When the growth of urban communities began its dramatic rise in the mid-eighteenth century, a little over 5 percent of the world's population lived in cities. Today, more than 75 percent of the population of developed countries lives in urban communities (Dodman et al. Reference Dodman, Brown, Francis, Hardoy, Johnson and Satterthwaite2013:1–4), and this rapid urbanization has been accompanied by ecological changes whose social implications are less well documented (Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). Understanding current and future urban ecosystems involves determining the causal relationships that may exist between biological, physical, and cultural variables, an important consideration given the kinds of wealth disparities that commonly exist in cities (Alcoforado and Andrade Reference Alcoforado, Andrade, Marzluff, Shulenberger, Endlicher, Alberti, Bradley, Ryan, ZumBrunnen and Simon2008:259). Archaeological contexts, with their potential to address both culture and environment simultaneously, afford a unique perspective for understanding the trajectory and interplay of these forces.
In this paper, we explore the intersection of a variety of biological and cultural variables that have helped shape the rich mosaic of culturally influenced microenvironments that constitute urban areas, and we link these to consequences for the health of people with disparate social standings. As cities developed, people altered urban ecologies and changed the ways they created and used their landscape. Such changes are manifest on a variety of scales, from alterations in soil microfauna to the increase in the geographic range of plant species due to the heat island effect (Gilbert Reference Gilbert1989; Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). Urban environments, in turn, had an effect on health that was recognized in North America during the eighteenth and nineteenth centuries (Melosi Reference Melosi2008). These impacts were significant from both biological and cultural perspectives. For example, the sanitary reform movement in the nineteenth century evolved as a response to outbreaks of epidemics diseases, which were viewed as being caused by environmental conditions (Duffy Reference Duffy1990).
We examine the processes of urbanization from the vantage of the archaeological remains of two parasites that inhabit the human body—Ascaris lumbricoides (roundworm, maw worm) and Trichuris trichiura (whipworm). People become infected with these parasites when their fertile eggs are ingested, often with contaminated food and water (Ash and Orihel Reference Ash and Orihel1990:134, 138). Once eggs are ingested and pass through the stomach, they hatch in the intestines. Trichuris larvae mature in the small intestines and establish themselves in the colon. Ascaris larvae move outside the gut, into the circulatory system, and travel through other organs of the body, spending about two weeks in the lungs before moving into the trachea and finally entering the small intestines, where they mature (Davey Reference Davey1966:168–169, 177).
Female Ascaris worms mature to produce eggs within 60 to 90 days, female Trichuris worms after 60 to 70 days. Adult Ascaris worms live for approximately one to two years, but data on Trichuris are less consistent, with lifespan estimates varying from one to 10 years (Ash and Orihel Reference Ash and Orihel1990:134, 138). Each Ascaris female has the potential to produce 200,000 to 240,000 eggs per day; a female Trichuris, on the other hand, produces many fewer—from 3,000 to 20,000 eggs per day (World Health Organization [WHO] 1981:59). The ova of both species are expelled from the human body in feces and must spend two to three weeks outside of their host and in soil in order to become infective (Ash and Orihel Reference Ash and Orihel1990). During this time, parasite ova embryonate or undergo larval development. Undeveloped eggs are not infective; that is, if they are consumed, they will not produce disease. During this process, the larva is sheltered within the egg, but the period in the soil exposes them to environmental conditions, which can affect their development, viability, and transmission (Maya et al. Reference Maya, Ortiz and Jiménez2010; Pecson et al. Reference Pecson, Barrios, Jiménez and Nelson2007). These parasites are useful for understanding changing environmental conditions because they spend part of their life cycle in the soil and are therefore susceptible to the conditions of anthropogenic environments.
Although these parasites rarely cause the death of their hosts, heavy parasite loads can cause a variety of illnesses and affect children's development. Trichuris infections tend to be mild, possibly asymptomatic in cases of light parasite loads, but very heavy loads may cause intestinal obstruction (Davey Reference Davey1966). Ascaris infections also tend to be mild, but they can be more dangerous in part because the worms leave the gut during their life cycles and migrate through the body. Ascaris can cause blockages of the liver, bile, and pancreatic ducts, peritonitis, and liver failure, as well as contributing to pneumonia and appendicitis (Davey Reference Davey1966). The parasites can cause allergic reactions and may be involved in reduced immune responses, and malabsorption of nutrients is associated with Ascaris infections (Bradley and Keymer Reference Bradley and Keymer1984). The mortality associated with the parasites may be low, but its contribution to other diseases is not well studied and is probably significant (Bradley and Keymer Reference Bradley and Keymer1984). Epidemiologists working on current infections suggest that the real costs of these diseases are unknown and probably underestimated (WHO 1981). Significantly, both parasites appear to affect children more heavily.
Archaeological evidence indicates that these parasites have plagued people for millennia, having evolved with hominids at least since Homo erectus (Bouchet et al. Reference Bouchet, Harter and Bailly2003; Leles et al. Reference Leles, Reinhard, Fugassa, Ferreira, Iñiguez and Araújo2010; Reinhard and Araújo Reference Reinhard, Araújo, Buikstra and Roberts2012), and they are among the most common parasites today (WHO 2012). They commonly co-occur, but a number of researchers have noted a shift through time in the numerical dominance of one parasite, Trichuris, to the other, Ascaris (Leles et al. Reference Leles, Reinhard, Fugassa, Ferreira, Iñiguez and Araújo2010; Trigg and Jacobucci Reference Trigg and Jacobucci2008)—a transition, we argue, that is related to changing environmental conditions brought about by both intentional and unintentional activities in cities. Because of the long relationship between these parasites, people, and the environment, we gain a window onto the complex processes of urbanization over the past 400 years. Using parasite data from a number of archaeological contexts, we explore the timing and causes of this shift in northeastern North America, focusing on the complex anthropogenic ecosystems that were deliberately and inadvertently created in cities and the impact of this shift on human health.
Archaeological Analysis, Culture, and Environment
Data on these and other parasites gleaned from archaeological contexts help complete biological and cultural pictures of urban life (Mrozowski Reference Mrozowski2006; Reinhard Reference Reinhard and Yamin2000). The relationship between parasites, health, status of households, and household composition provides a contrast with material culture representations of identity (Fisher et al. Reference Fisher, Reinhard, Kirk and DiVirgilio2007; Mrozowski Reference Mrozowski2006; Reinhard, Mrozowski, and Orloski Reference Reinhard, Mrozowski and Orloski1986). In colonial Newport, Rhode Island, parasite loads correlate with occupation and status levels (Reinhard, Mrozowski, and Orloski Reference Reinhard, Mrozowski and Orloski1986). In a study of sites in Albany, New York, Fisher and colleagues (Reference Fisher, Reinhard, Kirk and DiVirgilio2007) identified lower parasite loads in one household, which they attribute to the inhabitants’ knowledge of and access to medicines to treat intestinal parasites. Recent work by Reinhard et al. (Reference Reinhard, Ferreira, Bouchet, Sianto, Dutra, Iñiguez, Leles, Le Bailly, Fugassa, Pucu and Araújo2013) and others (Searcey et al. Reference Searcey, Karl, Eduard, Frank, Dario, Albert, Winjnand, Scott and Raffaella2013) shows that parasite diseases differed between North America and Europe. Parasites associated with meat consumption are much less common in New England than Europe, suggesting that colonists quickly developed methods of food preparation that eliminated such parasites (Reinhard et al. Reference Reinhard, Ferreira, Bouchet, Sianto, Dutra, Iñiguez, Leles, Le Bailly, Fugassa, Pucu and Araújo2013; Searcey et al. Reference Searcey, Karl, Eduard, Frank, Dario, Albert, Winjnand, Scott and Raffaella2013). In historic North America, the archaeoparasitological record is distinct for its lack of food-borne parasites, but the record in both North America and Europe is dominated by Trichuris trichiura and Ascaris lumbricoides.
While the adult worms are rarely recovered, the eggs of Ascaris and Trichuris (Figure 1) have been found in a number of archaeological deposits, particularly coprolites, privies, cesspools, and other features associated with human waste. Archaeoparasitological studies often rely on coprolite analysis from burials (Rácz et al. Reference Rácz, Pucu, Jensen, Mostek, Morrow, van Hove, Bianucci, Willems, Heller, Araújo and Reinhard2015), middens (Fugassa et al. Reference Fugassa, Reinhard, Johnson, Gardner and Vieira2011; Jiménez et al. Reference Jiménez, Gardner, Araújo, Fugassa, Brooks, Rácz and Reinhard2012), or privy-type deposits (Fisher et al. Reference Fisher, Reinhard, Kirk and DiVirgilio2007), although, in some instances, parasites have been found in mummies (Kumm et al. Reference Kumm, Reinhard, Piombino-Mascali and Araújo2010), wells, pits (Pike Reference Pike1968), and living surfaces in and around buildings (Jones Reference Jones, Fieller, Gilberston and Ralph1985; Jones et al. Reference Jones, Hutchinson and Nicholson1988). Because of the focus on coprolites and privy contexts, investigations have been useful for addressing parasitism on individual or household levels (Fisher et al. Reference Fisher, Reinhard, Kirk and DiVirgilio2007; Kumm et al. Reference Kumm, Reinhard, Piombino-Mascali and Araújo2010; Reinhard Reference Reinhard1988, Reference Reinhard and Yamin2000, Reference Reinhard and Reitz2008; Reinhard, Mrozowski, and Orloski Reference Reinhard, Mrozowski and Orloski1986). In historic contexts, where researchers can connect the social status of known individuals to the parasites, investigations are particularly powerful for understanding the relationship between health and status.
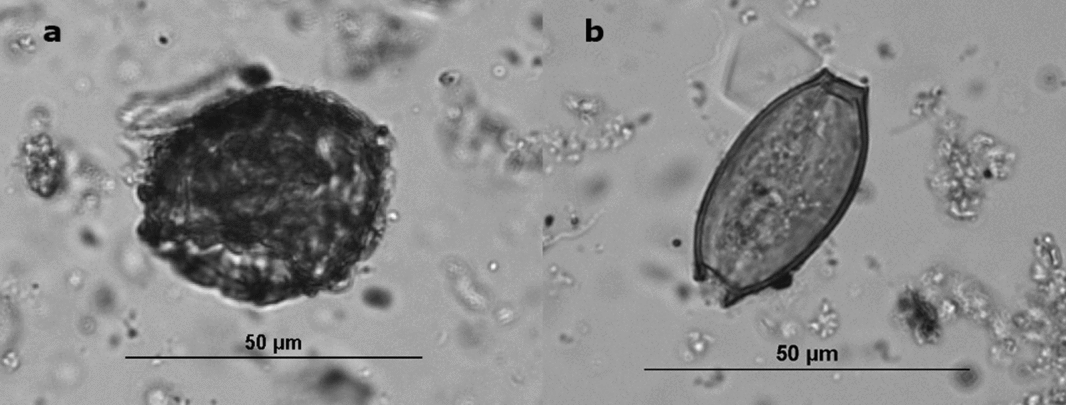
Figure 1. (a) Ascaris ovum from Boston's Cross Street Privy, (b) Trichuris ovum from Boston's Cross Street Privy—note the two opercula.
While much archaeoparasitological research has focused on individuals and households, parasites are also useful for examining interactions between people and their environments on a larger scale (Reinhard Reference Reinhard1992). Because geoparasites such as Ascaris and Trichuris require human hosts for reproduction and must spend some of their life cycle outside the human body, they can be used to investigate peoples’ migration through (Araújo et al. Reference Araújo, Reinhard, Ferreira and Gardner2008) and occupation of (Reinhard Reference Reinhard, Merbs and Miller1985, Reference Reinhard1988, Reference Reinhard1992) different habitats. For example, Hevly and colleagues (Reference Hevly, Kelly, Anderson, Olsen, Sheets and Grayson1979) determined that prehistoric inhabitants of Arizona may have built their homes in relatively arid locations but had at some point visited moister microenvironments of canyon bottoms, where they acquired parasite infections. While parasites generally reflect local environmental conditions, aggregated data of their presence can be used to track changing conditions on a larger scale. Reinhard (Reference Reinhard, Beaudry and Mrozowski1992) used the fact that Strongyloides requires moist soil conditions to argue that eggs recovered from prehistoric contexts in the American Southwest indicate that the environment was wetter in the past than in the present.
Because geoparasites, such as Ascaris and Trichuris, spend part of their life cycle in the soil, changes in the environment may affect their distribution and abundance, and the research presented here investigates the relationship between the peoples’ use of urban habitats and parasitic infections they may carry. By synthesizing archaeoparasitological data from a number of historic sites in the eastern United States, we examine the relationship between the development of urban spaces and the changing proportions of Trichuris trichiura and Ascaris lumbricoides. Today, Ascaris is the more common of the two parasites, infecting an estimated one billion people worldwide (Bethony et al. Reference Bethony, Brooker, Albonico, Stefan, Geiger, Diemert and Hotez2006), but this has not always been the case. Data from archaeological sites suggest that Trichuris was more common in prehistoric European and North American contexts (Bouchet et al. Reference Bouchet, Harter and Bailly2003; Bundy and Cooper Reference Bundy and Cooper1989; Leles et al. Reference Leles, Reinhard, Fugassa, Ferreira, Iñiguez and Araújo2010).
These parasites have similar although not identical environmental tolerances, life cycles, and histories, and the differences may have important implications for the relationships these parasites have with their human hosts and the environment. Ultimately, however, the reasons for the shift from Trichuris to Ascaris are not well understood. Leles and colleagues (Reference Leles, Reinhard, Fugassa, Ferreira, Iñiguez and Araújo2010) suggest several reasons for the lack of Ascaris ova in South American archaeological samples and the change from the archaeological to the present-day distribution, including the use of medicines, prehistoric human behavior, and taphonomic processes. What they do not explore is the possibility that changing environmental conditions may have influenced parasite distributions. Using well-dated archaeological deposits, we identify when the shift from Trichuris to Ascaris occurred in the cities of northeastern North America and offer some additional reasons that link the change to a set of biological, ecological, and cultural variables that had wide-ranging influence on urban life.
Archaeological Data
To trace the shift from Trichuris to Ascaris and the underlying causes, we surveyed archaeoparasitological literature for sites in the northeast United States and gathered data on parasite remains from archaeological contexts dating from the seventeenth through the early twentieth centuries, at which time privies declined in use in urban areas in favor of sewers (Driscoll Reference Driscoll1994; Geismar Reference Geismar1993; Mrozowski Reference Mrozowski2006, Reference Mrozowski2014). These data, although collected by several researchers, follow protocols developed by Karl Reinhard (see Warnock and Reinhard Reference Warnock and Reinhard1992). Hevly and colleagues (Reference Hevly, Kelly, Anderson, Olsen, Sheets and Grayson1979) first used these protocols when they found parasite eggs in latrine samples processed for pollen analysis. Reinhard, Mrozowski, and Orloski (Reference Reinhard, Mrozowski and Orloski1986) modified typical pollen extraction methods, which optimized parasite recovery with less damage to parasite eggs. Although other methods for recovering parasite eggs exist, Reinhard's procedure is considered to be the most effective (Dufour and Le Bailly Reference Dufour and Le Bailly2013). We follow this procedure, and therefore data presented here are directly comparable between contexts and cities, making this the largest quantified data set derived from the most effective parasite recovery method.
Ova recovery rates are influenced by the amount of fecal material in the deposits (Jones Reference Jones, Fieller, Gilberston and Ralph1985), the depositional environment (Reinhard, Confalonieri, et al. Reference Reinhard, Confalonieri, Herrmann, Ferreira and Araújo1986), and peoples’ parasite loads. In the case of latrine sediments, the rapid deposition in deep, protected, and anaerobic environments optimized preservation. Privies, especially those in the Northeast, provide anaerobic conditions that favor the preservation of parasite ova (Karl Reinhard, personal communication 2016). However, taphonomic conditions have been implicated in the differential preservation of Trichuris and Ascaris ova (Rácz et al. Reference Rácz, Pucu, Jensen, Mostek, Morrow, van Hove, Bianucci, Willems, Heller, Araújo and Reinhard2015). Ascaris eggs have several separable layers, whereas Trichuris eggs have one layer, and their bi-opercular morphology (Figure 1) makes them more susceptible to fracture. In general, we found very good preservation of both types of eggs in our study sites. We controlled for taphonomic factors by using data only from privy contexts rather than from the full suite of archaeological contexts, such as yards, trenches, and living surfaces, which have yielded parasite eggs.
We selected only those data where egg counts for both parasites were given. Although other parasites were identified in some samples, Ascaris and Trichuris were by far the most common taxa, and our analysis considers only these parasites. For each site or deposit, we used reported dates or estimated the date of the deposit based on the information given.
The selected sites are primarily urban or at least constitute more densely populated areas than their surroundings (Figure 2; Table 1). From the seventeenth and eighteenth centuries, we have deposits from four sites in New England. The earliest data come from the Cross Street privy in Boston, Massachusetts (Heck and Balicki Reference Heck and Balicki1998; Jacobucci Reference Jacobucci2009). The deposits analyzed are associated with the household of Katherine Naylor, a merchant's widow, and date to between 1670 and 1703. Information was also gathered from three households from the eighteenth century in Newport, Rhode Island. The earliest of these, dating to about 1730, is a privy associated with a shopkeeper (Gallagher Reference Gallagher2006; Hodge Reference Hodge2007). The second, dating to about 1750, is associated with a merchant's house (Mrozowski Reference Mrozowski2006; Reinhard, Mrozowski, and Orloski Reference Reinhard, Mrozowski and Orloski1986). The third privy, associated with a middle class household, dates to about 1770 (Mrozowski Reference Mrozowski2006; Reinhard, Mrozowski, and Orloski Reference Reinhard, Mrozowski and Orloski1986).

Figure 2. Cities yielding parasite ova data used in this study.
Table 1. Sources of Parasite Data.
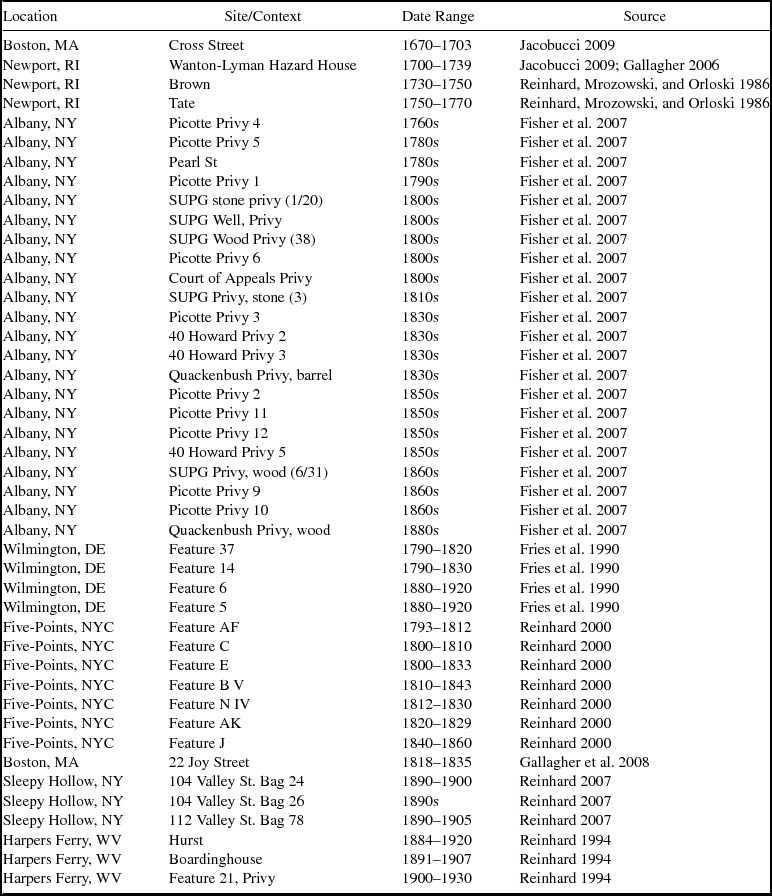
Privies from Albany, New York, span the mid-eighteenth through nineteenth centuries and represent a diverse population that included boardinghouse residents, laborers, merchants, ship captains, and politicians (Fisher et al. Reference Fisher, Reinhard, Kirk and DiVirgilio2007). Fisher and colleagues (Reference Fisher, Reinhard, Kirk and DiVirgilio2007) investigated eight sites for parasites, and we use data from 23 of the excavated privies. Eight privies from New York City's Five Points neighborhood date from the mid-eighteenth to the early twentieth centuries and provide parasite data from households of varying economic class (Reinhard Reference Reinhard and Yamin2000). Four privies in Wilmington, Delaware, provide data from the late eighteenth century to early twentieth centuries. These contexts were associated with laborers who rented their residences (Fries et al. Reference Fries, Katherine Beidleman and Custer1990). From the nineteenth century, we have data from the middle-class African American families who occupied a tenement behind Boston's African Meeting House (Gallagher et al. Reference Gallagher, Jacobucci, Trigg and Landon2008). Samples examined from Harpers Ferry, West Virginia, date from the late nineteenth to early twentieth centuries and yielded parasite data from a wealthy household and a boardinghouse (Reinhard Reference Reinhard1994). Three samples, one from each of three privies in Sleepy Hollow, New York, yielded parasite ova (Reinhard Reference Reinhard2007). These deposits dated to late nineteenth to early twentieth centuries. Where there were multiple samples from a particular privy or layer, we examined each sample separately.
Results
The parasite egg counts indicate a great deal of variability among samples, but in general there is a decline through time in the number of total eggs per sample (Table 2). Preservation and context can dramatically affect the quantities of eggs recovered, which is demonstrated in the wide variation in the number of eggs recovered (Table 2). To mitigate variables such as preservation and differences in parasite egg production, we focus on the ubiquity and relative proportions of ova rather than the absolute numbers of eggs.
Table 2. Counts and Proportions of Ascaris and Trichuris Ova in Each Context.
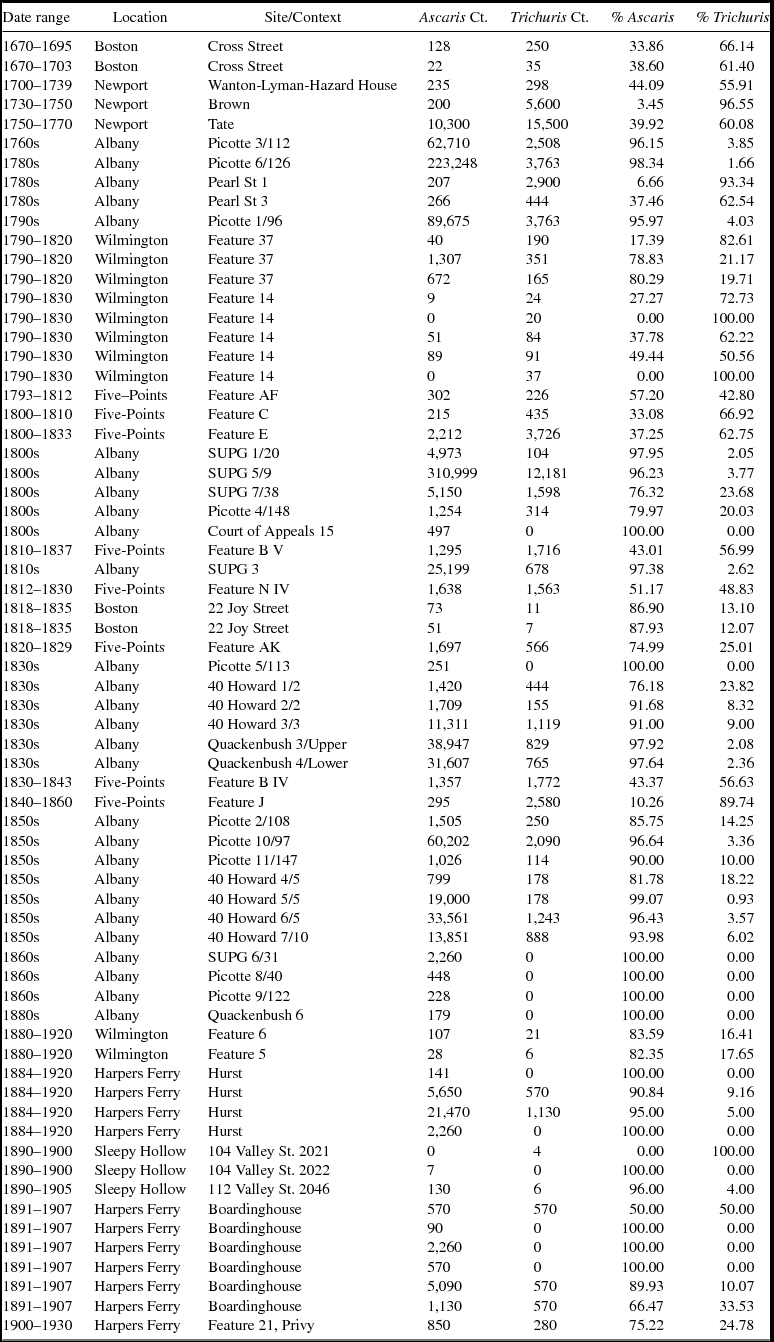
The ubiquity of the different parasites provides one indication of their prevalence and suggests an increase in the presence of Ascaris and a decrease in Trichuris thorough time. In samples dating to 1800 or earlier, 92 percent have Ascaris eggs and 96 percent have Trichuris eggs (Table 2). After 1800, 73 percent of samples have Trichuris eggs and 98 percent have Ascaris eggs. The prevalence of these taxa as measured by their ubiquity indicates a shift from Trichuris to Ascaris.
Relative proportions of Ascaris and Trichuris ova were calculated from the sum of both taxa and exclude the small number of other parasites identified (Table 2). We aggregated these data by decade using the earliest possible decade in ranges with large spans, ordered the proportions chronologically, and plotted the results (Figure 3). Data from individual sites show variation, but the general trend is an increasing proportion of Ascaris eggs through time (Figure 3). Prior to the last decades of the eighteenth century, Trichuris eggs dominate the assemblage. Around 1800, a predominance of Trichuris eggs gave way to a predominance of Ascaris eggs, and this shift appears to have been largely complete by 1850.
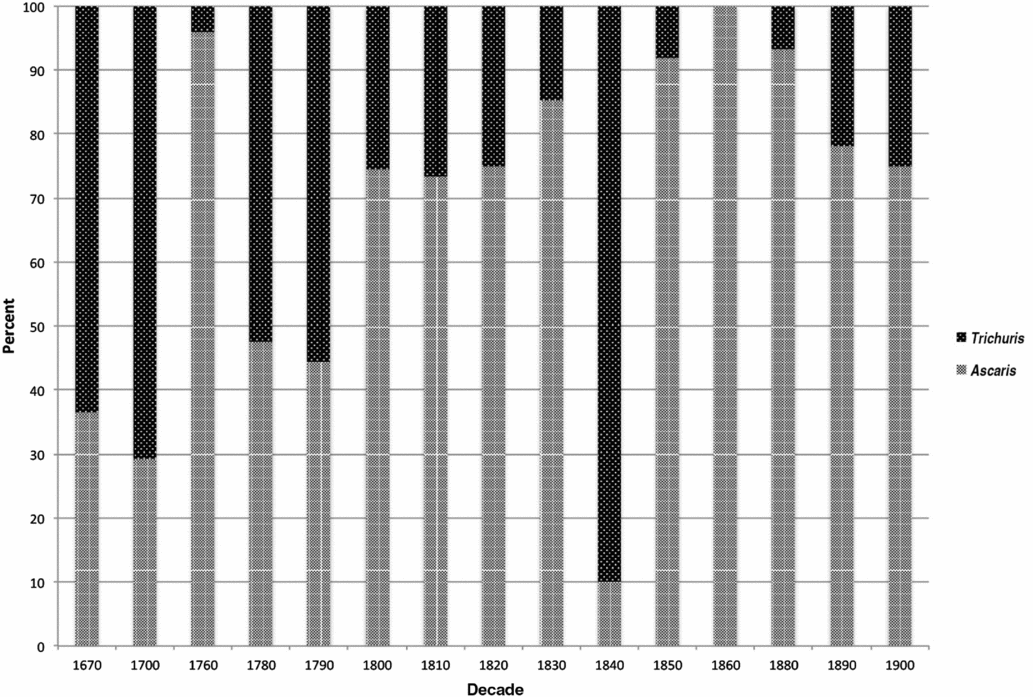
Figure 3. Proportions of Ascaris and Trichuris ova by decade. Note that the 1760 and 1840 data points are each represented by a single sample. Small sample size may account for their lack of fit with the pattern.
The difference in egg production between these taxa may have a bearing on the number of eggs recovered, but is not the cause of the shift from one taxon to the other. A female Ascaris produces 10 times more eggs than a female Trichuris (WHO 1981). The largest number of Trichuris eggs in a sample is 15,500, and the largest number of Ascaris is 310,999 eggs, more than 20 times greater—twice as many as would be expected from a simple difference in egg production. Moreover, Ascaris and Trichuris have been in association for thousands of years in Europe and at least hundreds of years in North America. Were the shift in proportions seen in the archaeological record merely the result of ascarids outproducing trichurids, this transition should have taken place earlier in their coexistence.
To explore the timing of the decline in more detail, we grouped the proportion data into 50-year intervals (Figure 4). The percentage of Ascaris is below 50 percent during the first 100 years of the study period and is greater than 80 percent during roughly the last 100 years. The middle 50-year period appears to be transitional. The median percentage of Ascaris eggs for both periods before 1750 hovers around 40 percent, while the median percentage of Ascaris eggs for both periods after 1800 is over 85 percent. There are a few samples with a low percentage of Ascaris eggs in all of the periods after 1750, but these samples tend to be outside the interquartile range. In the 1850–1900 period, those samples without Ascaris are outliers.
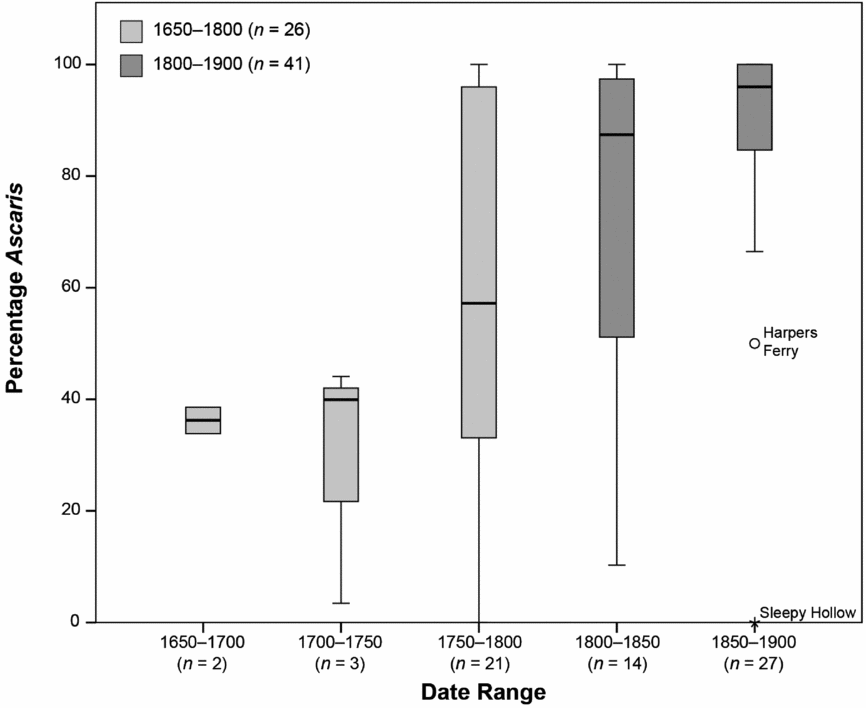
Figure 4. Box and whisker plot of the percentage of Ascaris eggs in 50-year intervals (Table 1). The box is the interquartile (IQ) range. The line in the center of each box is the median, and the whisker (line) is the case farthest from the median but still within 1.5 times the IQ. Harpers Ferry is an outlier (greater than 1.5 times the IQ range) and Sleepy Hollow is an extreme outlier (greater than 3 times the IQ range).
The shift occurs from 1800 to 1850, a period of increasing urbanization in the northeast United States (Melosi Reference Melosi2008; Mrozowski Reference Mrozowski, McGuire and Paynter1991, Reference Mrozowski2006; Shammas Reference Shammas2000). There are few large urban areas prior to the nineteenth century, but there are changes in urban population densities occurring by the 1790s through the 1820s (Shammas Reference Shammas2000). Population levels in the United States’ largest cities, Boston, New York, Baltimore, and Philadelphia, were at their peak densities during this period (Shammas Reference Shammas2000). Given the timing of the shift, we suggest that cultural and ecological changes accompanying urbanization may have played a role, perhaps differentially impacting the viability and transmission of Ascaris and Trichuris.
There are individual samples that deviate from the general trend of increasing proportion of Ascaris (Table 1). These may be the result of cultural factors such as variability among households in wealth, status, or household composition (Mrozowski Reference Mrozowski2006; Reinhard Reference Reinhard and Yamin2000; Reinhard, Mrozowski, and Orloski Reference Reinhard, Mrozowski and Orloski1986). Fisher and colleagues (Reference Fisher, Reinhard, Kirk and DiVirgilio2007) found a few privies in Albany with higher proportions of Trichuris ova than Ascaris, which is contrary to the general trend in their samples. They suggest that the use of medicines was the cause, as most nineteenth-century medicines were more effective against Ascaris than Trichuris. They also suggest that trichurids are small and, even when the worms were expelled in feces, they might not have been noticed by the human hosts, whereas ascarids are larger, more likely to be observed, and consequently treated with vermifuges.
The trend we see toward an increasing proportion of Ascaris ova is all the more interesting because Trichuris infections were more difficult to treat with remedies of the eighteenth and nineteenth centuries, such as plant-based tonics and patent medicines (Mrozowski Reference Mrozowski2006; Reinhard Reference Reinhard and Yamin2000). Even today, Trichuris infections are difficult to treat (Keiser and Utzinger Reference Keiser and Utzinger2008), perhaps because the adult worm attaches itself to the intestine wall. If this pattern were merely the result of the increasing efficacy of medicinal treatments or diligence in treating infections, we would expect diminishing proportions of the more vulnerable Ascaris. This is not borne out by the data, so other factors must be influencing this shift.
Differential preservation may cause a decrease in Trichuris ova (Reinhard Reference Reinhard1988). These eggs may be particularly sensitive to depositional factors, particularly fungal attack, which in some cases may selectively increase the decomposition of Trichuris eggs (Reinhard Reference Reinhard1988). We acknowledge this possibility; however, if differential preservation were a consistent problem, the overall trend would be for the proportion of Trichuris eggs to be the lowest in the oldest samples, which would have been exposed to fungi and environmental factors longer than the most recent samples. Data presented here suggest the opposite.
Contemporary investigations of parasite transmission provide information relevant to this issue. Bakta and colleagues (Reference Bakta, Widjana and Sutisna1993:92) suggest that three types of factors contribute to helminth infections: ecological (the presence of ova in the soil), sociocultural (education, wealth), and genetic—specifically the human population's resistance to a particular parasite. Brooker and colleagues (Reference Brooker, Alexander, Geiger, Moyeed, Stander, Fleming, Hotez, Correa-Oliveira and Bethony2006:1149) suggest, however, that human genetic factors are not a major concern, whereas environmental conditions around homes are the most important consideration. We suggest that changing urban environmental conditions may have affected the parasites differently, and cultural practices may have influenced people's exposure to disease.
Discussion
Archaeoparasitological data from historic sites in the Northeast show a shift in the dominant parasite during the early to mid-nineteenth century, and both environmental and behavioral factors play a role in the increasing prevalence of Ascaris over Trichuris. Here we consider the relationship between parasite biology and the changing microenvironments that characterize cities, peoples’ attempts to deal with human waste, and their use of space within cities. These potentially interrelated factors influence the number and type of viable parasite eggs residing in the soil, and thus those available to infect people. Geoparasite eggs exist in the soil as a result of contamination with human waste. The WHO (1981) suggests that the main pathway to children's infection is soils in which they play, primarily the dirt surrounding their homes. The use of night soil (human fecal waste) as fertilizer is a major way in which Ascaris and Trichuris infections are spread. Connecting these sorts of practices to historic patterns of disease and infection relies upon an understanding of the specific environmental conditions required for successful parasite reproduction.
One explanation for the reduction in the proportion of Trichuris eggs evident in the archaeological data is that the environment changed such that either the development of Ascaris eggs are favored or the conditions more negatively impact Trichuris. Below we consider some of the factors influencing each parasite's development and then discuss the ways that environments changed as they become increasingly urbanized.
Parasite Biology and Environmental Conditions
Ascaris is generally considered the more durable of the two parasites (Hotez et al. Reference Hotez, de Silva, Brooker and Bethony2003; Maya et al. Reference Maya, Ortiz and Jiménez2010), but specific data on the differences between them are generally sparse. Of the two, the environmental tolerances of Ascaris are the better known (Jiménez Reference Jiménez2007), but emphasis is often placed on identifying lethal limits rather than conditions that are optimal for growth, which has led to somewhat ambiguous or contradictory data (Maya et al. Reference Maya, Ortiz and Jiménez2010). Studies on the environmental tolerances of the parasites have generally focused on temperatures needed to inactivate larvae, but soil moisture, humidity, precipitation, sunlight, soil pH, and other physical conditions also affect egg development (Hinz Reference Hinz and Mehlhorn1988). These conditions act synergistically (Maya et al. Reference Maya, Ortiz and Jiménez2010).
Temperature has a major impact on the speed of development and viability of parasite eggs (Pecson et al. Reference Pecson, Barrios, Jiménez and Nelson2007). Ascaris larvae require soil temperatures between 15° and 34°C for development, with 30° to 33°C being optimal (Hinz Reference Hinz and Mehlhorn1988). Temperatures approach lethal levels at 38° or 40°C (Hotez et al. Reference Hotez, de Silva, Brooker and Bethony2003:13) for short periods of time, but longer periods at lower temperatures are also effective. Brown and Cort (Reference Brown and Cort1927) found that Ascaris eggs developed faster than Trichuris eggs, and Nolf (Reference Nolf1932) found that Trichuris was slightly more susceptible to high temperatures than Ascaris. However, a more recent study indicates that optimal temperature for Trichuris egg development is higher than for Ascaris (Bundy and Cooper Reference Bundy and Cooper1989:111). It is clear that data on soil temperatures that promote growth or inactivate the eggs are somewhat contradictory, but these conditions are similar for both parasites.
Other factors impact egg viability but are less understood. Beaver (Reference Beaver1975) found significantly higher mortality of Ascaris and Trichuris eggs in sun-exposed as opposed to unexposed soil. Nolf (Reference Nolf1932) found that Trichuris is more tolerant of ultraviolet light, which might indicate greater resistance to sunlight, but Ndamukong (Reference Ndamukong2005) found that Trichuris is more sensitive to sun exposure than Ascaris. Both parasites are susceptible to drying (Bundy and Cooper Reference Bundy and Cooper1989), but, due to their protective coating, Ascaris eggs are more resistant to drying than Trichuris (Jones Reference Jones, Fieller, Gilberston and Ralph1985; Spindler Reference Spindler1929). In a field study, Spindler (Reference Spindler1929) found that Trichuris infections correlated with soil moisture, the result of yard vegetation that kept the soil moist. Trichuris requires more moisture than Ascaris to begin development (Rudolfs et al. Reference Rudolfs, Falk and Ragotzkie1950) and Ascaris survives longer into the dry season (Ndamukong Reference Ndamukong2005).
Contemporary Ascaris infection rates are higher in urban areas than nearby rural areas (Hotez et al. Reference Hotez, de Silva, Brooker and Bethony2003:11) because the urban environment, along with its higher population densities and more closely packed homes, lends itself to increasing rates of infection. Brooker and colleagues (Reference Brooker, Alexander, Geiger, Moyeed, Stander, Fleming, Hotez, Correa-Oliveira and Bethony2006) found that the microenvironmental conditions, such as the presence of vegetation, humidity, and soil temperature, immediately around homes were the most important factor for transmission, and Ascaris infections, in particular, were acquired locally. A reduction in moisture and an increase in temperature found in urban environments, along with the sticky coating on Ascaris eggs, which helps prevent drying and allows them to adhere to fingers and other objects, may select for Ascaris over Trichuris. Below we consider some of the conditions in cities that may account for an environmental reduction in humidity and an increase in temperature.
Changing Urban Environments and Their Impact on Parasites
Studies of urban ecosystems over the last 50 years document a number of differences between the biogeophysical environments of urban and rural areas (Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). Urban areas are much more patchy environments, with a variety of microclimates. Changes to city environments include alterations in temperature, humidity, soil chemistry, soil permeability, vegetation density, numbers and types of plant and animal species, air quality and particulates (Gilbert Reference Gilbert1989; Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001), and such complex and interrelated processes as organic matter decomposition, water cycling, and soil composition (Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). The factors that appear to be the most important for Ascaris and Trichuris are those that relate to temperature, humidity, the presence of vegetation, and light. These are interrelated, as vegetation cover influences temperature and humidity, and temperature shapes plant species distributions.
A dramatic effect of urbanization is the creation of the urban heat island. Gilbert (Reference Gilbert1989), for example, reports a 5°C difference between adjacent rural areas and the center of London on clear days. The average difference between rural and urban areas ranges between 0.5° and 1.5°C, but this difference is magnified on clear days, when such differences might reach 10 degrees (Gilbert Reference Gilbert1989:25–26). Temperature differentials are not the only factor distinguishing urban from surrounding rural areas. Contemporary cities also have relative humidities that are up to 10 percent lower than surrounding areas (Gilbert Reference Gilbert1989:26), and soils are generally drier (Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001:139). Soil temperature and moisture regimes are important factors in the viability and speed of parasite egg development.
People also dramatically change the types and densities of vegetation (Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). Not only do they intentionally cultivate exotic ornamentals and selectively remove plants that are not desired, but they also introduce alien weeds and alter growing conditions such that some types of plants are enhanced, others inhibited. In addition, people and their activities modify the structure of plant communities, suppressing or altering succession, or creating microclimates that change plant densities and distributions (Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). Urban areas, located on formerly forested lands, tend to have less vegetation (Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). According to Pickett et al. (Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001:136) these impacts, particularly on the flora, depend on a variety of cultural constructions, including building densities and differing land use. In general, there is less vegetation and fewer trees, except where they are deliberately conserved in parks or estates. The nature of vegetation cover, which may directly influence parasite ova development, is also an important factor because it relates to soil temperature and soil moisture.
Although studies of urban ecosystems generally investigate the impacts of urbanization for the last 50 years or so, these conditions are the result of ongoing processes and feedback loops that have influenced the structure of plant and animal communities over the long term. In some places, the urban ecosystem dynamics we see today have developed over hundreds of years. Longer-term evidence for the warming of urban environments is indicated by documentary and ethnographic data showing that certain plants in cities are flowering earlier (Gilbert Reference Gilbert1989; Mrozowski Reference Mrozowski and Staski1987; Pickett et al. Reference Pickett, Cadenasso, Grove, Nilon, Pouyat, Zipperer and Costanza2001). Changes in urban flora and fauna have been documented in Liverpool since the industrial revolution (Greenwood Reference Greenwood1999) and Warsaw since the eighteenth century (Mackin-Rogalska et al. Reference Mackin-Rogalska, Pinowski, Solon and Wojcik1988). These processes were acting as urban areas developed.
The intersection between peoples’ choices and the environment can be seen in the urbanization process as both deliberate interventions and accidental changes with unintended consequences. Both reflect the prevailing perceptions of the time. During the nineteenth century, the ways people thought of the relationship between health and the environment had an impact on urban ecosystems. Szczygiel and Hewitt (Reference Szczygiel and Hewitt2000) argue that the miasmic theory of disease, popular in the mid-nineteenth century, had direct implications for the character of cities. This theory held that the environment was the source of disease. Especially problematic were damp places, mud, swamps, areas with rotting or thick vegetation, and lack of air circulation. This notion led to the perception that urban spaces, especially unkempt urban spaces, were unhealthy. Sanitary and progressive reform movements considered dense foliage and dampness in cities to be two sources of disease and mounted campaigns to rehabilitate such environments (Melosi Reference Melosi2008; Szczgiel and Hewitt Reference Szczygiel and Hewitt2000). Late in the nineteenth century, Fredrick Law Olmstead and others sought to redress society's ills by creating healthy environments (Szczgiel and Hewitt Reference Szczygiel and Hewitt2000) and improving on the aesthetic quality of the landscapes for the good of their communities. The concern about the health particularly in urban spaces eventually led to the parks movement, which created spaces such as Central Park in New York, the Emerald Necklace in Boston, and Riverside Park in Chicago (Szczgiel and Hewitt Reference Szczygiel and Hewitt2000). The emphasis on trees and well-drained landscapes that were shaded, but not excessively so, and landscapes that were manicured, dry, and controlled would have selected for the more resistant Ascaris, which could tolerate the drier conditions and whose sticky outer coating allowed it to adhere better to vegetation.
The population density of urban spaces and the increasing pace of urbanization in the early nineteenth century would also have increased the temperature of cities. While the heating effect alone is unlikely to cause a decline in the proportion of Trichuris, the environmental changes that accompanied the development of densely occupied urban areas would have, as a matter of consequence, reduced vegetation and humidity. These would have produced an environment that was less conducive to parasites, especially the more vulnerable Trichuris.
Cultural Factors Relating to the Change in Parasites: The Use of Space and Sanitation
In addition to the alterations in physical environment that characterize urbanized areas, peoples’ attempts to use or deal with waste altered their exposure to infective parasite ova. Until the late nineteenth century, people used urban yard space for waste and water management, as well as for gardening and the keeping of animals such as pigs (Mrozowski Reference Mrozowski, McGuire and Paynter1991, Reference Mrozowski2006). The use of night soil as fertilizer may also play a role in the transmission of human parasites (Mrozowski Reference Mrozowski2006; Reinhard, Mrozowski, and Orloski Reference Reinhard, Mrozowski and Orloski1986). The use of backlots for food production may have been the source of parasite infection for an entire household.
By the nineteenth century, this practice subsided in urban areas. Mrozowski (Reference Mrozowski2006) found that the backyards of the nineteenth-century boardinghouse lots in Lowell, Massachusetts, were not used for food production and not many parasite ova were recovered (Reinhard Reference Reinhard, Beaudry and Mrozowski1989). When backlots became infrequently used for food production, parasites remained a problem, particularly for younger children who played in the dirt. The change in the use of backlots may have also selected for parasites such as Ascaris, which were more persistent in the soil, could adhere to objects and fingers, and could tolerate the lower level of vegetation that characterized urban backlots. Parasite biology suggests that a reduction in vegetation and the decreased use of yards for food production, along with night soil as fertilizer, would have favored Ascaris eggs even while providing an unfavorable environment for either parasite.
From the late eighteenth century well into the nineteenth century, nightmen used bucket and cart methods to remove human waste from homes and privies in the Northeast (Geismar Reference Geismar1993). Cleaning privies may have introduced eggs into the soil if contents spilled as the privy was being emptied. Fisher and colleagues (Reference Fisher, Reinhard, Kirk and DiVirgilio2007) suggest that changes in privy technology and waste disposal in nineteenth-century Albany resulted in the reduction in parasite loads evident in their data. The use of plumbing and sewerage, as long as it functioned properly, would have helped to reduce parasitic infections, as fecal material and eggs were removed from yards where children played.
By the first quarter of the nineteenth century, cities in the northeast attempted to deal with human waste by systematically removing and compositing it. Beginning around 1840, northeast cities created legislation designed to reduce human waste, sometimes by collecting and dumping it into large piles at the edge of or outside cities (Roberts and Barrett Reference Roberts and Barrett1984). The high internal temperatures of such compost piles can destroy parasite eggs (Jensen et al. Reference Jensen, Phuc, Konradsen, Klank and Dalsgaard2009). By the mid-nineteenth century, human waste was sold as a component of poudrette, a fertilizer made from a combination of dried human waste mixed with charcoal and gypsum (Roberts and Barrett Reference Roberts and Barrett1984). In eastern cities such as Baltimore and Philadelphia, human waste, along with other refuse, was collected in piles, shipped from urban centers, and made into poudrette (Roberts and Barrett Reference Roberts and Barrett1984). The use of poudrette as fertilizer would probably have reduced both parasites but may have favored Ascaris, which is more tolerant of changes to pH and drying.
The transfer of food production from household backlots and the rise of specialized truck farms and larger-scale agricultural production may have also contributed to the reduction in parasites in general and the selection of the more durable Ascaris. The early to mid-nineteenth century saw the removal of food production from the cities to the countryside (Mrozowski Reference Mrozowski2006; Tarr Reference Tarr1975). Shifting food production from urban yards into rural farms may have impacted the parasites in two ways. First, the deliberate use of feces as fertilizer in house lots would have stopped, and parasite eggs would no longer be transferred from produce grown in yards to the table (Mrozowski Reference Mrozowski2006). Second, moving food production outside cities would have increased the time between when parasites are shed and when they would have been consumed. This may have selected for the more hardy Ascaris eggs, but ultimately may have allowed eggs to pass through their infective period without being consumed. The production of poudrette, although a nightsoil product and possibly contaminated with eggs, might likewise have created conditions that were too hot, alkaline, or dry for egg development.
Landscape and behavioral changes intertwined to modify environmental conditions that differentially impacted the health of urban dwellers. Those with the means to adopt better sanitary technology, move food production out of their back lots, or live in areas with more groomed vegetation were less likely to be parasitized. The nature of urban spaces, however, selected for the more dangerous Ascaris parasite, and for the people vulnerable to acquiring such infections, especially children of the urban poor, those infections may have taken an increasing toll.
Conclusions
Archaeoparasitological data from sites in the Northeast provide evidence of the shift from Trichuris trichiura to Ascaris lumbricoides, the two most common human parasites recovered archaeologically. Changes in the nature and use of urban spaces differentially influenced these parasites and corresponded to dimensions of class and status as changes in food production strategies and access to sanitation were influenced by wealth and social standing. In his study of New England towns, Mrozowski (Reference Mrozowski2006) found these changes were foreshadowed in the eighteenth century but became particularly apparent in the nineteenth century. In New York, sewer installation began in 1853, with wealthy areas connected first (Geismar Reference Geismar1993). Serviced last, the urban poor continued their reliance upon privies and cesspools, which may have meant more opportunities to become exposed to parasite eggs. Changes to urban landscapes meant that the overwhelming majority of poor urban dwellers found themselves living in increasingly more densely settled urban enclaves, where sanitation remained essentially “premodern” and did not feel the benefits of improving domestic technologies until the turn of the century or sometime thereafter (Beaudry and Mrozowski Reference Beaudry, Mrozowski, Mayne and Murray2001; Mrozowski Reference Mrozowski, McGuire and Paynter1991, Reference Mrozowski2006). Some households resisted technology, such as indoor plumbing, because their inhabitants thought that sewer gases contributed to disease and because they considered it an unwanted governmental intrusion into private affairs (Ford Reference Ford1994). Such beliefs may have contributed to the continued presence of parasites and uneven distribution of parasite loads among households.
Cultural practices, such as changing the use of space around homes, where most parasite infections are acquired, for food production, waste disposal, or ornamental purposes, may have reduced the transmission of parasites for those households with the ability to make such changes. Likewise, deliberate attempts to address health problems—removal of waste outside of cities, the use of sewers, and the creation of highly managed parks, which changed vegetation structure, would have reduced the exposure to parasites or created environments that did not favor their development.
Among the greatest impacts are both the inadvertent and deliberate changes to the distribution of vegetation, which may impact parasite viability and transmission. The nature of changing urban conditions, while affecting the transmission of both, appears to be more detrimental to Trichuris. Given the biology and environmental tolerances of the parasites, the increasing heat and dryness of urban areas may have selectively culled Trichuris eggs that were in the soil. Ascaris eggs may have been better able to survive urbanizing environments and, therefore, more likely to have been able to cause infections. Another characteristic of urban environments is the presence of highly varied microclimates. Such variation may provide local reservoirs for Trichuris ova and may influence the diversity of parasite loads from individual to individual or household to household, thus accounting for the continued presence of Trichuris infections and variability that we see in the archaeological record at different sites.
The shift from Trichuris to Ascaris has implications for the health of those vulnerable to parasite diseases. The acquisition of parasites from the immediate area in and around the house would have meant that poorer households, especially the children, would more likely have been exposed than wealthier households (Mrozowski Reference Mrozowski, McGuire and Paynter1991, Reference Mrozowski2006). Brooker et al. (Reference Brooker, Alexander, Geiger, Moyeed, Stander, Fleming, Hotez, Correa-Oliveira and Bethony2006) notes that contemporary parasite burdens are uneven, with a few heavily infected individuals often contributing the most to community parasite loads. These conditions, and the shift to the more dangerous Ascaris, may have played a role in the perception of densely inhabited urban areas as unhealthy and unsanitary. In the nineteenth century, such portrayals were perpetrated for political purposes (Mayne Reference Mayne1993; Yamin Reference Yamin2000), but the environmental reality of urban spaces may have nonetheless aided in this view, as urban children may have been at greater risk for serious disease. Children playing outdoors, especially if sickly because of parasites, may have provided a visible indication of the health of the neighborhood.
As data presented by Fisher and colleagues (Reference Fisher, Reinhard, Kirk and DiVirgilio2007) and our broader analysis suggest, it may well be that human populations are becoming increasingly less parasitized through time. Yet today's world remains one in which class and wealth continue to impact the biological well-being of many, especially those living in cities. Parasitological data from current populations illustrate that Ascaris in particular remains a problem in poor urban areas that lack modern sanitation (Hotez et al. Reference Hotez, de Silva, Brooker and Bethony2003:11). The archaeoparasitological data reveal the intricate way that social and biological variables intersected to create landscapes of inequality. Nineteenth-century slums may have been a political construction, but biological realities influenced the types of parasites people carried. Parasite data reveal the intimate nature of biological patterns that contribute to the inequities that were a critical part of nineteenth-century urbanization. At an even broader level, these data reflect the way large-scale human action can affect the close interaction of parasites and their human hosts. One of the more profound characteristics of urbanization was the shift from mixed commercial-residential areas that were economically diverse to more homogeneous but more rigidly class-differentiated neighborhoods in which social inequalities increasingly resulted in inferior living conditions for the poor, patterns we would today label as examples of environmental and spatial injustice (Soja Reference Soja2010). The spatially uneven but nevertheless accelerated quality of the changes revealed in the parasite data point to the close relationship between humans and their surroundings. They also suggest trends that could prove more troublesome in the future as more and more people live in cities.
Acknowledgments
The authors are indebted to Karl Reinhard, Pathoecology and Palynology Lab, School of Natural Resources, University of Nebraska, Lincoln, who collected a considerable percentage of data used in this paper and graciously offered additional data. His extensive comments greatly improved the manuscript.
Data Availability Statement
All data used in this study are presented here.








