As a result of global warming, the spread of non-indigenous species is one of the major drivers of change in marine ecosystems, with drastic consequences on their biodiversity (Bonanno & Orlando-Bonaca, Reference Bonanno and Orlando-Bonaca2019). Considering the number of non-indigenous species and the rate of introduction, the Mediterranean is one of the most affected seas by biological invasions (Zenetos et al., Reference Zenetos, Gofas, Verlaque, Cinar, Garcia Raso, Bianchi, Morri, Azzurro, Bilecenoglu, Froglia, Siokou-Frangou, Violanti, Sfriso, San Martín, Giangrande, Katagan, Ballesteros, Ramos-Esplá, Mastrototaro, Ocaña, Zingone, Gambi and Streftaris2010). Spatio-temporal information on non-indigenous species and monitoring their geographic range expansion represent valuable tools for conservation of marine ecosystems and biodiversity (Katsanevakis et al., Reference Katsanevakis, Poursanidis, Hoffman, Rizgalla, Rothman, Levitt-Barmats and Espinosa Torre2020).
Following a taxonomic revision of the Order Cephalaspidea, Haminoea cyanomarginata Heller & Thompson, Reference Heller and Thompson1983 was described from individuals of the Red Sea (Heller & Thompson, Reference Heller and Thompson1983; Dekker & Orlin, Reference Dekker and Orlin2000) and initially considered junior synonym of Lamprohaminoea cyanomarginata (Oskars & Malaquias, Reference Oskars and Malaquias2019). Further taxonomic studies demonstrated that both H. cyanomarginata and L. cyanomarginata are junior synonyms of Lamprohaminoea ovalis (Pease, 1868; Oskars & Malaquias, Reference Oskars and Malaquias2020). This heterobranch, characterized by highly variable body colour pattern, was first described from Tahiti (French Polynesia, France). Nowadays it is widespread from the Red Sea to the Indo-West Pacific. Its fairly recent records from Greece, Turkey, Malta, Italy, Cyprus, Croatia and Spain, more than 20 years after its first finding in the Red Sea, suggest that it is an invasive Lessepsian species that entered and established in the Mediterranean Sea via the Suez Canal (Rizgalla et al., Reference Rizgalla, Fridman, Ben Abdallah, Bron and Shinn2018; Oskars & Malaquias, Reference Oskars and Malaquias2020). Nowadays, L. ovalis has been reported from the whole Mediterranean basin (Mifsud, Reference Mifsud2007; Crocetta & Vazzana, Reference Crocetta and Vazzana2009; Zenetos et al., Reference Zenetos, Gofas, Verlaque, Cinar, Garcia Raso, Bianchi, Morri, Azzurro, Bilecenoglu, Froglia, Siokou-Frangou, Violanti, Sfriso, San Martín, Giangrande, Katagan, Ballesteros, Ramos-Esplá, Mastrototaro, Ocaña, Zingone, Gambi and Streftaris2010; Fernández-Vilert et al., Reference Fernández-Vilert, Giménez, Mas, Figueroa and Moles2018 and reference therein; Ragkousis et al., Reference Ragkousis, Abdelali, Azzurro, Badreddine, Bariche, Bitar, Crocetta, Denitto, Digenis, El Zrelli, Ergenler, Fortič, Gerovasileiou, Grimes, Katsanevakis, Koçak, Licchelli, Loudaros, Mastrototaro, Mavrič, Mavruk, Miliou, Montesanto, Ovalis, Pontes, Rabaoui, Sevingel, Spinelli, Tiralongo, Tsatiris, Turan, Vitale, Yalgin, Yapici and Zenetos2020), except from the Gulf of Lion and the Ligurian Sea.
With the aim of investigating L. ovalis range expansion in the Mediterranean Sea in the last 20 years, all available records have been collected. The expansion rate of L. ovalis has been evaluated by measuring the geographic distances (kilometres) between the location of its first record in 2001 and the locations of the following new records from other Mediterranean areas. The expansion rate of the species has been compared with change in time of the sea surface temperature (SST), to evaluate if the spread of this non-indigenous species is a direct consequence of seawater warming. The first record of L. ovalis from the Ligurian Sea (NW Mediterranean) has also been reported.
The first occurrence of L. ovalis in the Mediterranean Sea was reported at Porto Germano (Greece) in 2001 (Zenetos et al., Reference Zenetos, Gofas, Russo and Templado2003) (Figure 1A, Table 1). In the following years this species remained confined to the eastern basin of the Mediterranean, where Lessepsian species more typically settle (Gambi et al., Reference Gambi, Barbieri and Bianchi2009). Since the second half of the 2010s the species crossed the 20° meridian East, reaching the westernmost portions of the Mediterranean, and since 2014 it crossed the 40° parallel North, starting to approach the northernmost and coldest sectors of the basin (Figure 1A, Table 1). During the last 20 years the yearly mean SST continuously increased, and the isotherms shifted northwards (Pisano et al., Reference Pisano, Marullo, Artale, Falcini, Yang, Leonelli, Santoleri and Buongiorno Nardelli2020) (Figure 1B, C), favouring the north-western expansion of L. ovalis (Bianchi, Reference Bianchi2007).
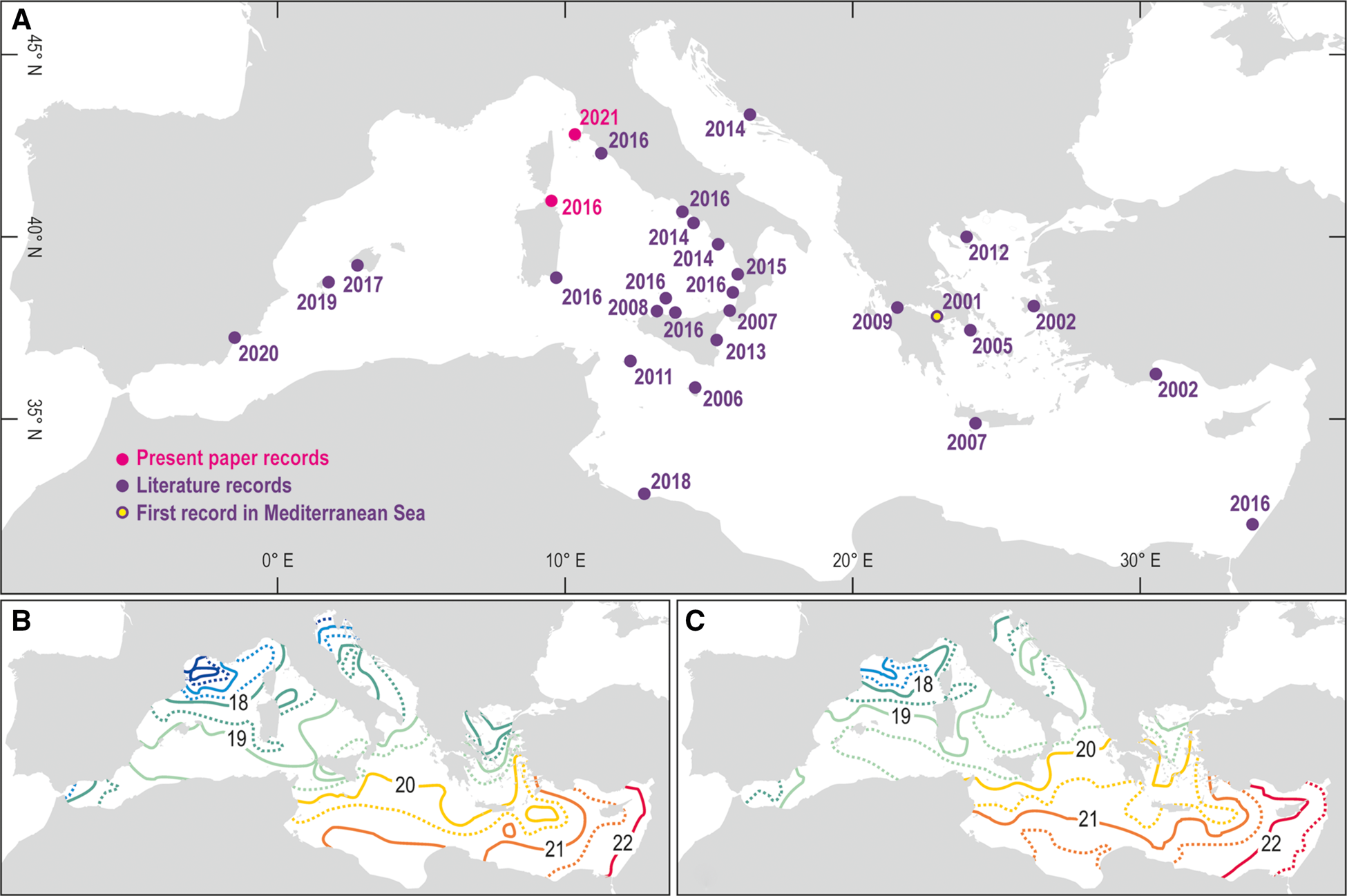
Fig. 1. Map of the available records of Lamprohaminoea ovalis in the Mediterranean Sea (a). Yearly mean SST from 1994 to 2005 (b) and from 2006 to 2018 (c) (modified from Pisano et al., Reference Pisano, Marullo, Artale, Falcini, Yang, Leonelli, Santoleri and Buongiorno Nardelli2020).
Table 1. List of records of Lamprohaminoea ovalis in the Mediterranean Sea (see also Figure 1)

Since 2016 the geographic range of L. ovalis expanded northward in the Tyrrhenian Sea (NW Mediterranean) and an individual has been recorded in 2016 at Porto Ercole, Tuscany (Figure 1A, Table 1). In the same year the species has been observed multiple times by the authors in the Tavolara-Punta Coda Cavallo Marine Protected Area (MPA), which represents its first record in North Sardinia (Tyrrhenian Sea) (Figure 1A, Table 1). Four individuals have been observed at Reulino (Isola Rossa) at 13 m depth in a mixed habitat with rocks, detritus and algae (Figure 2A). In the following years two other individuals have been observed in the MPA, one at 30 m depth on a coralligenous outcrop at Punta Papa (Tavolara Island), and one at Porto San Paolo at 3 m depth (Figure 2B). In the subsequent years L. ovalis became common in the MPA along the south-eastern coast of Tavolara Island.

Fig. 2. Individuals of Lamprohaminoea ovalis observed at: (a) Isola Rossa in 2016 (Sardinia, Tyrrhenian Sea), (b) Porto San Paolo in 2017 (Sardinia, Tyrrhenian Sea), (c, d) Elba Island (Tuscany, Ligurian Sea). Photo credit: Egidio Trainito (a, b), Lorenzo Moscia (c), Monica Montefalcone (d).
On 21 October 2021, during a scientific expedition conducted by the University of Genova in collaboration with Greenpeace to monitor non-indigenous species, two L. ovalis individuals of about 10 mm in length were observed and photographed at Portoferraio, Elba Island (Ligurian Sea) (Figures 1A & 2C, D, Table 1). The individuals were found at 26 m depth on a sub-vertical rocky bottom, covered by photophilic algae and Posidonia oceanica. They were observed in potential trailing behaviour. In Heterobranchia trailing action is aimed at reproduction: an individual follows the mucous trail of a conspecific and keeps contact with it by touching the tail. In this way, rows of up to four individuals can be formed (Betti et al., Reference Betti, Giuliano and Mojetta2011; Rizgalla et al., Reference Rizgalla, Fridman, Ben Abdallah, Bron and Shinn2018). The unambiguous determination of the species from the photographs taken in Sardinia and at Elba Island has been possible thanks to the distinctive unornamented bubble shell and body colour pattern. The mantle bordered with purple perfectly matches the ‘L. cyanomarginata purple morph’ (Figure 3) reported in the recent systematic revision of the genus Lamprohaminoea Habe, 1952 and ascribed to L. ovalis species (Oskars & Malaquias, Reference Oskars and Malaquias2020).
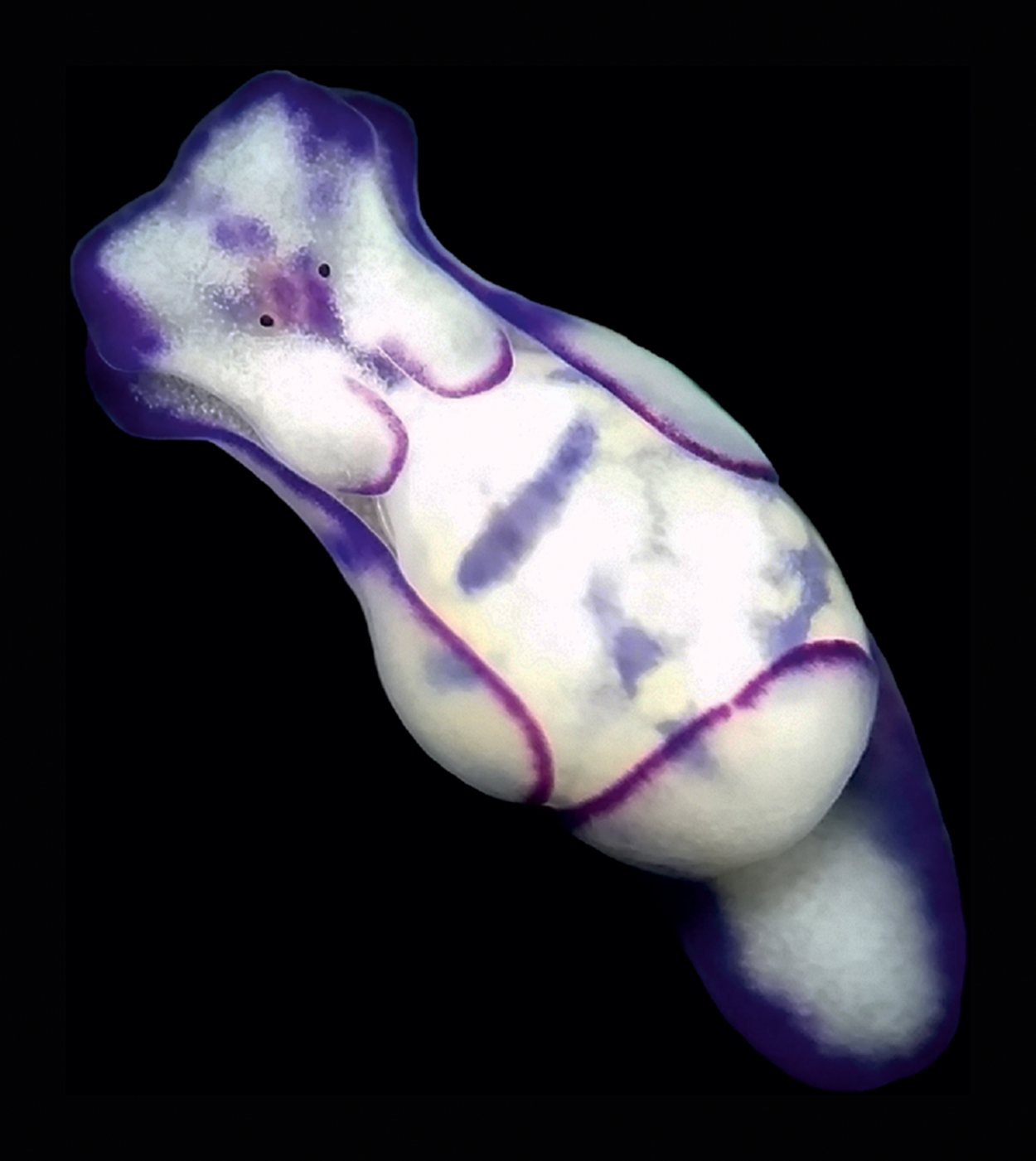
Fig. 3. An individual of Lamprohaminoea ovalis collected at 10 m depth at Cala Grande, Argentario (Tuscany, Tyrrhenian Sea) in October 2017 and photographed in laboratory. Photo credit: Giulia Furfaro.
The expansion rate of L. ovalis showed a significant increasing trend since 2001 (Figure 4A), and two distinct phases can be identified: (i) 2001–2013, when the species spread slowly westward; and (ii) 2014–2021, when its spread was faster and northward (Figures 1A & 4A). The mean distance ‘travelled’ by L. ovalis in the second period (2014–2021) was significantly higher than in the first one (Figure 4A). This species increased its range expansion in correspondence of a warming phase of the SST started in 2014 (Bianchi et al., Reference Bianchi, Azzola, Bertolino, Betti, Bo, Cattaneo-Vietti, Cocito, Montefalcone, Morri, Oprandi, Peirano and Bavestrello2019). As it is known that high summer temperatures favour the spread of alien species and mild winter temperatures allow for their establishment (Osland et al., Reference Osland, Stevens, Lamont, Brusca, Hart, Waddle, Langtimm, Williams, Keim, Terando, Reyier, Marshall, Loik, Boucek, Lewis and Seminoff2021), both minimum and maximum mean SST in the two considered periods showed the same significant increase as L. ovalis expansion rate (Figure 4C, D). Although this close analogy does not represent a correlation, it is suggestive that the new thermal regime is making the Mediterranean Sea increasingly receptive to species of tropical origin.

Fig. 4. Expansion rate of Lamprohaminoea ovalis in the Mediterranean Sea since its first record in 2001 (a). Mean range expansion rate in the two investigated time periods: 2001–2013 and 2014–2021 (b). SST minimum (c) and maximum (d) yearly mean in the two investigated time periods 2001–2013 and 2014–2021.
The expansion of non-indigenous species is a growing and recurrent phenomenon, so that the Mediterranean Sea is going towards a phase of tropicalization (Bianchi & Morri, Reference Bianchi and Morri2003). In the Ligurian Sea, one of the northernmost portions of the Mediterranean Sea, reports of thermophilic southern species are becoming frequent (Parravicini et al., Reference Parravicini, Mangialajo, Mousseau, Peirano, Morri, Montefalcone, Francour, Kulbicki and Bianchi2015; Bianchi et al., Reference Bianchi, Caroli, Guidetti and Morri2018). The continuous observations of L. ovalis in the Mediterranean, particularly after the year 2014, demonstrate that its spread is mainly favoured by seawater warming. In the coldest Mediterranean sectors, where the yearly mean SST is still around 18°C or lower, this species is not yet widespread. Nevertheless, the first observation of L. ovalis in the Ligurian Sea reported in this paper represents the northernmost record of occurrence of this non-indigenous species in the Western Mediterranean Sea, providing further important evidence of the ongoing global water warming also in the coldest sectors of the Mediterranean.
Acknowledgements
Authors would like to dedicate this work in the memory of Professor Riccardo Cattaneo-Vietti, for his valuable contribution to marine biology and to the knowledge of the heterobranchs. Annalisa Azzola and Monica Montefalcone are grateful to Professor Carlo Nike Bianchi, a continuous source of inspiration and suggestions. Giulia Furfaro would like to thank the Italian Ministry of Education, University and Research (MIUR, PON 2014-2020, grant AIM 1848751-2, Linea 2) for support. Andrea Romoli and Nicola Rozzi (Il Careno Diving Center) and Marco Sartore (ElbaTech SRL) provided logistic support during field activities at Elba Island.
Financial support
Part of this work has been supported by Greenpeace within the framework of the project ‘Mare Caldo’ (contract no. 1959.2021) for monitoring global warming effects on marine benthic habitats.
Conflict of interest
The authors declare that they have no competing interests.







