CVD is the leading cause of death in men in many Western nations including the USA( Reference Kochanek, Xu and Murphy 1 ) and the UK( 2 ). In Australia, it accounts for one-third of all deaths and is now the leading contributor to direct health care expenditure( 3 , 4 ). Although notable improvements in survival rates have been observed over recent decades, the number of Australian men who are expected to die from repeat heart attacks is projected to increase by >40 % by 2020( 5 ). These figures highlight the limitations of current primary and secondary prevention strategies. As 80 % of the CVD-related burden is attributable to modifiable risk factors( 6 ), there is great potential for health promotion and economic savings through the evaluation of alternative prevention strategies.
Modern-day, obesogenic environments have played a crucial role in propelling the CVD epidemic. Specifically, poor diet quality has been strongly and repeatedly linked to mortality( 7 ). Indeed, dietary intake has long been recognised as a potent contributor to the pathogenesis of CVD and has received substantial attention( Reference Keys, Menotti and Karvonen 8 – Reference Dauchet, Amouyel and Hercberg 10 ). Although most of the studies and public health focus regarding CHD have been on its relationship with the intake of individual macronutrients, particularly SFA, new data have highlighted the deleterious effects of inflammation-inducing foods that are often used to replace SFA in Western diets (e.g. highly processed carbohydrates, sugar, trans fats, etc.)( Reference Wallace and Mozaffarian 11 , Reference Jakobsen, Dethlefsen and Joensen 12 ). Accumulating data suggest that CVD is a set of inflammatory disorders, with key processes now thought to lie with the oxidation of LDL-cholesterol and phospholipids. The consequent autoimmune responses are directed against newly formed epitopes, especially oxidised LDL-cholesterol and other oxidised phospholipids( Reference Maes, Ruckoanich and Chang 13 ).
To date, cardiovascular epidemiology has primarily examined specific nutrients or foods, population means or nutritional guidelines to assess dietary composition within CVD populations, thus neglecting both the potential of dietary-related inflammation and its relation to measures of inflammatory and oxidative stress. It is recognised that a shift away from assessing individual macronutrients or micronutrients towards an investigation of overall diet quality in the pathogenesis of disease is required( Reference Stanton 14 , Reference Jacobs, Tapsell and Temple 15 ). The utility of novel and innovative methodologies has, thus, been called for in assessing dietary composition as a determinant of disease( Reference Collins and Lanza 16 ). One such new methodology focuses on the components of diet that are empirically related to markers of immune processes and systemic inflammation in humans. ‘Diet-related inflammation’ refers to the inflammatory potential of the diet; this is a concept that has been recently utilised in other disciplines such as nutritional psychiatry( Reference Lucas, Chocano-Bedoya and Schulze 17 ). A pro-inflammatory diet has been linked to a range of somatic conditions and, most recently, indices of obesity( Reference Ruiz-Canela, Zazpe and Shivappa 18 ). We, therefore, aimed to examine the extent to which diet-related inflammation is a risk factor for 5-year cardiovascular incidence in males, using a dietary inflammatory index (DII). We further aimed to compare its predictive ability with that of SFA intake alone.
Methods
Study sample
Details of the Geelong Osteoporosis Study (GOS) have been published elsewhere( Reference Pasco, Nicholson and Kotowicz 19 ). In brief, GOS comprises an age-stratified, population-based sample of women and men, randomly selected from electoral rolls of the Barwon Statistical Division in south-eastern Australia. As voting is compulsory in Australia for adults aged ≥18 years, this sampling technique provides a random sample of citizens registered with the Australian Electoral Commission. Population characteristics of this Division are comparable with national levels. Individuals randomly selected from the electoral roll were mailed an invitation letter, with a request to contact the research centre. Those residing in the area for <6 months or those unable to provide informed consent were excluded. In 2001–2006, 3273 men (aged 20–97 years) were invited to participate in the GOS. Of these, 167 had died, 311 had left the region, 482 were unable to be contacted and seventeen were unable to provide informed consent. Of the 2296 remaining individuals, 756 declined to participate (most common reasons: personal 44 %, time constraints 26 %), leaving 1540 male participants at baseline (participation rate 67 %). Fig. 1 displays the recruitment flow chart, representing those included in this study.

Fig. 1 Recruitment flow chart of Geelong Osteoporosis Study.
Ethics
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects/patients were approved by the Barwon Health Human Research Ethics Committee. Written informed consent was obtained from all the subjects.
Procedure
Although the GOS study comprises ongoing, regular health assessments at the study centre based at Barwon Health (University Hospital Geelong), this study utilised nutrition, anthropometric, demographic and other (non-CVD) health data from GOS baseline assessments. Trained research assistants collected these data during face-to-face interviews with clinical assessments of the participants. Following an overnight fast, blood samples were collected from the participants at the time of baseline assessment at a local pathology laboratory and were stored until batch analysis. In 2011, CVD events data were extracted retrospectively from hospital medical records.
Study measurements
Outcomes
The primary outcome was the occurrence of a CVD event that resulted in hospital presentation over the 5-year follow-up period (post-baseline assessment), with a formal diagnosis of the following: cardiac death, non-fatal myocardial infarction (MI; ‘heart attack’) based on troponin levels and electrocardiogram readings (ST segment elevation MI or non-ST-segment elevation myocardial infarction (STEMI)), coronary intervention or percutaneous coronary intervention, coronary artery bypass grafting, amputation or transient ischaemic attack, ischaemic or haemorrhagic stroke. Data were extracted from catchment area hospital medical records held at Barwon Health by medically trained research fellows. This procedure was likely to capture the majority of CVD events in the region due to three key reasons: (i) Barwon Health was the sole national health service emergency facility in the regional catchment area at the time, (ii) admission was accessible to the general public with no out-of-pocket expense and (iii) only a small percentage of participants moved away from the Barwon region during follow-up. Participants gave consent for their hospital admission records to be accessed, allowing CVD events data to be recorded for all the participants, regardless of retention status at subsequent follow-up assessments.
Exposure: dietary inflammatory index
Details of the development of the DII have been documented elsewhere( Reference Shivappa, Steck and Hurley 20 ), including its validation against general inflammatory biomarkers( Reference Hodge, English and Itsiopoulos 21 ). Data were derived from Victorian Cancer Council FFQ completed by the participants at baseline used to assess participants’ usual consumption of seventy-four foods and six alcoholic beverages over the previous year on a ten-point frequency scale. Raw data provided detailed information about the consumption of common foods (fruit, vegetables, dairy products, meat, fish, pasta, rice, breads, nuts, legumes, breakfast foods, snack foods and alcohol). The FFQ provided estimated intakes of total carbohydrates and specific macronutrients (e.g. SFA, MUFA, PUFA, proteins, carbohydrates, sugars, starch, dextrins, fibre, alcohol and fatty acids), a range of micronutrients and glycaemic load.
DII scores were computed from these FFQ data by a trained research fellow. Using a regionally representative world database (food consumption from eleven populations globally), means and standard deviations were calculated for each parameter. The ‘standard mean’ was subtracted from the actual exposure and divided by its standard deviation. These Z scores were converted to percentiles (minimising effects of outliers/right-skewing)( Reference Shivappa, Steck and Hurley 20 ) and centred by doubling the value and subtracting 1 to achieve symmetrical distribution of the scores. The product for each food parameter and the adjusted article score was calculated and summed across the food parameters to generate an overall DII score for each participant( Reference Shivappa, Steck and Hurley 20 ), where higher values indicate a more pro-inflammatory diet. The steps to calculate DII are shown in Fig. 2. Overall, twenty-two of the forty-five possible food parameters used for DII calculation were available in this study, and those food parameters were carbohydrates, proteins, total fat, energy, fibre, cholesterol, SFA, MUFA, PUFA, n-3, n-6, niacin, thiamine riboflavin, Fe, Mn, Zn, vitamin A, vitamin E, folic acid and β-carotene.

Fig. 2 Sequence of steps in creating the dietary inflammatory index (DII) in the Geelong Osteoporosis Study (GOS). CRP, C-reactive protein.
Covariates
Data on family history of CVD, age, waist circumference, smoking status, frequency of alcohol use, mobility levels, systolic and diastolic blood pressures (BP), energy intake and diabetes status were collected at baseline assessment. Body weight (sd 0·1 kg) was measured using electronic scales, and height (sd 0·1 cm) was measured using a wall-mounted stadiometer (Harpenden). Waist circumference was measured with a tape measure around the smallest circumference between the lower rib and iliac crest, and BP (seated) was measured using a digital meter. Data on smoking status, alcohol use and exposure to drugs were obtained through self-report. Daily energy intake (kJ/d) was derived from the FFQ. Blood samples were stored in serum aliquots at −80°C from which blood glucose levels were calculated using standard laboratory procedures. For the purpose of this study, diabetes diagnosis at baseline was based on at least one of the following: a blood glucose reading of ≥7·0 mmol/l, use of anti-hyperglycaemic medication or self-report at baseline assessment. SFA consumption (mg/d) was computed from the dietary data by means of the nutrient composition tables in the NUTTAB95 database (Food Standards Australia New Zealand, Canberra, 1995).
Data analysis
Descriptive statistics were used to identify the key characteristics of the sample. As our a priori hypothesis was that a pro-inflammatory diet would be associated with greater odds of a CHD event when compared with (what has been shown to be a cardio-protective diet) an anti-inflammatory dietary intake, DII scores were dichotomised such that positive values represented more pro-inflammatory diets and negative scores reflected more anti-inflammatory diets. Using methods described by Hosmer & Lemeshow( Reference Hosmer and Lemeshow 22 ), logistic regression was performed to examine the predictive effect of DII grouping at baseline (where anti-inflammatory dietary intake was treated as the referent group) on the primary outcome of the occurrence of a 5-year CVD event.
Logistic regression was used, as the outcome of interest was not common in our study population (<10 %); seventy-six events were recorded out of 1363 participants. Further, as all the participants were followed-up to the outcome of interest (CVD admission data were available from 1993), we explored the time to event, ignoring censoring.
Participants with an admission for a CVD event (as defined by the primary outcome) at any time before study enrolment were identified and subsequently excluded (n 176) from the analyses in order to determine the directionality of the relationship of interest, leaving a sample size of 1363 for inclusion in the statistical models. Missing data for each covariate are displayed in Fig. 1. We were particularly interested in the magnitude of effect of SFA on CVD incidence, relative to the DII, given that it has been considered a putative risk factor for atherosclerotic progression. As this macronutrient was a constituent of the DII, we estimated its effect in an independent model, in order to prevent problems with inter-collinearity and to enhance model stability. Logistic regression was also used to examine the relationship of SFA intake with CVD incidence by applying the same technique, but substituting the independent variable, DII score, for recommended daily SFA intake( 23 ) (<25 g/d).
We first conducted a univariate analysis to examine the unadjusted association between the independent and dependent variables. The second iteration of the model adjusted for the traditional CVD risk factors of age, diabetes, systolic and diastolic BP, smoking history, activity level (sedentary v. active) and waist circumference, as well as total daily energy consumption. Alcohol intake was excluded from the statistical models as it forms a component of the DII. A sensitivity analysis was performed excluding those men who indicated that they were using hypocholesteraemic agents at baseline. OR and accompanying 95 % CI were presented for each model. Cohen’s d effect sizes were reported for each model, calculated from the R 2 values generated. As a secondary outcome, a survival analysis was conducted to explore the effect of pro- and anti-inflammatory dietary patterns on time to first CVD event over the study period (years), presented as a Kaplan–Meier plot. All the analyses were performed using Stata® version 13.
Results
Characteristics of sample
Key characteristics of the sample are shown in Table 1. Compared with those recording a more anti-inflammatory diet, participants with a more pro-inflammatory dietary intake were older and had high BP. A larger proportion of participants in this group was sedentary, smokers, used aspirin or statins, had diabetes and had a family history of CVD. With respect to nutritional profile, this group had lower daily macronutrient intake (overall, SFA, PUFA and MUFA, protein and carbohydrates), lower intake of all micronutrients and a higher glycaemic index (Table 1). In total, 131 men were using anti-inflammatory drugs at baseline. The overall range of DII scores for the sample was −2·96 to 2·63. Mean baseline DII scores for the two groups were 1·00 and −1·00, respectively. In total, seventy-six events were observed during the 5-year follow-up period.
Table 1 Key characteristics of the sample by baseline dietary inflammation status (Mean values and standard deviations; percentages and 95 % confidence intervals; n 1363)
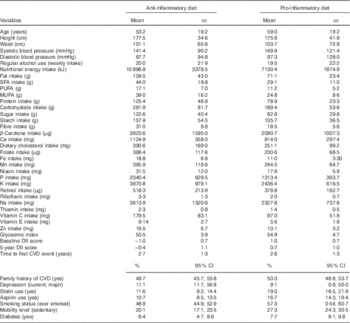
DII, dietary inflammatory index.
Impact of baseline dietary inflammatory index scores on the presence of 5-year CVD events
Univariate analyses revealed that men recording a pro-inflammatory diet were twice as likely to experience a CVD event over the 5-year observation period when compared with those consuming an anti-inflammatory diet (Table 2). Importantly, this association held after adjustment for traditional CVD risk factors and total energy intake (effect size d=0·20). As older age exerted a strong effect within the main effects model, we subsequently explored whether age was an effect modifier using an age-by-baseline DII score interaction variable. Although its inclusion strengthened the relationship between DII and CVD occurrence, the interaction effect was not ‘statistically significant’ (data not shown).
Table 2 Baseline dietary inflammatory index (DII) category (pro-inflammatory v. anti-inflammatory) as a predictor of 5-year cardiovascular event (presence/absence) (Odds ratios and 95 % confidence intervals)
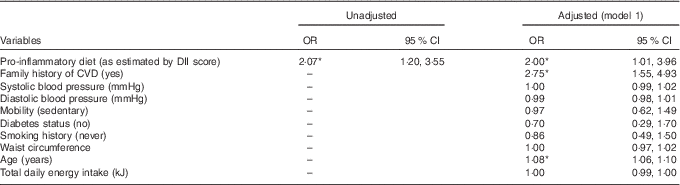
* P<0·05 level.
Finally, we adjusted for usage of medications with anti-inflammatory properties (e.g. aspirins, statins). The inclusion of this variable into the final model did somewhat attenuate the association between a pro-inflammatory diet and CHD (OR 1·95; 95 % CI 0·98, 3·87).
Relationship between baseline daily SFA intake and presence of 5-year CVD events
As a post hoc analysis, we were interested in the magnitude of the relationship between a pro-inflammatory diet and CVD events as it compared with that of SFA intake. Univariate analyses revealed that high SFA intake significantly predicted the occurrence of 5-year CVD events (OR 1·87; 95 % CI 1·12, 3·10); however, this relationship was no longer significant after the inclusion of traditional CVD risk factors (diabetes and lipid markers were excluded to avoid over-fitting the model) (adjusted OR 1·74; 95 % CI 0·92, 3·26). There were no significant interactions between DII scores and SFA scores.
Impact of dietary inflammatory index scores on time to first CVD event
The association between DII group and time to first CVD event was also investigated. Using a likelihood ratio test, the model met the proportional hazards assumption. The seventy-six observations comprised 204·5 years total analysis time at risk and under observation. Kaplan–Meier curves are displayed for the overall sample for the study period (Fig. 3a) and for the events occurring during the first 3 years after enrolment (Fig. 3b, in which it appeared that the inter-group differences were most pronounced).

Fig. 3 (a) Time to first event (years) by baseline (BL) dietary inflammatory index (DII) grouping. (b) Time to first event (0–3 years) by baseline DII grouping. ![]() , Pro-inflammatory diet;
, Pro-inflammatory diet; ![]() , anti-inflammatory diet.
, anti-inflammatory diet.
Discussion
In a longitudinal, nationally representative sample of Australian men, a pro-inflammatory diet (as estimated by the DII) was a significant predictor of CVD occurrence over 5 years. When compared with those consuming an anti-inflammatory diet, this dietary pattern more than doubled the likelihood of a CVD event that resulted in hospitalisation, even after adjustment for traditional CVD risk factors. The use of anti-inflammatory medication, however, did attenuate the observed relationships. The time to first CVD event did not significantly differ by dietary intake over the 5-year study period. When we examined the specific relationship of daily SFA consumption to 5-year CVD incidence, a non-statistically significant association was observed.
To date, evidence that a diet primarily characterised by its pro-inflammatory properties is a potential risk factor for CVD has been lacking. Although other studies have suggested that dietary patterns of inflammatory nature may be linked to heart disease( Reference Meyer, Döring and Herder 24 ), to our knowledge, this is the first study to investigate and, moreover, demonstrate the ability of the DII to predict CVD events. Our findings are intuitively consistent with results of epidemiological studies highlighting the benefits of an anti-inflammatory diet. The Mediterranean diet score, for example, has been shown to predict CVD mortality in Australian men with diabetes in whom a 6 % reduction in risk per unit of Mediterranean diet score (adjusted hazard ratio (HR) 0·94; 95 % CI 0·89, 0·99) was observed( Reference Hodge, English and Itsiopoulos 21 ). Similar effects have been observed under real-world, randomised conditions. PREDIMED investigators demonstrated that a Mediterranean diet supplemented with extra-virgin olive oil (adjusted HR 0·70; 95 % CI 0·54, 0·92) or nuts (adjusted HR 0·72; 95 % CI 0·54, 0·96), when compared with a low-fat diet, led to a reduction in the incidence of major cardiovascular events in those with high CVD risk( Reference Estruch, Ros and Salas-Salvadó 25 ). In that study, the DII was associated with higher average BMI, waist circumference and waist:height ratio after adjusting for known risk factors and remained associated with obesity after controlling for the effect that adherence to Mediterranean diet score had on inflammation( Reference Ruiz-Canela, Zazpe and Shivappa 18 ).
Our secondary finding suggests that the combined effect of inflammatory dietary constituents on the occurrence of CVD could be more potent than the effects of SFA alone. Traditionally, the impact of pro-inflammatory dietary patterns on disease onset has been somewhat obscured by the attention paid to putative, individual dietary risk factors such as SFA. Esposito & Giugliano( Reference Esposito and Giugliano 26 ) and others have provided evidence of the pro-inflammatory milieu as a mechanism through which unhealthy diets may induce cardio-metabolic diseases. They recommend that fully explicating the link between diet and inflammation could ‘elucidate the mechanisms by which dietary patterns improve cardiovascular health’. This study could not elucidate the mechanisms underpinning the relationship between diet, inflammation and CVD and, importantly, did not have the required lipid profile data. However, it provides preliminary evidence to support a movement towards viewing dietary intake in terms of totality of effect and not just as a source of specific culprit nutrients. Although SFA intake is part of the DII, which was associated with CVD outcomes, in isolation SFA was not strongly associated with these outcomes and, by inference, the atherosclerotic state.
Data from animal models, population-based studies and randomised trials provide some support for the key role of dietary intake on systemic inflammation. Epidemiological evidence has linked general markers of inflammation – including C-reactive protein, IL-6 and TNF – to greater consumption of ‘Western-style’ diets, which are high in sweetened soft drinks, refined grains, red meat, margarine and fish, but low in wine, coffee, olive oil, and green leafy and yellow vegetables( Reference Lopez-Garcia, Schulze and Fung 27 ). A sucrose-rich diet in overweight adults over 10 weeks resulted in significant increases in inflammatory markers (haptoglobin and transferrin) and small increases in C-reactive protein levels( Reference Jenkins, Kendall and Marchie 28 ). There is preliminary evidence of dysfunction of immune system activation induced by diet via the gut microbiota( Reference Moreira, Texeira and Ferreira 29 ).
Overall, the diet of the group consuming a more pro-inflammatory diet contained a range of nutritional deficiencies when compared with those consuming a more anti-inflammatory diet. Despite the latter group appearing to consume higher levels of macronutrients that could be considered inflammatory (e.g. SFA intake, sugar intake), closer examination of the characteristics of the anti-inflammatory group’s diet (at a micronutrient level) suggests that it is much more nutrient dense with phytochemicals and micronutrients commonly found in vegetables and fruits. For example, elevated levels of β-carotene, folate and vitamin C suggest higher fruit and vegetable intake, whereas low glycaemic intake indicates that whole grains are key constituents of their carbohydrate consumption. It is plausible that the nutritionally superior profile of the anti-inflammatory group is contributing to better overall health and well-being.
Strengths and limitations
The key strength of this study was that it is the first to explore the utility of a novel, DII as a key marker of CVD. To date, the DII has been found to be associated with inflammatory cytokines including C-reactive protein and IL-6( Reference Shivappa, Steck and Hurley 30 ), increased odds of asthma and reduced lung function in an Australian population( Reference Wood, Shivappa and Berthon 31 ), shiftwork( Reference Wirth, Burch and Shivappa 32 ), colorectal cancer among women in the Iowa Women’s Health Study( Reference Shivappa, Prizment and Blair 33 ), prostate cancer( Reference Shivappa, Bosetti and Zucchetto 34 ) and pancreatic cancer( Reference Shivappa, Bosetti and Zucchetto 35 ) in case–control studies in Italy. The longitudinal study design, nationally representative sample, use of comprehensive nutritional data (to both generate DII scores and inform statistical modelling), as well as the use of ‘hard’ clinical end points are additional strengths. Our primary finding adds to the burgeoning evidence base that dietary inflammation is associated with other chronic conditions. The major limitation of the study was the absence of lipid profile data for inclusion in our statistical models (for which other key risk factors for CVD were adjusted). Second, the absence of robust physical activity data is a limitation of the study. However, the inclusion of a proxy marker (mobility) in the adjusted model was not a significant predictor of CVD outcome.
A final limitation of the study was the likely lack of power to detect time to event differences over the study period due to the small number of cases. Future studies with adequate power to detect such differences between dietary inflammation groups and time to event should be carried out.
Conclusions
In conclusion, this study could be an important first step in broadening our understanding of the contribution of dietary intake to CVD risk to include dietary-promoted inflammation. Although there are numerous models of dietary interventions targeting CVD prevention (nutrient-based; e.g. Heart Foundation, Mediterranean diet), there is likely to be potential benefits associated with applying an approach to CVD prevention that is based on intricate inflammatory biological pathways from the whole diet. This moves away from a reductionist approach that depends on identifying specific nutrients or foods as risk factors, but rather identifies a cardio-protective diet that is independent of any pre-determined nutrient or cuisine models, population means or nutritional guidelines. Studies are now required to confirm these findings as well as those that can quantify the extent to which reducing dietary inflammation has health and economic benefits in the long term. Following recent calls, other future studies looking at this type of model on long-term CVD-related mortality are required( Reference Hoebeeck, Rietzschel and Langlois 36 ).
Acknowledgements
A. O’N. is a recipient of a Fellowship from the organisation that funded the original GOS study (National Health and Medical Research Council (NHMRC)). This activity exerted no influence on the findings. The authors wish to thank all GOS project staff and participants.
A. O’N. is supported by a Fellowship from the NHMRC (#1052865). J. R. H. was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975). The study was supported by NHMRC (projects 299831, 628582).
A. O’N. and F. N. J. designed the research; J. A. P. and M. A. K. oversaw all the aspects of the GOS study; K. K. collected the CVD data; F. N. J. coordinated the collection of dietary data; J. R. H. developed the DII instrument and DII scores were generated under his supervision by N. S.; and A. O’N. analysed the data, wrote the manuscript and had primary responsibility for its final content. All the authors read and approved the final version of the manuscript.
None of the authors has any conflicts of interest with the NHMRC.








