CVD has become the leading cause of mortality and morbidity in Western countries. It is one of the most significant diet-related health problems and there is a great deal of interest in how dietary fat composition influences the development of CVD. We have previously shown that dietary red palm oil (RPO) supplementation protects against ischaemia–reperfusion-induced injury in the isolated perfused rat heart; however, the effects of RPO supplementation on ischaemia–reperfusion-induced injury have not been investigated when supplemented with a high-cholesterol diet.
RPO is a natural oil obtained from oil palm fruit (Elaeis guineensis). It is high in palmitic (44 %) and oleic acid (40 %) with natural fat-soluble tocopherol, tocotrienol and carotonoids, which may act as antioxidants. Despite the high saturated fat content of RPO, several studies have demonstrated that RPO supplementation has beneficial or neutral effects on serum total cholesterol (Zhang et al. Reference Zhang, Ping, Chunrong, Shou and Keyou1997a , Reference Zhang, Wang, Dai, Chen and Ge b ; Niyongabo et al. Reference Niyongabo, Youyou, Leger, Descomps, Ammouche and Bellal1999; Nor Aini & Suria, Reference Nor, Suria, Basiron, Jalani and Chan2000). Other than its effect on lipoproteins, a few recent studies have shown that RPO has cellular signalling effects associated with protection and better recovery under conditions of ischaemia–reperfusion (Esterhuyse et al. Reference Esterhuyse, du Toit, Benade and van Rooyen2005; Engelbrecht et al. Reference Engelbrecht, Esterhuyse, du Toit, Lochner and van Rooyen2006). However, the signalling events in hearts supplemented with RPO in the presence of cholesterol remain to be elucidated.
Mitogen-activated-protein kinase (MAPK) pathways are among the best characterised signalling pathways in the heart. The major regulatory enzymes of the MAPK pathways, namely extracellular signal-regulated protein kinases (ERK1/2), c-Jun NH2-terminal kinases (JNK) and p38, have been implicated in cell proliferation, differentiation and apoptosis (Bhattacharya et al. Reference Bhattacharya, Ray and Johnson2004). Furthermore, various studies have shown that MAPK are regulated in direct response to ischaemia–reperfusion-induced injury (Maulik et al. Reference Maulik, Watanabe, Zu, Huang, Cordis, Schley and Das1996; Bernabe et al. Reference Bernabe, Tejedo, Rincon, Cahuana, Ramirez, Sobrino and Bedoya2001; Bhattacharya et al. Reference Bhattacharya, Ray and Johnson2004).
It is important to note, however, that discrepancies exist regarding the functions of the different MAPK in the heart. JNK mainly acts in association with p38-MAPK, which also plays a critical role in apoptosis when stimulated by environmental stressors (Matsukawa et al. Reference Matsukawa, Matsuzawa, Takeda and Ichijo2004). Unlike JNK though, p38-MAPK is highly expressed during ischaemia and reperfusion, whereas JNK is only activated during reperfusion (Whitmarsh et al. Reference Whitmarsh, Yang, Su, Sharrocks and Davis1997). The dynamic balance between the growth factor-activated ERK and stress-activated JNK and p38-MAPK are important in determining whether a cell survives or undergoes apoptosis.
The protective effect of RPO against ischaemia–reperfusion-induced injury in the isolated perfused rat heart was observed in animals consuming standard rat chow which was low in cholesterol (Esterhuyse et al. Reference Esterhuyse, du Toit, Benade and van Rooyen2005; Engelbrecht et al. Reference Engelbrecht, Esterhuyse, du Toit, Lochner and van Rooyen2006). In the present study we investigated whether RPO would counteract the negative effects of cholesterol in rats fed standard rat chow supplemented with 2 % cholesterol. A previous study (Engelbrecht et al. Reference Engelbrecht, Esterhuyse, du Toit, Lochner and van Rooyen2006) also showed that RPO-induced protection of the heart against reperfusion injury was associated with changes in MAPK phosphorylation. Therefore, in the present study, we correlated the effect of RPO supplementation of a high-cholesterol diet with MAPK phosphorylation and apoptosis.
Materials and methods
Antibodies and chemicals
All primary phospho-specific antibodies were purchased from Cell Signaling Technology (New England BioLabs, Ipswich, MA, USA), secondary antibodies from Amersham Biosciences (Piscataway, NJ, USA), and preGOLD prestained protein marker IV from PEQLAB Biotechnology GmbH (Erlangen, Germany). Chemiluminescence detection kits were purchased from Amersham Biosciences, whereas Western blotting systems used were obtained from Bio-Rad Laboratories Inc. (Hercules, CA, USA) and all chemicals from commercial companies.
Experimental model
All animals used received humane care in accordance with the Principle of Laboratory Animal Care of the National Society of Medical Research and the National Institutes of Health Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences (National Institutes of Health publication no. 80–23, revised 1978). Long–Evans rats aged 7 weeks were randomly allocated to three groups according to the dietary supplementation they received: group 1, standard rat chow (control group); group 2, standard rat chow enriched with 2 % cholesterol; group 3, standard rat chow enriched with 2 % cholesterol and RPO (7 g/kg diet) (Table 1).
Table 1 Approximate energy and macronutrient contents of rat diets
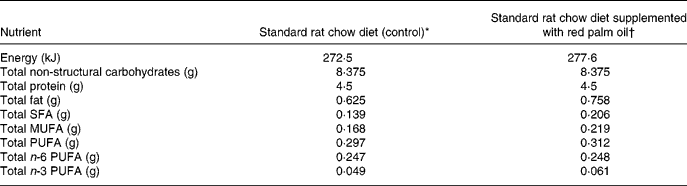
* 25 g standard rat chow per d.
† 25 g standard rat chow plus 0·175 g red palm oil per d.
The rats were fed a standard rat chow diet or 2 % cholesterol-enriched diet (based on previous studies by Giricz et al. Reference Giricz, Csonka, Onody, Csont and Ferdinandy2003) for 6 weeks. The approximate energy and macronutrient content of the two diets are indicated in Table 1. The rat chow was supplied by Atlas Animal Foods (Cape Town, South Africa) and regularly analysed to monitor possible variations between batches. Rats of the control group consumed an average of 25 g standard rat chow/d, containing 0·625 g fat, which provided 8·7 % of the energy intake. Protein intake was 4·5 g (28 % of energy intake). In the additional group the cholesterol-enriched rat chow diet was supplemented with RPO-baking fat (7 g RPO per kg diet) every morning for 6 weeks. Thus, there was a 21 % increase in fat intake in the RPO-supplemented experimental group. The RPO used in the present study provided an additional 70·0 μg carotenoids and 87·5 μg vitamin E (tocotrienols and tocopherols) to that present in the standard rat chow diet (antioxidant nutrient status of standard rat chow diet not provided by supplier due to confidentiality) (Nagendran et al. Reference Nagendran, Unnithan, Choo and Sundram2000). There were no significant differences in the serum total cholesterol or the HDL-cholesterol after the 6-week cholesterol and RPO supplementation period. Serum concentrations of TAG were increased in the RPO- and cholesterol-supplemented groups after 6 weeks on the diet (0·056 (sem 0·07) and 0·53 (sem 0·08) mmol/l, respectively before the 6-week diet and 0·87 (sem 0·08) and 1·05 (sem 0·19) mmol/l; P < 0·05, respectively after the 6-week diet). In the control group it was 0·54 (sem 0·07) mmol/l before and 0·83 (sem 0·09) mmol/l after the 6-week diet (Esterhuyse et al. Reference Esterhuyse, du Toit, Benade and van Rooyen2005).
Rats weighing 300–400 g were anaesthetised with sodium thiopentone before the hearts were rapidly excised and placed in ice-cold Krebs–Henseleit buffer. Hearts were transferred to the standard working heart perfusion apparatus and perfused with a Krebs–Henseleit buffer (pH 7·4) containing: 119 mm-NaCl, 24·9 mm-NaHCO3, 4·74 mm-KCl, 1·19 mm-KH2PO4, 0·6 mm-MgSO4, 0·59 mm-Na2SO4, 1·25 mm-CaCl2 and 10 mm-glucose. The buffer was oxygenated and kept at pH 7·4 by gassing with O2–CO2 (95:5, v/v). The aorta was cannulated and retrograde perfusion was initiated. During this initial perfusion in the Langendorff mode, the opening to the left atrium was cannulated. Following a 5 min stabilisation period in the Langendorff mode, hearts were switched to the working heart mode for 20 min. The temperature of both the perfusate and the air surrounding the heart was thermostatically controlled and checked at regular intervals to ensure that the temperature was maintained at 37°C irrespective of coronary flow. Hearts were then subjected to 25 min of total global ischaemia. At the end of ischaemia, hearts were reperfused in the Langendorff mode for 10 min, followed by 15 min working heart perfusion. In order to reduce the incidence of reperfusion arrhythmias, 2 % lignocaine solution was used for the initial 3 min of reperfusion of all hearts. To assess myocardial MAPK activity, hearts were freeze-clamped with Wollenberger clamps pre-cooled in liquid N2 at the end of the pre-ischaemic working heart perfusion (four rats per group), after 10 min ischaemia (four rats per group) and 10 min into reperfusion (four rats per group), and samples were stored at − 80°C (Fig. 1).
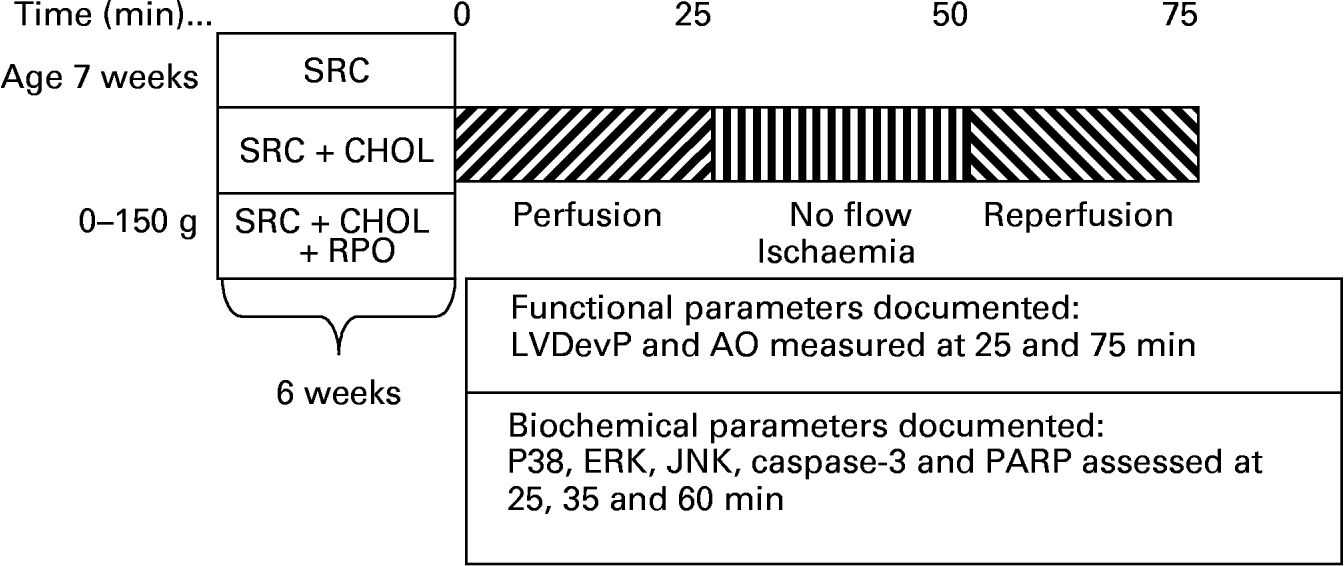
Fig. 1 A diagrammatic representation of the rat feeding programme and heart perfusion protocol employed for the study. SRC, standard rat chow; SRC+CHOL, standard rat chow enriched with 2 % cholesterol; SRC+CHOL+RPO, standard rat chow enriched with 2 % cholesterol and red palm oil; LVDevP, left ventricular developed pressure; AO, aortic output; ERK, extracellular signal-regulated protein kinase; JNK, c-Jun NH2-terminal kinase; PARP, poly(ADP-ribose) polymerase.
Functional parameters measured
Coronary and aortic flow rates were measured by collecting 1 min samples of the respective effluents 25 min into the pre-ischaemic perfusion and 25 min into reperfusion. Aortic output recovery was calculated by dividing the aortic output measured after ischaemia by that measured before ischaemia and expressing these values as percentage recovery (Fig. 1).
Western blot analysis
Cardiac MAPK and protein kinase B/Akt as well as caspase-3 and poly(ADP-ribose) polymerase (PARP) protein were extracted with a lysis buffer containing: 20 mm-tri(hydroxymethyl)-aminomethane (Tris), 20 mm-p-nitrophenylphosphate, 1 mm-EGTA, 50 mm-sodium fluoride, 0·1 mm-sodium orthovanadate, 1 mm-phenylmethyl sulfonyl fluoride, 1 mm-dithiothreitol, aprotinin (10 μg/ml) and leupeptin (10 μg/ml). The tissue lysates were diluted in Laemmli sample buffer, boiled for 5 min and 10 μg (MAPK and protein kinase B/Akt) or 50 μg protein (caspase-3 and PARP) were separated by 10 % PAGE–SDS-gel electrophoresis. The lysate protein content was determined using the Bradford technique (Bradford, Reference Bradford1976). The separated proteins were transferred to a PVDF membrane (Immobilon™ P; Millipore Corp., Bedford, MA, USA). These membranes were routinely stained with Ponceau Red for visualisation of proteins. Non-specific binding sites on the membranes were blocked with 5 % fat-free milk in Tris-buffered saline–0·1 % Tween 20 and then incubated with the primary antibodies that recognise phosphospecific ERK p42/p44 (Thr202/Tyr204), p38-MAPK (Thr180/Tyr182), JNK p54/p46 (Thr183/Tyr185), protein kinase B (Ser473and Thr308), caspase-3 (p17 fragment pAb) and PARP (p85 fragment pAb). Membranes were subsequently washed with large volumes of Tris-buffered saline–0·1 % Tween 20 (5 × 5 min) and the immobilised antibody conjugated with a diluted horseradish peroxidase-labelled secondary antibody (Amersham Life Science). After thorough washing with Tris-buffered saline–0·1 % Tween 20, membranes were covered with ECL™ detection reagents and quickly exposed to an autoradiography film (Hyperfilm ECL, RPN 2103; Amersham Biosciences) to detect light emission through a non-radioactive method (ECL™ Western blotting). Films were densitometrically analysed (UN-SCAN-IT; Silk Scientific Corporation (SilkScience), USA, version 5.1) and phosphorylated protein values were corrected for minor differences in protein loading, if required. Experiments were performed (data not shown) to ensure that all signals were within the linear range of detection on the autoradiographs under our assay and gel-loading conditions.
Data analysis
Data are presented as mean values with their standard errors. Statistical significance was determined in multiple comparisons among independent groups of data in which ANOVA and Bonferroni's post hoc test indicated significant differences. P < 0·05 was considered statistically significant.
Results
Aortic output recovery
We used aortic output recovery as an indirect index of the severity of ischaemia–reperfusion injury. Cholesterol supplementation decreased aortic output recovery when compared with the control group (35·5 (sem 6·2) v. 55·4 (sem 2·5) %). However, when RPO was added to the cholesterol diet, the percentage aortic output recovery was significantly increased when compared with the cholesterol group (63·2 (sem 3·1) %; P < 0·05) (Fig. 2).
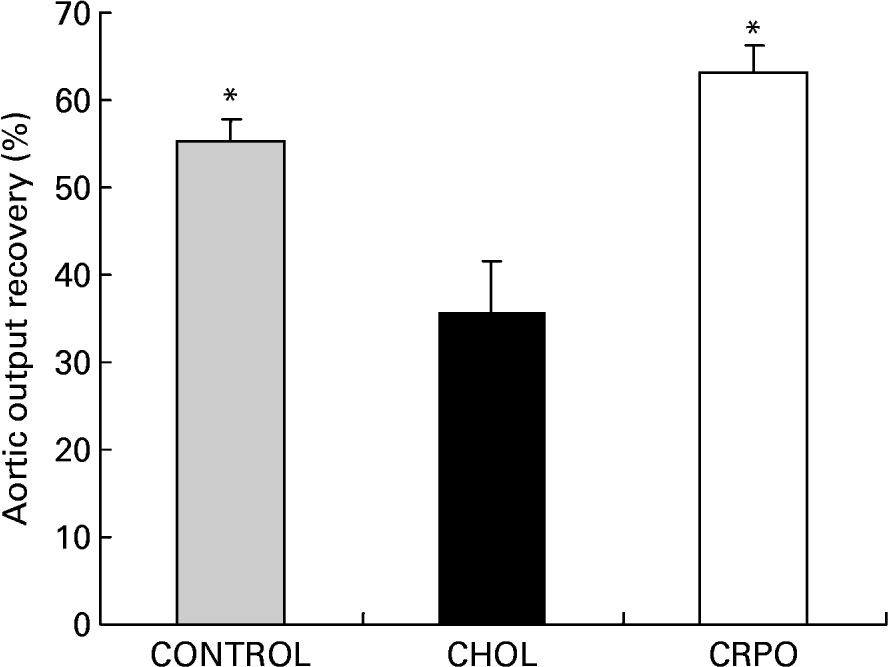
Fig. 2 The percentage aortic output recovery of cholesterol (CHOL)-supplemented hearts v. cholesterol–red palm oil (CRPO)-supplemented hearts v. control hearts. Data are means for seven independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the CHOL group (P < 0·05).
Effect of red palm oil supplementation on the phosphorylation of p38, c-Jun NH2-terminal kinase and extracellular signal-regulated protein kinase in cholesterol-fed rat hearts subjected to ischaemia and reperfusion
Phosphorylation of p38, JNK (p46/p54-MAPK) and ERK1/2 (p42/p44-MAPK) was determined by Western blotting using phospho-specific antibodies. There was a significant increase in p38 phosphorylation in the control group during reperfusion v. baseline perfusion before ischaemia (1·67 (sem 0·05)-fold; P < 0·01). As shown in Fig. 3 (A), p38 phosphorylation was significantly increased in the control group v. the cholesterol group during both ischaemia and reperfusion (0·86 (sem 0·03)- to 3·05 (sem 0·19)-fold; P < 0·001 for ischaemia and 1·68 (sem 0·05)- to 4·05 (sem 0·29)-fold; P < 0·001 for reperfusion). Ischaemia–reperfusion also caused significant increases in p38 phosphorylation in the cholesterol–RPO group when compared with the cholesterol group (0·88 (sem 0·20)- to 3·05 (sem 0·19)-fold; P < 0·001 for ischaemia and 2·07 (sem 0·26)- to 4·05 (sem 0·29)-fold; P < 0·001 for reperfusion). There was a significant increase in ERK44/42 phosphorylation in the control group during reperfusion v. the baseline perfusion group (4·02 (sem 0·51)-fold; P < 0·001 for ERK44 and 3·11 (sem 0·67)-fold; P < 0·001 for ERK 42). ERK44 and ERK 42 phosphorylation was significantly increased in the cholesterol–RPO group during reperfusion when compared with the cholesterol group (1·42 (sem 0·20)- to 2·60 (sem 0·76)-fold; P < 0·05 for ERK44 and 1·55 (sem 0·19)- to 3·33 (sem 0·03)-fold; P < 0·001 for ERK42). When compared with the control group, the phosphorylation of both subunits in the cholesterol group was significantly lower (1·42 (sem 0·20)- to 4·02 (sem 0·52)-fold; P < 0·001 for ERK44 and 1·55 (sem 0·19)- to 3·12 (sem 0·67)-fold; P < 0·001 for ERK42). No statistically significant differences occured between the cholesterol–RPO and control groups in the ERK42 fraction, but with regard to the ERK44 fraction, a significant reduction in ERK phosphorylation was found (2·60 (sem 0·76)- to 4·02 (sem 0·52)-fold; P < 0·01). There was a significant increase in JNK54/46 phosphorylation in the control group during reperfusion v. the baseline perfusion group (1·39 (sem 0·04)-fold; P < 0·001 for JNK54 and 1·29 (sem 0·09)-fold; P < 0·01 for JNK 46). Dietary RPO supplementation in the presence of cholesterol caused a significant decrease in phosphorylation of both JNK54 and JNK46 during reperfusion when compared with the cholesterol group (1·45 (sem 0·09)- to 1·21 (sem 0·04)-fold; P < 0·001 for JNK54 and 1·61 (sem 0·08)- to 1·23 (sem 0·04)-fold; P < 0·001 for JNK46). Cholesterol supplementation, on the other hand, only caused a significant increase in the JNK46 fraction when compared with the control group (1·61 (sem 0·08) to 1·30 (sem 0·10); P < 0·001), while the cholesterol–RPO group differed significantly from the control group in the JNK54 fraction during reperfusion (1·21 (sem 0·04)- to 1·4 (sem 0·04)-fold; P < 0·01). (Figs. 3 (A), (B) and (C))

Fig. 3 (A) The effect of red palm oil (RPO) supplementation on the phosphorylation of p38-mitogen-activated-protein kinase (MAPK) in cholesterol-fed rat hearts subjected to ischaemia and reperfusion. Samples were analysed by Western blotting with phospho-specific antibodies recognising dual phosphorylated MAPK. (
![]() ), Control; (■), cholesterol-fed; (□), cholesterol-fed RPO-supplemented. Data are means for six independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the 20 min perfusion control (P < 0·01). † Mean value was significantly different from that of the 10 min ischaemia cholesterol condition (P < 0·001). ‡ Mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001). (B) The effect of RPO supplementation on the phosphorylation of extracellular signal-regulated protein kinase (ERK) in cholesterol-fed rat hearts subjected to ischaemia and reperfusion. Samples were analysed by Western blotting with phospho-specific antibodies recognising dual phosphorylated MAPK. Data are means for six independent experiments, with their standard errors represented by vertical bars. ERK44: mean value was significantly different from that of the 10 min reperfusion control: *P < 0·01, **P < 0·001; † mean value was significantly different from that of the 10 min ischaemia control (P < 0·01); ‡ mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·05). ERK42: * mean value was significantly different from that of the 10 min reperfusion control (P < 0·001); † mean value was significantly different from that of the 10 min ischaemia cholesterol condition (P < 0·05); ‡ mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001). (C) The effect of RPO supplementation on the phosphorylation of c-Jun NH2-terminal kinase (JNK) in cholesterol-fed rat hearts subjected to ischaemia and reperfusion. Samples were analysed by Western blotting with phospho-specific antibodies recognising dual phosphorylated MAPK. Data are means for six independent experiments, with their standard errors represented by vertical bars. JNK54: mean value was significantly different from that of the 10 min reperfusion control: *P < 0·01, **P < 0·001; † mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001). JNK46: mean value was significantly different from that of the 10 min reperfusion control: *P < 0·01, **P < 0·001; † mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001).
), Control; (■), cholesterol-fed; (□), cholesterol-fed RPO-supplemented. Data are means for six independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the 20 min perfusion control (P < 0·01). † Mean value was significantly different from that of the 10 min ischaemia cholesterol condition (P < 0·001). ‡ Mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001). (B) The effect of RPO supplementation on the phosphorylation of extracellular signal-regulated protein kinase (ERK) in cholesterol-fed rat hearts subjected to ischaemia and reperfusion. Samples were analysed by Western blotting with phospho-specific antibodies recognising dual phosphorylated MAPK. Data are means for six independent experiments, with their standard errors represented by vertical bars. ERK44: mean value was significantly different from that of the 10 min reperfusion control: *P < 0·01, **P < 0·001; † mean value was significantly different from that of the 10 min ischaemia control (P < 0·01); ‡ mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·05). ERK42: * mean value was significantly different from that of the 10 min reperfusion control (P < 0·001); † mean value was significantly different from that of the 10 min ischaemia cholesterol condition (P < 0·05); ‡ mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001). (C) The effect of RPO supplementation on the phosphorylation of c-Jun NH2-terminal kinase (JNK) in cholesterol-fed rat hearts subjected to ischaemia and reperfusion. Samples were analysed by Western blotting with phospho-specific antibodies recognising dual phosphorylated MAPK. Data are means for six independent experiments, with their standard errors represented by vertical bars. JNK54: mean value was significantly different from that of the 10 min reperfusion control: *P < 0·01, **P < 0·001; † mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001). JNK46: mean value was significantly different from that of the 10 min reperfusion control: *P < 0·01, **P < 0·001; † mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001).
The effect of red palm oil supplementation on caspase-3 activation and poly(ADP-ribose) polymerase cleavage in cholesterol-fed rat hearts subjected to ischaemia and reperfusion
In Fig. 4 (A), total caspase-3 was measured during perfusion, ischaemia and reperfusion of the control, cholesterol and cholesterol–RPO groups. All groups showed a marked reduction in total caspase-3 during ischaemia and reperfusion compared with baseline perfusion. An increase in total caspase-3 is associated with less cleaved caspase-3 and thus a reduction in apoptosis. The expression of total caspase-3 in the cholesterol–RPO group was significantly higher compared with the control and cholesterol group during both ischaemia and reperfusion (0·71 (sem 0·02)- to 0·28 (sem 0·06)-fold; P < 0·001 for control ischaemia and 0·24 (sem 0·01)-fold; P < 0·001 for cholesterol ischaemia; 0·56 (sem 0·06)- to 0·27 (sem 0·01)-fold; P < 0·001 for control reperfusion and 0·42 (sem 0·05)-fold; P < 0·01 for cholesterol reperfusion). It is only during reperfusion that a significant difference in caspase cleavage was observed between the control and cholesterol group (P < 0·01). In Fig. 4 (B), cleaved PARP was measured during perfusion, ischaemia and reperfusion of the control, cholesterol and cholesterol–RPO groups. A significant increase in cleaved PARP was found in both the cholesterol–RPO and cholesterol groups during ischaemia when compared with the control group (P < 0·001 for both), where the amount of cleaved PARP in the cholesterol–RPO supplemented group was significantly lower than the cholesterol group (1·74 (sem 0·04)- to 1·40 (sem 0·11)-fold; P < 0·001). During reperfusion an even greater amount of cleaved PARP was observed in all the groups, where a significant difference was found between all three groups (P < 0·001).

Fig. 4 The effect of red palm oil (RPO) supplementation on caspase-3 activation (A) and poly(ADP-ribose) polymerase (PARP) cleavage (B) in cholesterol-fed rat hearts subjected to ischaemia and reperfusion. Samples were analysed by Western blotting with antibodies recognising cleaved PARP and caspase-3. (
![]() ), Control; (■), cholesterol-fed; (□), cholesterol-fed RPO-supplemented. Data are means for six independent experiments, with their standard errors represented by vertical bars. Caspase-3: * mean value was significantly different from that of the 10 min ischaemia cholesterol–RPO condition (P < 0·001); † mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·01); ‡ mean value was significantly different from that of the 10 min reperfusion control (P < 0·001). PARP: * mean value was significantly different from that of the 10 min ischaemia control (P < 0·001); † mean value was significantly different from that of the 10 min ischaemia cholesterol condition (P < 0·001); ‡ mean value was significantly different from that of the 10 min reperfusion control (P < 0·001); § mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001).
), Control; (■), cholesterol-fed; (□), cholesterol-fed RPO-supplemented. Data are means for six independent experiments, with their standard errors represented by vertical bars. Caspase-3: * mean value was significantly different from that of the 10 min ischaemia cholesterol–RPO condition (P < 0·001); † mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·01); ‡ mean value was significantly different from that of the 10 min reperfusion control (P < 0·001). PARP: * mean value was significantly different from that of the 10 min ischaemia control (P < 0·001); † mean value was significantly different from that of the 10 min ischaemia cholesterol condition (P < 0·001); ‡ mean value was significantly different from that of the 10 min reperfusion control (P < 0·001); § mean value was significantly different from that of the 10 min reperfusion cholesterol condition (P < 0·001).
Discussion
We have demonstrated for the first time that RPO supplementation of a cholesterol-enriched diet offers protection against ischaemia–reperfusion-induced injury in the isolated perfused working heart as reflected by improved myocardial functional recovery when compared with the cholesterol-supplemented control group (Fig. 2). This decreased myocardial functional recovery in the cholesterol-supplemented groups was also observed by Golino et al. (Reference Golino, Maroko and Carew1987), who demonstrated that hypercholesterolaemia caused an increase in myocardial infarct size in rabbits after 30 min of ischaemia. Furthermore, Ferdinandy et al. (Reference Ferdinandy, Szilvassy, Horvath, Csont, Csonka, Nagy, Szentgyorgyi, Nagy, Koltai and Dux1997) demonstrated for the first time that the cardioprotection conferred by classical preconditioning was lost when rats became hypercholestrolaemic. Kyriakides et al. (Reference Kyriakides, Psychari, Iliodromitis, Kolettis, Sbarouni and Kremastinos2002) also showed that the anti-ischaemic effect of preconditioning is abolished in the presence of hypercholesterolaemia, emphasising the necessity of developing new cardioprotective drugs capable of preventing increased susceptibility of hearts to ischaemic stress, resulting in decreased functional recovery. Although there appears to be some conflicting data in the literature, the majority of studies show that hyperlipidaemia, independently from the development of coronary atherosclerosis, worsens the outcome of ischaemia–reperfusion injury and attenuates the cardioprotective effect of preconditioning (for a review, see Ferdinandy, Reference Ferdinandy2003).
Engelbrecht et al. (Reference Engelbrecht, Esterhuyse, du Toit, Lochner and van Rooyen2006) previously demonstrated that RPO supplementation offered significant protection against ischaemia–reperfusion-induced injury in the isolated perfused heart, but no evidence exists for RPO-induced protection in a cholesterol-supplemented diet. Although some evidence exist for an effect of some of the major components of RPO on cardiac function, it is still not clear whether the beneficial effect of RPO can be attributed to one single component or if it is the combined effect of the ingredients of RPO that induced the protection. For example, Serbinova et al. (Reference Serbinova, Khavaja, Catudioc, Ericson, Torres and Gapor1992) showed that RPO vitamin E was more efficient than tocopherols in protecting against ischaemia–reperfusion injury in the isolated perfused rat heart. Furthermore, Bilgin-Karabulut et al. (Reference Bilgin-Karabulut, Ademoglu, Aydin, Erer and Gokkusu2001) showed that pre-treatment with a combination of vitamins A and E gave protection against venous ischaemia–reperfusion-induced injury. Interestingly, these vitamins were not effective when used as single agents. On the other hand, Meehan & Higgins (Reference Meehan and Higgins1994) demonstrated that oleic acid (another major component of RPO) improved functional recovery in ischaemic–reperfused rat hearts.
Various signal transduction pathways are activated in the heart in response to ischaemia–reperfusion-induced injury. It has also been suggested that the MAPK are important regulators of apoptosis in response to myocardial ischaemia–reperfusion (Hreniuk et al. Reference Hreniuk, Garay, Gaarde, Monia, McKay and Cioffi2001). We characterised the phosphorylation pattern of the three major MAPK subfamilies after 20 min of perfusion, 10 min of ischaemia and 10 min of reperfusion and correlated it with markers of apoptosis at the same time points. Our data show that reperfusion in control hearts caused significant increases in p38, ERK and JNK phosphorylation compared with the 20 min baseline perfusion control groups. This is in agreement with results of Bogoyevitch et al. (Reference Bogoyevitch, Gillespie-Brown, Ketterman, Fuller, Ben-Levy, Ashworth, Marshall and Sugden1996) and Marais et al. (Reference Marais, Genade, Strijdom, Moolman and Lochner2001) who reported attenuation of MAPK phosphorylation during ischaemia–reperfusion. This increase in MAPK phosphorylation also correlated with an increase in apoptosis demonstrated by caspase-3 and PARP cleavage in our model.
We have demonstrated that dietary cholesterol supplementation significantly increased p38 phosphorylation during ischaemia and reperfusion compared with the control group (Fig. 2 (A)) and that RPO supplementation caused a significant decrease in p38 phosphorylation. This down regulation of p38 with RPO supplementation was associated with improved function of the isolated perfused heart after ischaemia–reperfusion-induced injury. Despite reports to the contrary (Weinbrenner et al. Reference Weinbrenner, Liu, Cohen and Downey1997), most evidence (as well as our own data) supports the concept that p38 activation is harmful to the ischaemic heart (Bogoyevitch et al. Reference Bogoyevitch, Gillespie-Brown, Ketterman, Fuller, Ben-Levy, Ashworth, Marshall and Sugden1996; Mackay & Mochly-Rosen, Reference Mackay and Mochly-Rosen2000; Engelbrecht et al. Reference Engelbrecht, Niesler, Page and Lochner2004). These opposing results can be attributed to the different isoforms (α and β) expressed in the heart (Saurin et al. Reference Saurin, Martin, Heads, Foley, Mockridge, Wright, Wang and Marber2000), which appear to mediate opposing functions. The p38α isoform is implicated in apoptosis, whereas p38β is anti-apoptotic (Minet et al. Reference Minet, Michel, Mottet, Raes and Michiels2001).
In our model, JNK phosphorylation was also significantly increased during reperfusion in the cholesterol-supplemented group but was attenuated by dietary RPO supplementation. This is in agreement with other results which showed that JNK phosphorylation appears to be pro-apoptotic in many cell types; however, its exact role in regulating cell death is still unclear (Wang et al. Reference Wang, Su, Sah, Brown, Han and Chien1998; Obata et al. Reference Obata, Brown and Yaffe2000; Park et al. Reference Park, Seol, Kim, Hyun, Jung, Lee, Binderup, Koeffler, Kim and Lee2000; Hreniuk et al. Reference Hreniuk, Garay, Gaarde, Monia, McKay and Cioffi2001).
Dietary RPO supplementation of the cholesterol-enriched diet also significantly increased ERK phosphorylation when compared with the cholesterol-supplemented group. It is suggested that the ERK pathway is required for survival signalling in response to ischaemia–reperfusion in cardiomyocytes (Abe et al. Reference Abe, Baines and Berk2000). Among the substrates of ERK, p90 ribosomal S6 kinase (p90RSK) is a ubiquitous and versatile mediator of ERK signalling and one of its functions include the phosphorylation of the pro-apoptotic protein BAD at serine 112, which specifically suppresses BAD-mediated apoptosis (Bonni et al. Reference Bonni, Brunet, West, Datta, Takasu and Greenberg1999).
It was also demonstrated that the increase in functional recovery induced by RPO supplementation was associated with a significant inhibition of apoptosis in our model. Apoptosis has been consistently observed in the myocardium after ischaemia–reperfusion-induced injury and may represent a direct mechanism by which myocytes are killed (Yue et al. Reference Yue, Wang, Gu, Ma, Kumar, Lee, Feuerstein, Thomas, Maleeff and Ohlstein2000; Hreniuk et al. Reference Hreniuk, Garay, Gaarde, Monia, McKay and Cioffi2001). Indeed, ischaemia–reperfusion-induced injury resulted in the cleavage of PARP, a phenomenon that is well known to result from caspase-3 activation. Inhibition of apoptosis by RPO may, therefore, offer a unique approach towards the amelioration of ischaemia–reperfusion-induced injury.
The present results have shown that dietary RPO supplementation significantly protected the heart from the adverse effects of a high-cholesterol diet. Our data suggest that its beneficial effects are associated with decreased p38 and JNK phosphorylation and increased ERK phosphorylation which may contribute to the decrease in apoptosis in our model. Therefore, RPO might offer an alternative, non-pharmacological strategy to protect the heart against ischaemia–reperfusion-induced injury in the presence of cholesterol, one of the risk factors ultimately leading to IHD.
Acknowledgements
The present study was supported by the University of Stellenbosch (Subcommittee B), National Research Foundation of South Africa and the Cape Peninsula University of Technology. Carotino Palm Oil was supplied by Carotino SDN BHD (company no. 69 046-T), Johore Bahry, Malaysia. We also thank Dirk Bester from the Cape Peninsula University of Technology for his technical support.







