Heat undissipated from high-power electronics reduces the lifetime and efficiency of the devices. Materials with high thermal conductivities, such as diamond and graphite, could help shed heat. However, the cost of synthetic diamond and the low thermal conductivity of graphite normal to the layer plane of the material limit their utility, so alternative materials are needed. A trio of independent thermal-conductivity measurements on high-quality crystals of boron arsenide (BAs) now confirms predictions of the semiconductor’s high thermal conductivity, κ, as recently reported in Science (doi:10.1126/science.aat5522, 10.1126/science.aat7932, 10.1126/science.aat8982).
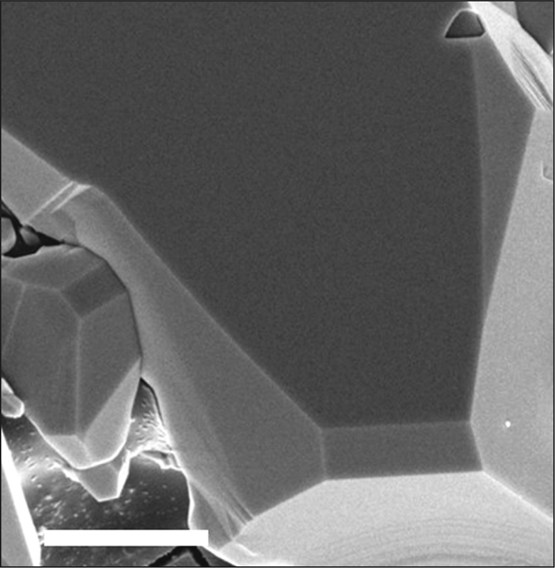
Scanning electron microscope image of a single-crystal boron arsenide. Scale bar is 5 µm. Credit: Science.
BAs was first predicted to have a thermal conductivity as high as that of diamond or graphite—about 2000 W/m∙K—in 2013. This was surprising at the time because BAs does not contain light atoms packed in a simple crystal, features classically thought to be important for materials with high thermal conductivity. But the calculations of the fundamental vibrational properties in BAs suggested several reasons for its high thermal conductivity: bunching of optical phonons, the quasi-particles that carry heat as vibrations through a crystal lattice, and a large frequency gap between acoustic and optical phonons suppresses scattering. But when researchers grew BAs crystals, they measured thermal conductivities roughly 10 times lower than predicted. It was thought that point defects and grain boundaries in the crystals scattered phonons and reduced the material’s actual thermal conductivity.
Over the past few years, researchers refined their crystal-growing methods for BAs, overcoming challenges with working with solids that have high melting points and the tendency of BAs to form a polycrystalline phase around 920°C.
Three teams independently synthesized very pure BAs crystals using chemical vapor transport. They sealed solid boron and arsenide, along with a vapor-transport agent such as iodine, inside a quartz tube and heated one end of the tube to vaporize the solids. The gaseous atoms traveled to the other side of the tube, held at a cooler temperature. Pure BAs crystals, 500 µm to 4 mm wide, slowly grew from a single nucleation site over weeks to months.
The researchers characterized the purity of their crystals using various x-ray and electron diffraction techniques, along with electron microscopy methods. They measured thermal conductivity at various locations in the crystals using time-domain thermoreflectance measurements, a technique that can measure thermal conductivity with 10 µm resolution.
The cross-study average thermal conductivity was 1140 W/m∙K, which was within ±15% of the predicted value of 1250 W/m∙K. A team led by David Broido, at Boston College; Li Shi, at The University of Texas at Austin; Gang Chen, at the Massachusetts Institute of Technology; and Zhifeng Ren, at the University of Houston, synthesized one of the pure crystals, measured its thermal conductivity, and updated its theoretical conductivity. The most recent prediction accounts for four-phonon interactions, rather than the lowest-order three-phonon interactions first used in the calculations.
The theory and the experiments studying BAs thermal conductivity have coevolved, says Yongjie Hu, who led a team of researchers at the University of California, Los Angeles. “This is a good example of combining first-principles modeling and experiments for new materials discovery,” Hu says.
With measured BAs thermal conductivity verifying the prediction, “now we know that the theory works,” says Bing Lv, at The University of Texas at Dallas. Along with David Cahill, at the University of Illinois, Urbana-Champaign, Lv and colleagues synthesized one of the pure crystals and measured its thermal conductivity. But there is more work to be done to integrate these crystals into devices, starting with finding a cost-effective method of synthesizing them on larger scales, Lv says.
In a Perspective article (doi:10.1126/science.aau4793), Chris Dames, at the University of California, Berkeley, writes, “These results … earn BAs long-overdue recognition as an ultrahigh-κ material.” Dames, who was not involved with the latest thermal-conductivity measurements, agrees that synthetic methods need further development for electronics applications, and suggests BAs powder might be added to a metal-matrix composite so that only nanoscale crystals, rather than wafers of thin films, would be needed.


