range of older adults and caregiver characteristics, supporting their robustness.
Nonetheless, our study has its limitations. First, the delineated profiles depend on the indicators identifying multiple symptoms among older adults with severe dementia. Second, the selected indicators were based on caregivers’ reports of older adults’ condition and may have varied by their reporting behavior (caregivers may over- or underestimate patients’ symptoms) and relationship with the older adult. However, we did not find any difference between the symptom profiles based on caregiver–older adults relationship (spousal versus adult child caregivers), thus strengthening our findings. Finally, given the cross-sectional nature of the analysis, we cannot assign a causal direction to the association between the symptom profiles and caregiver outcomes.
Conclusion
We delineated 3 distinct symptom profiles of older adults – “primarily responsive behaviors,” “physical and emotional symptoms with responsive behaviors,” and “high functional deficits with loss of speech and eye contact.” These profiles were associated with older adults’ use of potentially burdensome interventions and prognosis and with adverse caregiver outcomes. The delineated symptom profiles will be used to plan or optimize care plans for older adults and their caregivers.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1478951523000068.
Conflicts of interest
The authors declare that there is no conflict of interest








