The commercial practice of abrupt weaning exposes piglets to several environmental and nutritional challenges, including the absence of sows, new littermates, and changes in the source and delivery of nutrition( Reference Pluske, Hampson and Williams 1 ). Among these, the rapid dietary transition from liquid milk to solid feed principally disrupts digestive processes, temporarily impeding growth performance. However, frequently the weaning process results in a physiological damage to the gastrointestinal tract as microbial pathogens take advantage of a turbulent immune status. The consequences of this microbial dysbiosis are poor nutrient utilisation, faecal scouring, increased number of faecal coliforms such as Escherichia coli, and impaired feed conversion efficiency (FCE).
The practice of in-feed antibiotic inclusion was popular as a means of buffering the weaning transition in piglets to overcome the disruption of growth performance after weaning. However, concerns of increased microbial tolerance to antibiotics eventually resulted in a ban on prophylactic antibiotic use, upheld in the European Union since 2006. Consequently, the prescribed inclusion of ZnO at rates above nutritional requirements for the maintenance and growth of pigs has increased gradually, in an attempt to contain the aforementioned problems associated with weaning by pig producers. The biological activity of Zn and associated compounds is diverse( Reference Vallee and Falchuk 2 ). For example, in enterocytes, ZnO exerts a protective effect, inhibiting the adherence of and invasion by E. coli ( Reference Roselli, Finamore and Garaguso 3 ). In piglets, the mechanisms by which ZnO modulates the gastrointestinal tract during the weaning transition period include modifications to the inflammatory transcriptome during chronic inflammation( Reference Sargeant, McDowall and Miller 4 ) and also complex interactions with the profile and metabolism of the gut microbiota( Reference Molist, Hermes and de Segura 5 , Reference Højberg, Canibe and Poulsen 6 ). However, concerns have been mooted about the accumulation of Zn in pig manure-applied soils( Reference Buff, Bollinger and Ellersieck 7 , Reference Meyer, Lindemann and Cromwell 8 ), and this may restrict ZnO use in the future.
Thus, considerable efforts are being made to identify suitable alternatives that can form natural components of the starting diet and promote growth performance. The dietary inclusion of seaweed extracts (SWE) from brown algae containing predominately the polysaccharides laminarin and fucoidan has shown considerable promise in stimulating body weight gain and FCE in piglets during the weaning period( Reference O'Doherty, Dillon and Figat 9 , Reference Gahan, Lynch and Callan 10 ). Laminarin consists of a group of hexose polymers comprising a β-(1 → 3)-linked glucan backbone with intermittent β-(1 → 6)-linked glucan side chains. In contrast, fucoidan consists of a group of complex and heterogeneous sulphated polysaccharides, typically having the hexose fucose as a repeating unit( Reference Berteau and Mulloy 11 ). The favourable improvements in growth performance following consumption of SWE can be observed in tandem with greater nutrient utilisation, reductions in coliform bacterial counts and faecal scouring, and greater abundance of lactic acid bacteria( Reference O'Doherty, Dillon and Figat 9 ). The physiological effects of SWE reflect the bioactive properties of laminarin and fucoidan. Laminarin has been reported to modify the gastrointestinal physiology of pigs, influencing the expression of genes involved in cytokine and mucin production during weaning, with a reduction in the faecal counts of Enterobacteriaceae and E. coli ( Reference Smith, O'Doherty and Reilly 12 , Reference Reilly, O'Doherty and Pierce 13 ). Fucoidan has been shown to exert a lactic acid bacteria-promoting effect, which may contribute to nutrient digestibility( Reference O'Doherty, Dillon and Figat 9 , Reference Swanson, Grieshop and Flickinger 14 ).
Pigs do not produce a suite of endogenous enzymes capable of hydrolysing complex NSP. Therefore, the mechanism whereby SWE containing laminarin and fucoidan stimulate gastrointestinal structure and function and contribute to growth performance in the post-weaning pigs is probably mediated through interactions with the microbiota. Thus, as the dietary inclusion of pharmacological doses of ZnO has pronounced inhibitory effects on microbial growth and abundance in the gastrointestinal tract( Reference Højberg, Canibe and Poulsen 6 ), it is difficult to predict the physiological response of pigs to SWE in the presence of dietary ZnO. The objective of the present study was to evaluate the effect of dietary SWE when compared with that of ZnO and investigate the effect of the interaction between SWE and ZnO on growth performance and selected nutrient digestibility and faecal variables in newly weaned piglets.
Materials and methods
In the present study, all the experimental procedures were carried out under experimental licence from the Irish Department of Health in accordance with the Cruelty to Animals Act 1876 and the European Communities (Amendments of the Cruelty to Animals Act, 1876) Regulations (1994).
Animal management and experimental dietary treatments
The growth performance study commenced at weaning (0 d) and concluded on day 40 of weaning. The experimental period consisted of two phases separated by a diet change: the dietary treatments started with a starter diet followed by a transition diet from 21 d to accommodate changing nutrient requirements. A 2 × 2 factorial design of the experimental dietary treatments was employed throughout the study (0–40 d) comprising SWE inclusion (yes or no) and ZnO inclusion (yes or no). The dietary treatments were as follows: (1) control diet; (2) control diet+ZnO; (3) control diet+SWE; (4) control diet+ZnO+SWE. A total of 192 piglets (progeny of Large White × (Large White × Landrace)) weaned at 24 (sem 2) d, with a starting body weight of 6·5 (sem 0·8) kg, were penned (fully slatted 1·68 m × 1·22 m) in groups of 4 on the basis of litter of origin, starting body weight and sex. The pen served as the experimental unit (n 12), and one of the four dietary treatment groups was assigned to each pen. The inclusion rates of ZnO (Zincotec, Provimi) were 3·1 and 2·5 g/kg in the starter diet and transition diet, respectively, and were derived from the study of Carlson et al. ( Reference Carlson, Hill and Link 15 ) and Davis et al. ( Reference Davis, Brown and Maxwell 16 ). The SWE used were extracted from the brown algae spp. Laminaria (Bioatlantis) and were formulated to contain 300 mg/kg laminarin and 240 mg/kg fucoidan per kg of diet, based on previous optimisation data( Reference Gahan, Lynch and Callan 10 , Reference Reilly, O'Doherty and Pierce 13 ). The SWE contained 455 mg laminarin/g, 360 mg fucoidan/g, 90 mg crude protein/g and 50 mg crude ash/g and were included in the relevant dietary treatments at a rate of 660 mg/kg diet. All the diets were given in meal form for 40 d after weaning. The starter diet was formulated to contain 1·3 % standardised ileal digestible lysine and 15 MJ digestible energy/kg, and the transition diet was formulated to contain 1·1 % standardised ileal digestible lysine and 14 MJ digestible energy/kg. All additional amino acid requirements were met relative to lysine( Reference Sauvant, Perez and Tran 17 ). Pen temperature was maintained at 30°C for the first week after weaning and then reduced by 2°C each week. Body weight was recorded at the beginning of the experiment (day of weaning = 0 d) and on days 7, 14, 21, 28, 35 and 40. The piglets were fed ad libitum using a four-space feeder with precautions taken to avoid feed wastage. Water was available ad libitum from nipple drinkers. Feed was available up to weighing, and then uneaten feed was weighed for the purpose of calculating FCE.
Faecal scoring
The piglets were observed for clinical signs of diarrhoea from 0 to 40 d of the experiment by a single blinded operator, and a scoring system was applied to indicate the presence and extent of severity( Reference Pierce, Callan and McCarthy 18 ). Faecal scoring began on day 0 on the experimental diets and continued daily until day 40. Scores were obtained for individual pens, and the average faecal score per pen was calculated. The following scoring system was used: 1, hard firm faeces; 2, slightly soft faeces; 3, soft, partially formed faeces; 4, loose, semi-liquid faeces (diarrhoea); 5, watery, mucus-like faeces (severe diarrhoea).
Laboratory analysis
Representative feed samples were collected at regular intervals throughout the experimental period. Multiple fresh faecal samples were collected daily from all the pens (n 12 pen per treatment) from day 10 to day 15 and either promptly frozen at − 20°C for microbial and SCFA analyses or dried as described below for chemical analysis. The DM content of the feed and faecal samples was determined after drying at 103°C for 16 h. For composition analysis, feed and dried faecal samples were milled through a 1 mm screen (Christy and Norris Hammer Mill). The crude ash content of the feed and faecal samples was determined using a muffle furnace (Nabertherm) at 500°C for 4 h. The crude protein content of fresh faecal samples was calculated from Kjeldahl N (N × 6·25) by the macro-Kjeldahl technique (Buchi digestion (K-437) and distillation (K-360) apparatus, Buchi Labortechnik) and that of the feed samples using the LECO FP 528 instrument (Leco Instruments, UK Limited). Neutral-detergent fibre (NDF) content was determined using a fibretec extraction unit (Tecator). The gross energy content of the feed and faecal samples was determined using a Parr 1201 oxygen bomb calorimeter (Parr). The total laminarin content of the SWE and feed was determined using a commercial assay kit (K-YBGL; Megazyme International Ireland Limited) as described by Leonard et al. ( Reference Leonard, Sweeney and Bahar 19 ). Fucoidan content was determined using the method of Usov et al. ( Reference Usov, Smirnova and Klochkova 20 ). Briefly, this procedure involved acid hydrolysis (0·2 m-HCL) of the ground algal biomass, decolourisation of the acid extract using sodium chlorite, and spectrophotometric determination of fucoidan content after the removal of inorganic salts by dialysis. Profiling of SCFA was carried out using thawed faecal samples by GC( Reference O'Connell, Callan and O'Doherty 21 ). The acid-insoluble ash technique was used for the determination of nutrient digestibility( Reference McCarthy, Bowland and Aherne 22 ).
Microbial analysis
Microbial genomic DNA was extracted from the faecal samples using a QIAamp DNA stool kit (Qiagen) in accordance with the manufacturer's instructions. The quantity and quality of DNA were assessed using a Nanodrop apparatus (ND1000, Thermo Scientific). For the real-time PCR assay, standard curves were prepared with pooled aliquots of faecal DNA as described previously( Reference O'Shea, Sweeney and Bahar 23 ) and used for the absolute quantification of bacteria based on gene copy number( Reference Lee, Kim and Shin 24 ). Genus- and species-specific primers (Table 2) were used for the estimation of select bacterial groups in the faecal matter using quantitative real-time PCR on the ABI 7500 Real-Time PCR System (Applied Biosystems Limited). For bacterial groups, real-time PCR were carried out at a final reaction volume of 20 μl containing 1 μl of template DNA, 1 μl of forward and reverse primers (100 pm), 10 μl of SYBR Green PCR Master Mix (Applied Biosystems Limited) and 8 μl of nuclease-free water. The thermal cycling conditions involved an initial denaturation step at 95°C for 10 min followed by forty cycles of 95°C for 15 s and 65°C for 1 min. Dissociation analyses of the PCR products were carried out to confirm the specificity of the resulting PCR products. The mean threshold cycle values from the triplicate of each sample were used for calculations. The estimates of gene copy numbers for select bacteria were log-transformed, and they are presented as gene copy numbers/g faeces.
Table 1 Composition and chemical analysis (g/kg) of the starting diet and transition diet, as fed

SWE, seaweed extract.
* SWE provided 300 mg/kg of laminarin and 240 mg/kg of fucoidan in the both starter and transition diets.
† ZnO included at 3·1 and 2·5 g/kg in the starter and transition diets, respectively.
‡ Unless otherwise indicated.
§ Lactofeed 70 (source of lactose approximately 70 %; Volac Limited).
∥ Provided in the starter diet (mg/kg): Cu 175; Fe 140; Mn 47; Zn 120; I 0·6; Se 0·3; retinol 1·8; cholecalciferol 0·025; tocopherol 67; menaquinone 4; cyanocobalamin 0·01; riboflavin 2; nicotinic acid 12; pantothenic acid 10; choline chloride 250; thiamin 2; pyridoxine 0·015. Provided in the transition diet (mg/kg): Cu 175; Fe 140; Mn 47; Zn 120; I 0·6; Se 0·3; retinol 1·8; cholecalciferol 0·025; tocopherol 67; menaquinone 4; cyanocobalamin 0·01; riboflavin 2; nicotinic acid 12; pantothenic acid 10; choline chloride 250; thiamin 2; pyridoxine 0·015.
Table 2 Oligonucleotide primers used for real-time PCR to profile select bacteria in the faecal samples

* Annealing temperature.
Statistical analysis
All the data were checked initially for normality using the univariate procedure of the Statistical Analysis System Institute (SAS)( 25 ). The experimental data were analysed as 2 × 2 factorial using the general linear model procedure of SAS( 25 ). The statistical model used included the main effects of SWE and ZnO and the associated interaction between SWE and ZnO. The individual pen represented the experimental unit. The growth performance data were adjusted for initial body weight by covariance analysis. Where not significant, covariance adjustment was excluded from the model. The microbial counts were log-transformed before statistical analysis. Pearson's correlation coefficients among the growth performance measurements were determined using the correlation procedure of SAS( 25 ). The probability level denoting significance was P< 0·05. The data are presented as least square means with their standard error of the mean.
Results
Animal growth performance
Adjustment for initial body weight as a covariate was significant for average daily feed intake from day 0 to day 7 (P= 0·043) and for average daily body weight gain from day 0 to day 7 (P= 0·050) (Table 3).
Table 3 Effect of seaweed extracts (SWE) and zinc oxide inclusion on average daily feed intake, average daily body weight gain and feed conversion efficiency (Least square means with their standard errors)

There was no significant effect of the starter diet (0–21 d) containing SWE or ZnO either alone or in combination on the average daily feed intake, average daily body weight gain or FCE of the piglets. Average daily gain was not significantly affected by the dietary treatments during the transition diet period (21–40 d).
There was an interaction between SWE and ZnO supplementation on the average daily feed intake of the piglets from day 28 to day 40 (P= 0·023) and during the entire transition diet period (21–40 d; P= 0·016). There was no effect of diets containing SWE on average daily feed intake when compared with the control diet. However, when piglets were fed the diet containing SWE in combination with ZnO, feed intake increased significantly.
There was an interaction between SWE and ZnO supplementation on the FCE of the piglets from day 21 to day 28 (P= 0·048), from day 28 to day 40 (P= 0·009), and during the entire transition diet period (P= 0·005). Piglets fed the diet containing only SWE or ZnO had improved FCE compared with those fed the control transition diet. However, there was no effect of SWE on the FCE of the piglets when given in combination with ZnO.
Coefficient of total tract apparent digestibility
There was an effect of the interaction between SWE and ZnO supplementation in the starter diet on the coefficient of total tract apparent digestibility (CTTAD) of organic matter (P= 0·001), N (P= 0·001), NDF (P= 0·001), crude ash (P= 0·001) and gross energy (P= 0·001) (Table 4). Piglets fed the diet containing only SWE had a greater CTTAD of organic matter, N, NDF, crude ash and gross energy compared with those fed the control diet. However, there was no effect of SWE on CTTAD when given in combination with ZnO.
Table 4 Effect of seaweed extracts (SWE) and zinc oxide inclusion on total tract apparent digestibility, faecal DM and faecal scores (Least square means with their standard errors)
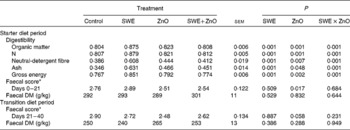
* 1 = hard faeces progressing to 5 = watery, mucus-like faeces.
Faecal scores
Piglets fed diets supplemented with ZnO had a reduced faecal score during the starter diet period (0–21 d; P= 0·017) and during the transition diet period (21–40 d; P= 0·050) when compared with those fed diets without ZnO supplementation. There were no significant effects (P>0·05) of SWE and ZnO inclusion on faecal DM.
Microbial and SCFA analyses
There was an effect of the interaction (P= 0·022) between SWE and ZnO supplementation in the transition diet on the faecal counts of E. coli (Tables 5 and 6). Piglets fed diets containing SWE had decreased faecal counts of E. coli when compared with those fed the control diet; however, this effect was not observed when piglets were fed the diet containing both SWE and ZnO. There was an effect of the interaction between SWE and ZnO supplementation on the ratio of Lactobacilli: E. coli in the faeces of piglets fed the starter diet (P= 0·050) and the transition diet (P= 0·050). There was no effect of the diet containing only SWE when compared with the control diet on this ratio. However, when piglets were fed the diet containing both SWE and ZnO, the Lactobacilli:E. coli ratio in the faeces was significantly decreased. Piglets fed the transition diet containing SWE had reduced faecal counts of Lactobacillus sp. (P= 0·001) when compared with those fed the transition diet containing no SWE. Piglets fed the starter diet containing ZnO had lower concentrations of total SCFA (P= 0·020), acetic acid (P= 0·010), isovaleric acid (P= 0·030), valeric acid (P= 0·040), and short, branch-chained fatty acids+valeric acid (P= 0·020) when compared with those fed the starter diet containing no ZnO. Piglets fed the transition diet containing ZnO had lower concentrations of isobutyric acid (P= 0·027) when compared with those fed the transition diet containing no ZnO.
Table 5 Effect of seaweed extracts (SWE) and zinc oxide inclusion on faecal SCFA concentrations (Least square means with their standard errors)

Table 6 Effect of seaweed extracts (SWE) and zinc oxide inclusion on faecal counts of bacteria (Least square means with their standard errors)

rRNA, ribosomal RNA.
Discussion
Recent studies demonstrate that dietary SWE containing laminarin and fucoidan can stimulate body weight gain and improve FCE and thus offer a means to maintain progressive growth in piglets after weaning( Reference O'Doherty, Dillon and Figat 9 , Reference Reilly, O'Doherty and Pierce 13 ). However, in tandem with the reduction in antibiotic use, greater usage of therapeutic doses of ZnO has occurred( Reference Dybkjær, Jacobsen and Tøgersen 26 ). The objective of the present study was to evaluate the effect of dietary SWE when compared with that of ZnO and investigate the effect of the interaction between SWE and ZnO on the growth performance and selected nutrient digestibility and faecal variables of newly weaned pigs.
The experimental period spanned 40 d after weaning consisting of two dietary phases to accommodate the evolving nutritional requirements of piglets as they progressed from the weaning to the transition growth period. No effect of experimental treatments was observed on growth performance variables during the starter diet period. This probably reflects the composition of the starter diet when compared with the transition diet (Table 1). The starter diet contained substantial quantities of milk by-products and lactose-rich ingredients, which may have stimulated optimal growth conditions in the immediate post-weaning period. Comparable complex rations have been shown to induce greater growth performance when compared with simpler cereal and protein by-product dietary regimens( Reference Dritz, Owen and Nelssen 27 ), although there is a greater feed formulation cost associated with the former that must be reconciled. In comparison with the starter diet, the transition diet primarily comprised a wheat/barley and soyabean meal admixture and notably did not contain milk by-product ingredients. During the entire transition diet period, supplementation with either SWE or ZnO improved FCE by 14·5 and 13·3 %, respectively, than when feeding the control diet, demonstrating the capacity for either supplement to singly promote feed utilisation. However, when the diet containing SWE was fed in combination with ZnO, FCE remained unchanged in the transition diet period when compared with that observed after feeding the control diet. Conversely, during the same period, an interaction effect was observed, whereby the feed intake of piglets fed the diet containing both SWE and ZnO was greater by 17 % when compared with that of piglets fed the diet containing only SWE (Table 3), which equated to approximately 3 kg of extra feed consumed for the duration of the transition diet period. These alternating effects of SWE and ZnO in improving FCE when given alone and in increasing feed intake when given in combination are promising evidence in the context of stimulating greater growth performance in young piglets. Disrupted appetite and poor feed utilisation are well-defined events that characterise the post-weaning period( Reference Pluske, Hampson and Williams 1 ). However, to provide some insight into the possible practical improvements in feed intake and FCE in response to experimental treatments Pearson's correlations were executed to determine the statistical dependence of body weight gain on feed intake and FCE during the transition diet period. Interestingly, feed intake during the transition diet period was not statistically associated with body weight gain during the same period (r 0·18), while FCE during the same period was positively associated with body weight gain (r 0·47; P< 0·05). This implies an imperative towards stimulating the FCE rather than feed intake during the transition diet period towards improving body weight gain.
To assess the impact of experimental dietary regimens on feed utilisation, total tract apparent nutrient digestibility was determined during 10–15 d of the starter diet period, a time frame during which the growth of piglets may be suppressed and persistence of diarrhoea may be observed( Reference Taras, Vahjen and Macha 28 ) (Table 4). Supplementation with dietary SWE alone increased N digestibility by 8·9 % and organic matter by 8·8 % when compared with the control diet. This constitutes a tangible improvement in nutrient digestibility and corroborates the findings of previous studies where greater nutrient digestibility in response to dietary SWE supplementation has been observed( Reference O'Doherty, Dillon and Figat 9 ). This increase in nutrient digestibility probably reflects an alteration in digestion and absorption dynamics in the small intestine, where N and organic matter are predominately captured by the host( Reference Just, Fernández and Jørgensen 29 , Reference Wilfart, Montagne and Simmins 30 ). Supporting this premise, recent studies carried out by this research group in which SWE has been incorporated into piglet diets have reported increased villous height in the duodenum( Reference Walsh, Sweeney and O'Shea 31 ) and an increased villous height:crypt depth ratio in the jejunum( Reference Leonard, Sweeney and Bahar 19 ) when compared with the controls, events which are involved in the retention of the structure and function of the small intestine( Reference Pluske, Thompson and Atwood 32 ). The improvement in nutrient digestibility is worth noting, for while there was no effect of experimental treatments on growth performance during the starter diet period, it suggests a prompt biological response to dietary supplementation with SWE, which can occur at least approximately 10 d after weaning. This greater nutrient capture may contribute to the FCE of the animal during the post-weaning period.
Similar to the observed response of FCE to the co-consumption of SWE and ZnO during the transition diet period, N and organic matter digestibility was markedly lower when the diet containing both SWE and ZnO was fed to the piglets. To elaborate on this interaction between SWE and ZnO, the effect of experimental treatments on the total tract digestibility of crude ash and NDF was also determined. The assessment of the digestibility of these dietary components is informative, as dietary minerals may be absorbed at various sites along the gastrointestinal tract, including the large intestine( Reference Wilfart, Montagne and Simmins 30 , Reference Walsh, Sweeney and O'Shea 33 ), while NDF, which largely comprises hemicelluloses, such as arabinoxylans, and cellulose( Reference Zijlstra, Lange and Patience 34 ), is mostly fermented by the post-ileal enteric microbiota( Reference Wilfart, Montagne and Simmins 30 ). A pronounced effect of the interaction between dietary SWE and ZnO was observed on crude ash and NDF digestibility, whereby supplementation with dietary SWE alone increased crude ash digestibility by 82·4 % and NDF digestibility by 57·5 %, an effect which was again markedly diminished when SWE were given in combination with ZnO. Because piglets lack endogenous enzymes to effectively hydrolyse the complex polysaccharides of the NDF fraction and rely instead on mutualism with the enteric microbiota to achieve this, the increase in NDF digestibility suggests a stimulating effect on the metabolic activity of saccharolytic microbes in response to dietary SWE supplementation alone. Inversely, that NDF digestibility decreased substantially on feeding the diet containing both SWE and ZnO points to a change in fermentation dynamics in the large intestine of this experimental treatment group.
To explain this concept, profiling of SCFA in the faecal matter was carried out. The SCFA are by-products of carbohydrate and protein degradation following microbial fermentation, thus SCFA concentrations and relative proportions provide important insights into microbial activity in the large intestine( Reference Macfarlane, Macfarlane and Cummings 35 , Reference Marounek, Adamec and Skivanova 36 ). Interestingly, piglets fed diets containing ZnO during the starter diet period had reduced total SCFA concentrations per g of faecal matter. This decrease in total SCFA concentrations in the faecal matter is largely due to a decrease in the concentration of acetic acid, the principal anion found in faeces, and also reflects a reduction in valeric acid and isovaleric acid concentrations. Acetic acid is derived from the fermentation of both proteins and carbohydrates, while the latter SCFA derive from the fermentation of only proteins. The reduction in the concentrations of several protein- and carbohydrate-derived SCFA implies that microbial activity on various fermentable substrates was quelled in response to dietary ZnO, given the close stoichiometric relationship of SCFA production with other microbial fermentation processes( Reference Blümmel, Aiple and Steingaβ 37 ). Previously, Højberg et al. ( Reference Højberg, Canibe and Poulsen 6 ) have reported a reduction in microbial activity as assessed by ATP accumulation in the digesta of pigs fed diets containing doses of ZnO comparable to those used in the present study. Further evidence of the altered faecal content of piglets fed diets containing ZnO was observed in the present study when the faecal matter was scored for consistency. While the assessment of faecal consistency indicated that none of the experimental treatment groups had diarrhoea throughout the experimental period (range from 2·48 to 2·9 equating to slightly soft faeces; Table 4), dietary supplementation with ZnO reduced faecal scores during the starter diet and transition diet periods. Importantly, faecal DM content remained comparable across the dietary treatment groups, indicating that dietary ZnO decreased faecal moisture. A reduction in faecal moisture as a consequence of dietary ZnO supplementation has been observed previously in piglets during the weaning-growing phase( Reference Hill, Cromwell and Crenshaw 38 , Reference Owusu-Asiedu, Nyachoti and Marquardt 39 ). Recently, Medani et al. ( Reference Medani, Bzik and Rogers 40 ) have contributed towards our understanding of the underlying mechanism of this effect, demonstrating that Zn attenuates chloride secretion in colonic mucosa. The attenuation of chloride secretion reduces consequent fluid secretion, contributing to the dehydration of digesta in the lumen.
Thus, that dietary ZnO can impede microbial activity, dehydrating digesta and reducing faecal moisture, has been relatively well established, both in the present study and in the aforementioned supporting work. This biological effect of ZnO, both when given alone and when given in combination with other substances, provides an important insight into the interaction between SWE and ZnO in the present study. Accumulating evidence on the effect of dietary SWE on the distal gastrointestinal tract of the newly weaned piglets points to the consequences for mucosal processes( Reference Smith, O'Doherty and Reilly 12 ), inflammatory cytokine gene expression and, importantly, colonic microbiota modification( Reference Walsh, Sweeney and O'Shea 31 ). These effects may have bearing on the health of piglets and have been aligned with improvements in animal growth performance( Reference Walsh, Sweeney and O'Shea 33 , Reference McDonnell, Figat and O'Doherty 41 ). This capacity of SWE to modify both the function of the distal gastrointestinal tract and its contents reflects the principal SWE components, the polysaccharides laminarin and fucoidan, that are not hydrolysed by the host and thus come in contact with the enteric microbiota. However, it is evident from the present study that the biological effects observed when SWE were given alone were not apparent in the unique environment induced by the consumption of pharmacological doses of ZnO. This absence of an effect in the combination treatment may reflect the dehydrating effect of dietary ZnO and decreased faecal SCFA concentrations, pointing to altered microbial activity and perhaps SWE functionality loss in the distal gastrointestinal tract.
To develop this theory further, select microbial populations in the faecal matter were enumerated. Lactobacillus spp. and E. coli populations and their comparative ratio were chosen based on tentative relationships with gastrointestinal tract function( Reference Casey, Gardiner and Casey 42 ) and pathology( Reference Heo, Kim and Hansen 43 ), respectively. During the transition diet period, the diet containing only SWE induced a 30-fold decrease in E. coli counts in the faecal matter. Recent research carried out by this group provides evidence that this SWE-induced effect on E. coli is due to the effect of both the laminarin and fucoidan components of SWE( Reference Walsh, Sweeney and O'Shea 31 ). However, the effect of SWE on these coliforms was not observed in the faecal matter of piglets fed either the starter diet or the transition diet in combination with ZnO. In addition, the Lactobacillus spp.:E. coli ratio, which serves as a useful single indicator of the relative proportions of these two microbial groups( Reference Castillo, Martín-Orúe and Manzanilla 44 ), was higher in the faecal matter of piglets fed the diet containing only ZnO, but was diminished in that of piglets fed the diet containing ZnO and SWE. This dichotomy in growth performance, nutrient digestibility and faecal microbiological measurements between single and combined consumption of the experimental diets points to the antagonism or at least negation of the biological activity of SWE when given along with ZnO. Debon & Tester( Reference Debon and Tester 45 ) have shown the capacity of complex polysaccharides to chelate with various minerals in vitro, in physiological conditions comparable with the distal gastrointestinal tract. While speculative, this tendency for complex formation may have occurred in piglets fed the combination diet containing SWE and ZnO in the present study, impeding the biological activity of either dietary supplement. Interestingly, Becker et al. ( Reference Becker, Osterloh and Schäfer 46 ) reported a decrease in Zn absorption in rats in response to jejunal infusions of fucoidan and ascribed this to complex formation between the mineral and the polysaccharide.
In summary, the supplementation of SWE and ZnO in post-weaning diets had different biological effects in the weaning-growing piglets as assessed by nutrient digestibility and faecal composition and faecal consistency. Dietary supplementation with SWE alone improved N, organic matter, ash and NDF digestibility during the starter diet period and FCE during the transition diet period, with these improvements being completely negated when SWE was given in combination with ZnO. The addition of ZnO improved the FCE during the transition diet period when given alone and reduced faecal scores throughout the experimental period and faecal SCFA concentrations during the starter diet period when given either alone or with SWE. Dietary SWE supplementation had no effect on SCFA concentrations in the faecal matter. The dietary inclusion of SWE alone reduced the faecal counts of E. coli during the starter diet period, but again this effect was not observed when SWE was given in combination with ZnO. Thus, in the present study, SWE or ZnO when given alone led to similar improvements in FCE during the transition diet period, although this was accompanied by contrasting biological activity. The combination of SWE and ZnO negated any improvements observed in growth performance, nutrient digestibility or microbiological measurements.
Acknowledgements
The authors thank Bernie Flynn, Paddy Reilly and Denise Cunningham for their technical assistance.
The present study was funded by Enterprise Ireland and BioAtlantis Limited under the Innovation Partnership Programme.
Neither Enterprise Ireland nor BioAtlantis Limited had a role in the design and analysis of the present study or in the writing of this article. None of the authors has any conflicts of interest.








