According to the concept of ‘metabolic programming’, alteration of nutrition at a critical period of development in early life affects the subsequent pattern of growth and development of tissues and organs and may predispose individuals to metabolic disorders in later life(Reference Dauncey1–Reference Widdowson and McCance5). In humans, it is recognised that neonates born with small birth weights exhibit an increased risk of developing these disorders(Reference Hales and Barker2, Reference Godfrey and Barker6–Reference Saenger, Czernichow and Hughes8). Moreover, these babies are often fed formula enriched in protein to promote catch-up growth and brain development. Based on some observational and epidemiological studies, it has been hypothesised that a high protein (HP) intake during early life may further increase the risk of subsequent obesity(Reference Rolland-Cachera, Deheeger and Akrout9, Reference Koletzko, Rigo and Ziegler10). Some studies have tended to show a positive correlation between dietary protein:energy ratio in early life and BMI and subscapular skinfold in childhood(Reference Rolland-Cachera, Deheeger and Akrout9, Reference Scaglioni, Agostoni and Notaris11) and adulthood(Reference Kemper, Post and Twisk12). But other studies have failed to show such a relationship(Reference Dorosty, Emmett and Cowin13, Reference Hoppe, Molgaard and Thomsen14), indicating that available data are not entirely clear-cut. These discrepancies may result from heterogeneity in formula composition (energy-enriched or not, amount of extra protein, etc.), duration of formula feeding and/or inclusion criteria.
The use of animal models reared in well-controlled environments should clarify this issue that is of great interest for nutritionists(Reference Bertram and Hanson15). To date, the impact of an increase in protein intake during the suckling period on subsequent growth and development is poorly documented compared with the impact of protein restriction. Moreover, most of the few available data dealing with early protein intake have been obtained in rodents and are based on the modulation of protein intake of the dam and not of the offspring(Reference Daenzer, Ortmann and Klaus16–Reference Zambrano, Bautista and Deás18). The pig is recognised as an appropriate model for nutritional research(Reference Stahl, Lei and Larson19, Reference Litten-Brown, Corson and Clarke20). This model provides a natural form of intra-uterine growth retardation(Reference Morise, Louveau and Le Huërou-Luron21) and allows artificial rearing that is required to modulate and control food intake during the nursing period. Recently, we have shown that a rise in early protein intake between 7 and 28 d of age reduced adiposity in piglets(Reference Morise, Sève and Macé22). According to the hypothesis raised in humans(Reference Rolland-Cachera, Deheeger and Akrout9, Reference Koletzko, Rigo and Ziegler10), protein intake may induce modifications in regulatory axes and especially in the insulin-like growth factor (IGF) system during infancy and childhood, and these changes may alter subsequent development. Nevertheless, available data on mid- and long-term outcomes are rather limited in the literature. The present study was undertaken to evaluate the adaptation of animals fed such a diet and fed a standard diet thereafter. The phenotype of these animals was examined at 70 d of age through the evaluation of growth, body composition and hormonal status. Finally, we assessed whether the impact of early protein intake may differ between female and male pigs and/or between pigs born with normal or small weight.
Materials and methods
Animals, diets and experimental design
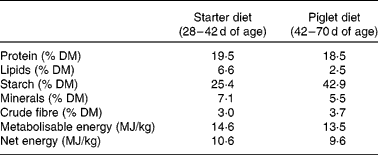
The care and use of animals were performed in compliance with the guidelines of the French Ministry of Agriculture and Fisheries (certificate of authorisation no. 7676 to experiment on living animals). All people involved in the experiment have an agreement for conducting experimental procedures on animals, delivered by the veterinary services. A total of fifty-six cross-bred (Pietrain × (Large White × Landrace)) pigs from sixteen litters were obtained from the experimental herd of INRA (St-Gilles, France). All piglets were weighed at birth. Piglets with a weight close to the average birth weight of the herd (1·40 kg) were identified as normal-birth-weight (NW) piglets, and those with a 30 % lower weight were defined as small-birth-weight (SW) piglets. The range of birth weights in the NW group was 1·28–1·58 kg, and that in the SW group was 0·76–1·12 kg. Piglets were allowed to suckle the dam naturally up to 7 d of age. At this stage, pairs of piglets with similar birth weight and sex were selected within a litter. In theory, we should have selected eight piglets from each litter to equilibrate sex, NW and SW within a litter. However, from a practical point of view, it was impossible. There were from one to three pairs per litter. Within a pair, piglets were randomly assigned to one of the two dietary groups. They were separated from their dam and were placed individually in stainless-steel metabolism cages in a temperature-controlled room (30 ± 0·5°C) up to 28 d of age. They were fed milk replacers formulated to provide an adequate protein (AP) or a HP supply with an automatic formula feeder as described previously(Reference Morise, Sève and Macé22) (Table 1). In brief, the ratio of protein:net energy was 46·5 % higher in the HP powder than in the AP powder. After weaning at 28 d of age, animals from both dietary groups had then free access to standard commercial diets up to slaughter 6 weeks later (Table 2). Body weights were recorded weekly and feed intake was measured daily.
Table 1 Composition of adequate-protein (AP) or high-protein (HP) formulae
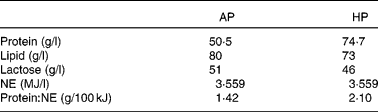
NE, net energy.
Table 2 Composition of standard diets
Sample collection
At 70 d of age, pigs were killed 3 h after the last meal in the experimental slaughterhouse by electrical stunning and exsanguination. Blood samples were collected, and EDTA–plasma samples were stored at − 20°C for later analyses. Half of the carcass (without head and tail) was devoted to chemical composition determination. This part was weighed and stored at − 20°C. In the other half of the carcass, tissue samples were collected within 15 min. The entire semitendinosus muscle and perirenal adipose tissue were collected and weighed. Samples of dorsal subcutaneous adipose tissue and of longissimus muscle were also collected. These samples were cut into small pieces, frozen in liquid N2 and then stored at − 75°C.
Chemical analysis
The frozen half-carcass was minced, sampled and freeze-dried for further chemical analyses. All analyses were then performed in duplicate on freeze-dried carcass samples according to the Association of Official Analytical Chemists methods(23). The residual water content after freeze-drying was determined on an aliquot sample by drying at +105°C to constant weight. All analytical results were expressed on a DM basis. Ash was determined by incineration at +550°C. Nitrogen was determined using the rapid nitrogen cube analyser (Elementar, Haunau, Germany). The total content of crude protein was calculated as total carcass nitrogen × 6·25. Lipid content was determined using the total lipid extraction procedure described by Folch et al. (Reference Folch, Lees and Sloane Stanley24).
Hormone assays
All samples were analysed within a single assay. Plasma IGF-I concentrations were determined using a validated RIA(Reference Louveau and Bonneau25) that used recombinant IGF-I (GroPep, Adelaïde, Australia). The intra-assay CV for a plasma sample with a mean IGF-I concentration of 28 ng/ml was 12 %. Plasma leptin concentrations were measured using the multi-species RIA kit (Linco Research, St Charles, MO, USA) previously validated for use in porcine plasma(Reference Qian, Barb and Compton26). The intra-assay CV for a porcine plasma sample with a mean leptin concentration of 1·82 ng/ml was 5 %.
Real-time RT-PCR
Total RNA was extracted from adipose tissues and skeletal muscle using TRIzol reagent (Invitrogen, Cergy-Pontoise, France). RNA integrity was then checked on an ethidium bromide-stained agarose gel. Treated-DNase total RNA (2 μg) was reverse-transcribed using random hexamer primers. Primers for selected genes (Table 3) were designed using Primer Express software (version 2.0; Applied Biosystems, Courtaboeuf, France). Real-time quantitative PCR analyses were performed in a final volume of 12·5 μl, starting with 5 ng of reverse-transcribed RNA using SYBR® Green I PCR core reagents in an ABI PRISM 7000 Sequence Detection System instrument (Applied Biosystems). Amplification was performed with forty cycles, with each cycle consisting of denaturation at 95°C for 15 s, followed by annealing and extension at 59°C for 1 min. Cycle threshold (C T) values are means of triplicate measurements. Negative controls were included on each ninety-six-well plate. Endogenous 18S ribosomal RNA amplifications were used for each sample to normalise the expression of the selected genes. A complementary DNA sample obtained from a pool of total RNA isolated from adipose tissues or skeletal muscle was used as an inter-plate calibrator for each gene. Since PCR efficiencies for target genes and 18S gene were close to 1, the relative expression of a target gene was calculated as follows(Reference Pfaffl27):
Table 3 Primers used for the analysis of gene expression by real-time RT-PCR
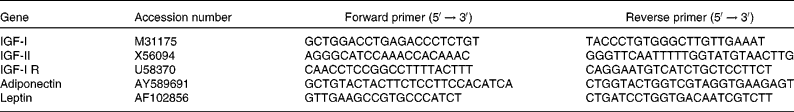
IGF-I and IGF-II, insulin-like growth factor-I and -II; R, receptor.
Statistical analysis
Data were analysed using the General Linear Model procedure of SAS (SAS Institute, Cary, NC, USA). The eight groups of pigs were compared. Dietary group (AP and HP), class of birth weight (small and normal), sex (female and male) and the interactions (dietary treatment × birth weight and dietary treatment × sex) were tested. Pair within birth weight and sex was also included in the model. All data are presented as means with their standard errors. Differences were considered significant at P ≤ 0·05.
Results
Growth and feed intake
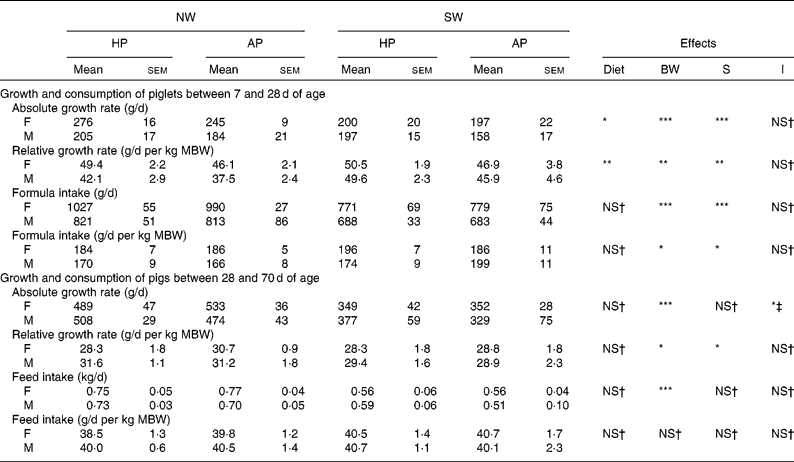
During the formula-feeding period, absolute (weight gain/d) and relative (weight gain/kg mean body weight) growth rates were higher (P < 0·05) in HP piglets than in AP piglets and in female piglets than in male piglets (Tables 4 and 5). With respect to birth weight, the absolute growth rate was higher (P < 0·001) in NW piglets than in SW piglets, while the opposite (P < 0·01) was observed for the relative growth rate. The average formula intake did not differ between the two dietary groups of piglets. The average absolute intake was higher (P < 0·001) in females than in males and in NW piglets than in SW piglets. When expressed per kg mean body weight, formula intake was still higher (P < 0·05) in females than in males but lower (P < 0·05) in NW piglets than in SW piglets.
Table 4 Body weights of female (F) and male (M) pigs fed adequate-protein (AP) or high-protein (HP) formulae between 7 and 28 d of age and fed a standard diet thereafter
(Mean values with their standard errors, n 7)
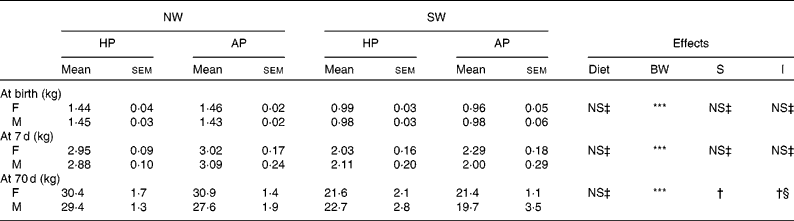
NW, normal birth weight; SW, small birth weight; BW, birth weight; S, sex; I, interactions (diet × S and/or diet × BW).
Mean values were significantly different: **P < 0·1, ***P < 0·001.
† Mean values tended to be significantly different (P < 0·1).
‡ Mean values were not significantly different (P>0·1).
§ Diet × S interaction.
Table 5 Growth and consumption of female (F) and male (M) pigs fed adequate-protein (AP) or high-protein (HP) formulae between 7 and 28 d of age and fed a standard diet thereafter
(Mean values with their standard errors, n 7)
NW, normal birth weight; SW, small birth weight; BW, birth weight; S, sex; I, interactions (diet × S and/or diet × BW); MBW, mean birth weight during the experimental period.
Mean values were significantly different: *P < 0·05, **P < 0·01, ***P < 0·001.
† Mean values were not significantly different (P>0·1).
‡ Diet × S interaction.
During the post-weaning period, there was an interaction (P < 0·05) between diet and sex for absolute growth rate (Table 5). This parameter was similar in AP and HP female pigs but was higher (P < 0·05) in male pigs receiving the HP diet than those receiving the AP diet. Moreover, absolute and relative growth rates were higher (P < 0·05) in NW pigs than in SW pigs.
Even though pigs were fed ad libitum, feed intake did not differ between AP and HP pigs. Feed intake was higher (P < 0·001) in NW pigs than in SW pigs, but when expressed per kg mean body weight, there was no significant difference between the birth-weight groups. Moreover, no difference was detected between the sexes.
Body composition and tissue features
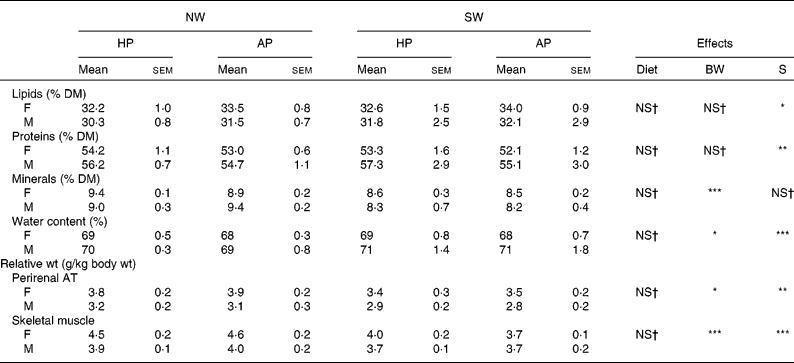
The lipid and the protein contents of the carcass were not affected by diet or birth weight but differed (P < 0·05) between female and male pigs (Table 6). Whereas the lipid content was higher in females (P < 0·05) than in males, the protein content was lower (P < 0·01) in females than in males. Mineral content was not influenced by diet or sex but was higher (P < 0·001) in NW pigs than in SW pigs. The relative weight of perirenal adipose tissue did not differ between AP- and HP-fed pigs, but it was higher (P < 0·05) in NW pigs than in SW pigs and in female pigs than in male pigs. Similarly, the relative weight of the semitendinosus muscle was not affected by the diet but was higher (P < 0·001) in NW pigs than in SW pigs and in female pigs than in male pigs.
Table 6 Body composition and tissue features of female (F) and male (M) pigs fed adequate-protein (AP) or high-protein (HP) formulae between 7 and 28 d of age and fed a standard diet thereafter
(Mean values with their standard errors, n 7)
NW, normal birth weight; SW, small birth weight; BW, birth weight; S, sex; AT, adipose tissue.
Mean values were significantly different: *P < 0·05, **P < 0·01, ***P < 0·001.
† Mean values were not significantly different (P>0·1).
Plasma hormones
Plasma IGF-I concentrations did not differ between the dietary and birth-weight groups but were lower (P < 0·05) in female pigs than in male pigs (Fig. 1). Plasma leptin concentrations were affected by diet and birth weight (Fig. 1). They were higher (P < 0·05) in HP pigs than in AP pigs. These concentrations were lower (P < 0·05) in NW pigs than in SW pigs.
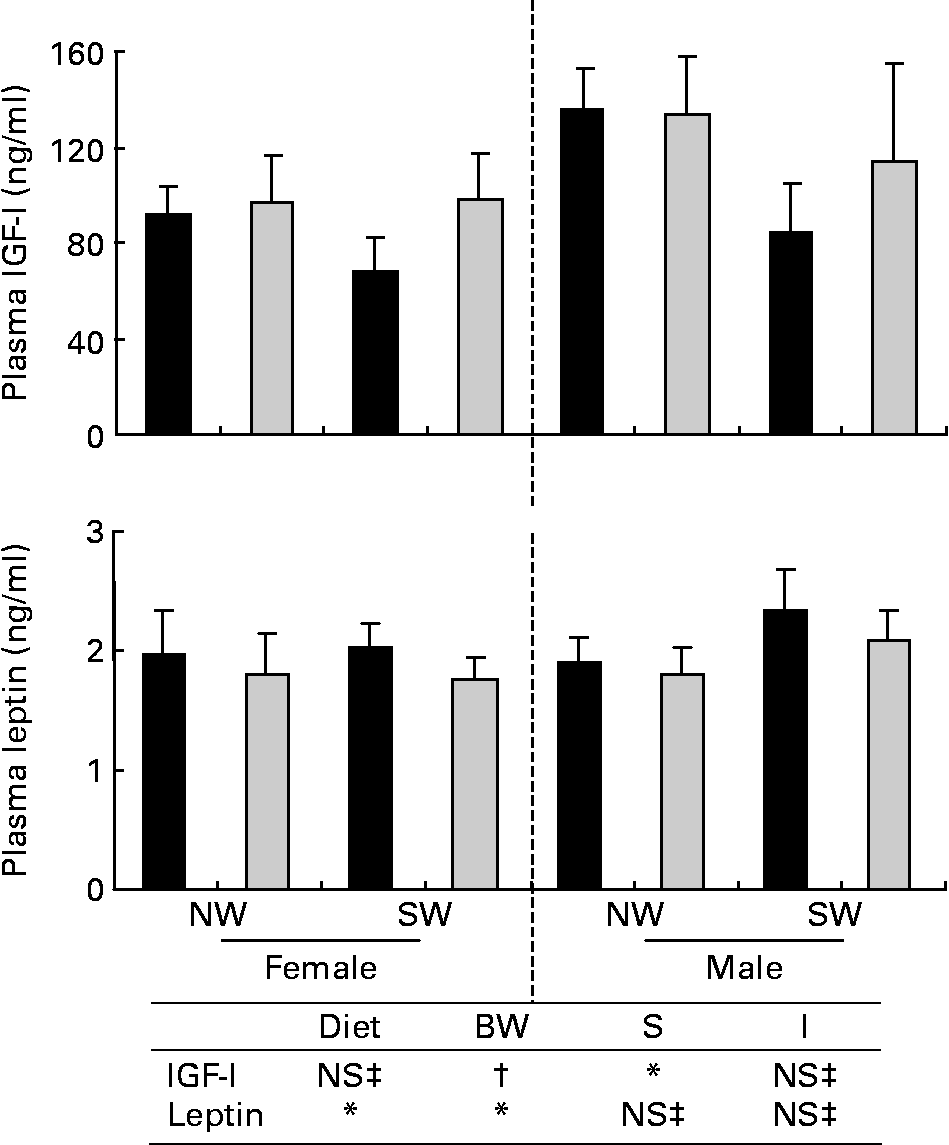
Fig. 1 Plasma insulin-like growth factor-I (IGF-I) and leptin concentrations in pigs fed adequate-protein (![]() ) or high-protein (■) formulae between 7 and 28 d of age and fed a standard diet thereafter. Values are means, with their standard errors (n 7) represented by vertical bars. * Mean values were significantly different (P < 0·05). † Mean values tended to be significantly different (P < 0·1). ‡ Mean values were not significantly different (P>0·1). NW, normal birth weight; SW, small birth weight. Diet, BW and S are the effects of diet, birth weight and sex; I are interactions (diet × S and/or diet × birth weight).
) or high-protein (■) formulae between 7 and 28 d of age and fed a standard diet thereafter. Values are means, with their standard errors (n 7) represented by vertical bars. * Mean values were significantly different (P < 0·05). † Mean values tended to be significantly different (P < 0·1). ‡ Mean values were not significantly different (P>0·1). NW, normal birth weight; SW, small birth weight. Diet, BW and S are the effects of diet, birth weight and sex; I are interactions (diet × S and/or diet × birth weight).
Expression of genes in adipose tissues and skeletal muscle
In subcutaneous adipose tissue, the relative levels of IGF-I and IGF-II mRNA were not influenced by diet or sex but were lower (P < 0·01) in NW pigs than in SW pigs (Fig. 2). For the relative levels of IGF-I receptor mRNA, there was an interaction (P < 0·05) between diet and sex. These levels were lower (P < 0·01) in NW pigs than in SW male pigs. In perirenal adipose tissue, the expressions of IGF-I, IGF-II and IGF-I receptor genes were not significantly influenced by diet, sex or birth weight (data not shown). As shown in Fig. 3, there was an interaction (P < 0·05) between diet and sex with respect to the relative levels of leptin mRNA in subcutaneous adipose tissue. Whereas these levels tended (P < 0·1) to be higher in HP pigs than in AP female pigs, they did differ in male pigs. Similar observations were made in perirenal adipose tissue (data not shown). The relative levels of adiponectin mRNA in subcutaneous and perirenal (data not shown) adipose tissues were not affected by diet or sex, but the levels were lower (P < 0·001) in NW pigs than in SW pigs.
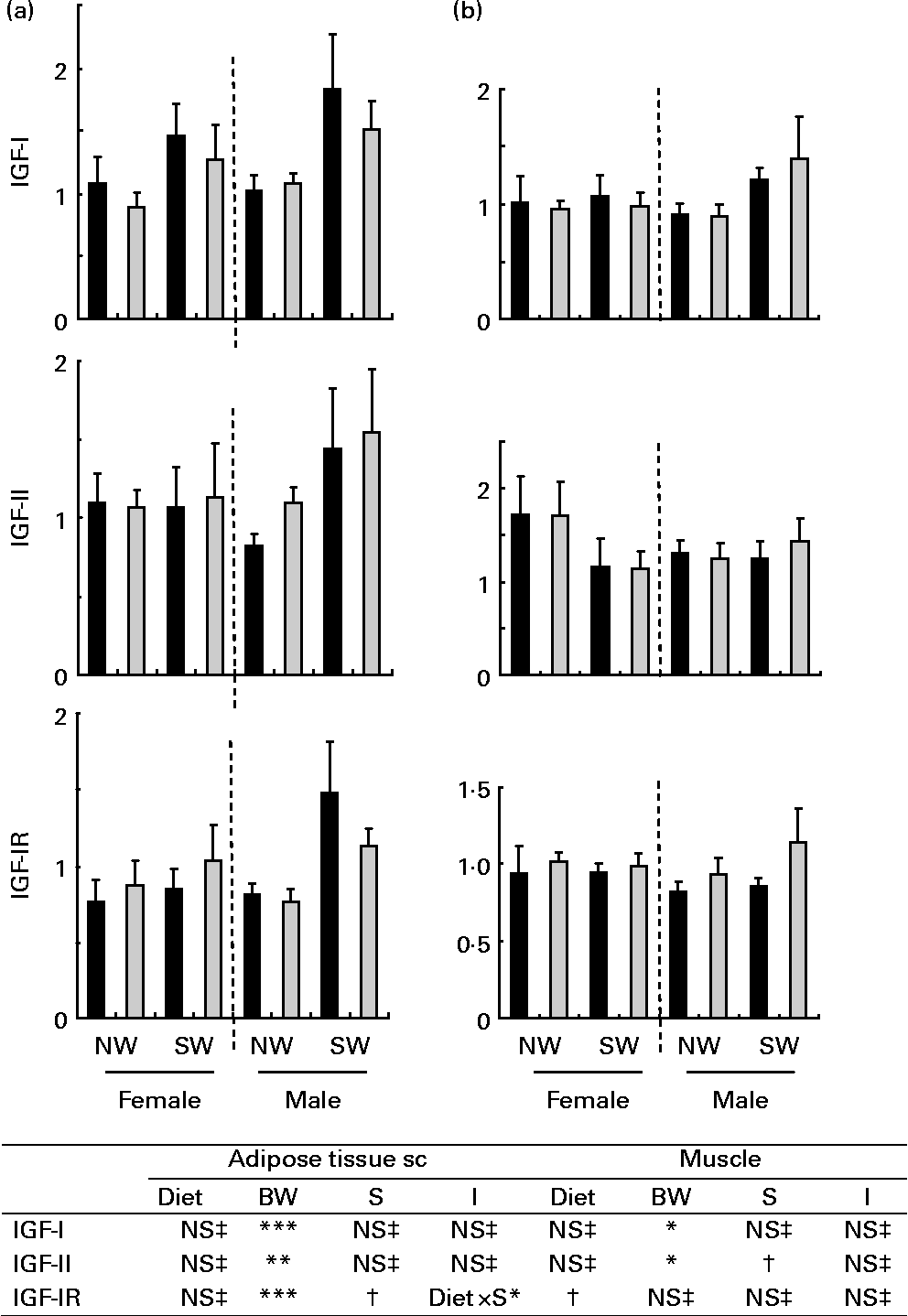
Fig. 2 Relative levels of insulin-like growth factor-I (IGF-I), IGF-II, IGF-I receptor mRNA in (a) subcutaneous (sc) adipose tissue and (b) in longissimus muscle in pigs fed adequate-protein (![]() ) or high-protein (■) formulae between 7 and 28 d of age and fed a standard diet thereafter. Values are means, with their standard errors (n 7) represented by vertical bars. Mean values were significantly different: *P < 0·05, **P < 0·01, ***P < 0·001. † Mean values tended to be significantly different (P < 0·1). ‡ Mean values were not significantly different (P>0·1). NW, normal birth weight; SW, small birth weight; R, receptor. Diet, BW and S are the effects of diet, birth weight and sex; I are interactions (diet × S and/or diet × birth weight).
) or high-protein (■) formulae between 7 and 28 d of age and fed a standard diet thereafter. Values are means, with their standard errors (n 7) represented by vertical bars. Mean values were significantly different: *P < 0·05, **P < 0·01, ***P < 0·001. † Mean values tended to be significantly different (P < 0·1). ‡ Mean values were not significantly different (P>0·1). NW, normal birth weight; SW, small birth weight; R, receptor. Diet, BW and S are the effects of diet, birth weight and sex; I are interactions (diet × S and/or diet × birth weight).
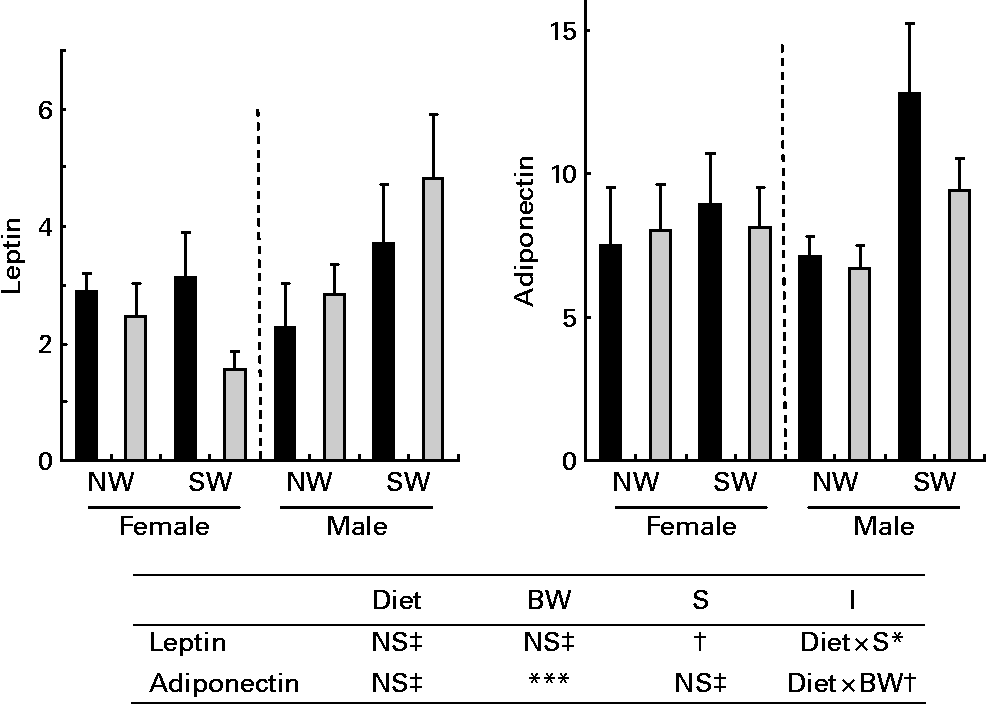
Fig. 3 Relative levels of leptin and adiponectin mRNA in subcutaneous adipose tissue in pigs fed adequate-protein (![]() ) or high-protein (■) formulae between 7 and 28 d of age and fed a standard diet thereafter. Values are means, with their standard errors (n 7) represented by vertical bars. Mean values were significantly different (***P < 0·001). † Mean values tended to be significantly different (P < 0·1). ‡ Mean values were not significantly different (P>0·1). NW, normal birth weight; SW, small birth weight. Diet, BW and S are the effects of diet, birth weight and sex; I are interactions (diet × S and/or diet × birth weight).
) or high-protein (■) formulae between 7 and 28 d of age and fed a standard diet thereafter. Values are means, with their standard errors (n 7) represented by vertical bars. Mean values were significantly different (***P < 0·001). † Mean values tended to be significantly different (P < 0·1). ‡ Mean values were not significantly different (P>0·1). NW, normal birth weight; SW, small birth weight. Diet, BW and S are the effects of diet, birth weight and sex; I are interactions (diet × S and/or diet × birth weight).
In longissimus muscle (Fig. 2), the expressions of the genes under study were not influenced by diet or sex. With respect to birth weight, slight differences (P < 0·05) were observed for IGF mRNA, with lower levels of IGF-I mRNA in NW pigs than in SW pigs and with higher levels of IGF-II mRNA in NW pigs than in SW pigs.
Discussion
The present study is one of the rare ones documenting the impact of a HP intake exclusively during the suckling period on subsequent growth, body composition and hormonal status of growing pigs reared in well-controlled conditions. The present findings demonstrate that an increase in early protein intake increased the growth rate during the intervention period in both female and male pigs whatever their birth weights. After weaning, the difference in growth rate persisted only in male pigs. With respect to the regulatory axes, the present study indicates that plasma leptin concentrations and leptin gene expression in adipose tissues were slightly affected by the diets. Nevertheless, these changes were not associated with any significant difference in adiposity between the two dietary groups at 70 d of age.
The finding of an increase in growth rates in HP piglets whatever their birth weight or sex between 7 and 28 d of age supports the conclusion of Premji et al. (Reference Premji, Fenton and Sauve28), suggesting in a systematic review that higher protein intake from formula accelerates growth of low-birth-weight infants. More recently, it has been reported in a large randomised controlled trial that infants receiving a formula with a higher protein content between 15·5 d (median age) and 12 months of age exhibited a higher growth at the end of the intervention period(Reference Koletzko, von Kries and Closa29). In contrast, a very recent study indicates that feeding a protein-enriched formula without extra energy for 6 months after term did not affect growth of pre-term infants(Reference Amesz, Schaafsma and Cranendonk30). In rats, body weights of pups receiving 130 % of the normal protein content of rat milk did not differ from those of pups receiving a control diet, whereas those of pups receiving 50 % of the normal protein content were lower in the short term(Reference Des Robert, Li and Caicedo31). In the post-formula-feeding period, the present findings clearly indicate that early protein intake did not influence the subsequent relative growth rate of pigs. In infants(Reference Koletzko, von Kries and Closa29), the difference in body weights induced by a rise in protein intake persisted in the year following the intervention period. Nevertheless, it should be noted that the reported differences tended to decrease during this period. In rats, no major impact of protein intake could be detected on later body weights(Reference Des Robert, Li and Caicedo31). Altogether, most of the few available data based on the comparison of early HP intake with AP intake are consistent with growth rate acceleration during the intervention period that does not persist in the post-weaning period.
With respect to adiposity, our observations clearly indicate that the reduction in adiposity that has been reported at the end of the intervention period in our animal model(Reference Morise, Sève and Macé22) as well as in pre-term infants(Reference Amesz, Schaafsma and Cranendonk30) is not detected at the end of the post-weaning period examined. Indeed, both the carcass lipid content and the perirenal adipose tissue mass were similar in the two dietary groups. This lack of difference in body composition agrees with recent observations in adult rats(Reference Des Robert, Li and Caicedo31) that have received different protein intakes during the suckling period. Altogether, these findings indicate that a HP intake in early life reduces adiposity only temporarily. Despite the lack of difference in adiposity of 70-d-old pigs, it cannot be excluded that evaluation of HP pigs for a much longer duration may lead to fatter pigs subsequently.
The evaluation of hormonal factors that have been described to play a role in the processes that link early nutrition and later growth may have provided some indications on later tissue development. However, there is no evidence of any effect of this early diet on the IGF system in the period examined. Therefore, these observations do not support the hypothesis that a HP intake may stimulate secretion of IGF-I that may lead to greater adiposity thereafter(Reference Rolland-Cachera, Deheeger and Akrout9). On the other hand, plasma leptin concentrations and leptin mRNA levels in adipose tissue were found to be slightly affected by the early diet. Whereas plasma leptin concentrations were higher in HP pigs, mRNA levels were not affected by the HP diet in adipose tissue of HP male pigs. Leptin is known to play an important role in the regulation of food intake and energy partitioning, and its plasma concentration is often considered as a good marker of adiposity in adults(Reference Maffei, Halaas and Ravussin32). As previously shown at the end of the intervention period(Reference Morise, Sève and Macé22), the present results confirm the lack of relationship between plasma leptin level and fat mass in young growing animals. Such a lack of a relationship has also been reported in young rats(Reference Passos, Toste and Dutra33). The meaning of the observed changes is not clear. The increase in leptinaemia may result from an increase in leptin production by tissues other than adipose tissue(Reference Wang, Liu and Hawkins34). It could be also hypothesised that this increase may represent an early phase in the development of leptin resistance that may occur in later development(Reference Attig, Solomon and Ferezou35).
It is possible that the difference in protein intake between the two groups in our investigation as well as in the recent study in rats(Reference Des Robert, Li and Caicedo31) may not be sufficient to affect subsequent tissue development. However, the hypothesis that an early exposure to a HP intake needs to be associated with other risk factors is more likely. For instance, an early HP intake may need to be associated with a Western-type diet to increase subsequent adiposity. Data based on nutrient restriction corroborate this hypothesis(Reference Bieswal, Ahn and Reusens36). Another possibility is that the early suckling period may not be so critical for the induction of later obesity in response to a HP intake. Some data in humans further document these points. According to Günther et al. (Reference Günther, Remer and Kroke37), there was no association between protein intake before 6 months and later adiposity, whereas a HP intake during complementary feeding has been shown to be associated with later adiposity.
The evaluation of the possible interaction between birth weight, sex and early protein intake constitutes another originality of the present study. Even though it is often considered that a HP intake may induce a catch-up growth in low-birth-weight individuals, no significant interaction between early protein intake and birth weight could be identified in our experimental conditions. Despite a higher relative daily weight gain in SW piglets than in NW piglets during the formula-feeding period, the growth retardation persisted in the two dietary groups during the whole period examined. This agrees with data indicating that pigs exhibiting small weight at birth stay lighter than their counterparts until at least 6 months of age(Reference Campbell and Dunkin38–Reference Gondret, Lefaucheur and Juin41). As suggested previously(Reference Davis, Fiorotto and Burrin42), it is possible that SW pigs cannot exhibit catch-up growth because they may have been undernourished throughout gestation.
With respect to sex, several expected parameters such as adipose tissue mass and plasma IGF-I concentrations differed between female and male pigs(Reference Louveau, Bonneau and Salter43). Moreover, the finding of interactions between diet and sex suggests that the impact of the diet may be sex-dependent. The HP diet increased both absolute growth rate and weight at 70 d in male pigs but not in female pigs. Altogether, our observations are consistent with the statement that male pigs may be more responsive to early protein intake than female pigs, at least in term of growth. In rats, studies that have investigated protein intake during lactation and pregnancy indicate that the impact of early nutrition is also sex-specific(Reference Zambrano, Bautista and Deás18, Reference Sugden and Holness44, Reference Thone-Reineke, Kalk and Dorn45). As mentioned in these studies, the reason for this sex specificity remains unknown.
Altogether, our data clearly indicate that an exposure to a high-protein diet induced an increase in growth rate during the intervention period. Our current data also support an interaction between diet and sex, with male pigs being more sensitive to the diet but do not support an interaction between diet and birth weight. The findings of similar adiposity in 70-d-old pigs of the two dietary groups suggest that the effects of early protein intake on subsequent body composition may not be as important as those suggested in some epidemiological studies(Reference Rolland-Cachera, Deheeger and Akrout9, Reference Koletzko, Rigo and Ziegler10). Of course, we cannot exclude that the period examined was too short to detect a significant effect. It is also possible that an early exposure to a HP intake needs to be associated with a Western-type diet to later increase adipose tissue development.
Acknowledgements
The present study was supported by INRA and Nestec. The authors wish to thank C. Tréfeu for her expert technical assistance and M. Alix, J. Liger and J. F. Rouaud for animal slaughtering and carcass homogenisation. They also acknowledge A. Chauvin, L. Gaillard, F. Le Gouevec and V. Piedvache for expert assistance with animal care as well as E. Bobillier and G. Savary for the conception, production and maintenance of formula feeders. A. M., B. S., K. M., C. M., I. L. H.-L. and I. L. were responsible for the study design; B. S. and C. M. designed the experimental diets; A. M., B. S., I. L. H.-L. and I. L. conducted the research; A. M. and I. L. analysed data and wrote the manuscript. I. L. and K. M. had primary responsibility for the final content. All authors read and approved the final manuscript. There are no conflicts of interest.











