Surveillance of the physical development of the newborn over infancy and early childhood is important as delays in physical development can indicate further problems in motor as well as neurocognitive development. It is well-known that low birth weight children have high risk for delayed motor development (de Kieviet et al., Reference de Kieviet, Piek, Aarnoudse-Moens and Oosterlaan2009), and delays in brain development, such as the total brain volume and the size of hippocampus, have been found (de Kieviet et al., Reference de Kieviet, Zoetebier, van Elburg, Vermeulen and Oosterlaan2012). There is also sound evidence that length or height and head circumference (HC) at birth and over childhood are positively associated with IQ (Broekman et al., Reference Broekman, Chan, Chong, Quek, Fung, Low and Saw2009; Fattal-Valevski et al., Reference Fattal-Valevski, Toledano-Alhadef, Leitner, Geva, Eshel and Harel2009; Heinonen et al., Reference Heinonen, Räikkönen, Pesonen, Kajantie, Andersson, Eriksson and Lano2008; Lira et al., Reference Lira, Eickmann, Lima, Amorim, Emond and Ashworth2010). However, less is known of how other anthropometric traits and different developmental trajectories are associated with neurodevelopment. Previous studies have shown that early rapid growth in height and HC is associated with higher IQ in mid-childhood in children with normal birth weight (Fattal-Valevski et al., Reference Fattal-Valevski, Toledano-Alhadef, Leitner, Geva, Eshel and Harel2009; Heinonen et al., Reference Heinonen, Räikkönen, Pesonen, Kajantie, Andersson, Eriksson and Lano2008; Lira et al., Reference Lira, Eickmann, Lima, Amorim, Emond and Ashworth2010). This association can go back to the prenatal life, as a positive association between HC in early gestation and IQ in mid-childhood was also found (Walker et al., Reference Walker, Thame, Chang, Bennett and Forrester2007). On the other hand, a review of 14 studies did not find evidence that weight gain during the first 2 years of life is associated with future IQ in children with normal birth weight (Beyerlein et al., Reference Beyerlein, Ness, Streuling, Hadders-Algra and von Kries2010).
The background mechanisms of these associations are, however, not clear. It is well-known that multiple maternal factors, such as smoking and low social position, can affect the birth weight and physical development of the newborn, as well as neurological development (Valero De Bernabe et al., Reference Valero De Bernabe, Soriano, Albaladejo, Juarranz, Calle, Martinez and Dominguez-Rojas2004). Also, gestational age has a longstanding effect on neurodevelopment and can thus partly explain these associations (de Kieviet et al., Reference de Kieviet, Piek, Aarnoudse-Moens and Oosterlaan2009). In this respect twin studies are important. As the postnatal environment is similar for both co-twins and they have the same gestational age, the association between physical and neurological development within twin pairs is likely to be due to intra-pair differences in prenatal conditions. In a US study, low birth weight was only weakly associated with cognitive and motor development within twin pairs (Datar & Jacknowitz, Reference Datar and Jacknowitz2009), while in a cohort of twin children in Chile, birth weight showed a clear association with school performance in mid-childhood within twin pairs (Torche & Echevarria, Reference Torche and Echevarria2011). Similar within pair associations between birth weight and IQ have also been found in the United Kingdom and New Zealand monozygotic (MZ) twins and United States twins (Edmonds et al., Reference Edmonds, Isaacs, Cole, Rogers, Lanigan, Singhal and Lucas2010; Newcombe et al., Reference Newcombe, Milne, Caspi, Poulton and Moffitt2007; Ross et al. Reference Ross, Krauss and Perlman2012). In a longitudinal study of Dutch twins, there was an inverse association between catch-up growth during the first 2 years of life and IQ in late childhood; however, this result can be partly explained by catch-up growth in low birth weight children (Estourgie-van Burk et al., Reference Estourgie-van Burk, Bartels, Hoekstra, Polderman, Delemarre-van de Waal and Boomsma2009).
Most previous studies have used IQ as an indicator of neurodevelopment. However, delayed motor development may be an equally good or better indicator of long-term neurodevelopmental problems (Diamond, Reference Diamond2000). Further, most of the longitudinal studies have used only one or two anthropometric indicators. Comparing several indicators gives information on which of them would be the best predictor of problems in neurocognitive and motor development. In this study, we analyze growth in weight, length, HC, and chest circumference (CC) over infancy in Japanese twins and how these are associated with different milestones of motor development until mid-childhood. Studying twins discordant for motor development allows us to account for shared maternal and postnatal environmental factors.
Material and Methods
The participants of this study were recruited from the West Japan Twins and Higher Order Multiple Births Registry (Yokoyama, Reference Yokoyama2013). Questionnaires were sent to 803 mothers of twins in the year 2009 (response rate 52%). To make the data more homogeneous, we removed the children born before 1989 or after 2002 (N = 9 pairs). Together, we had information on 740 twin children (370 pairs) in our study cohort (54% girls, 57% MZ twins). When answering the questions on the physical and motor development of their children, the mothers were advised to refer to the Maternal and Child Health Handbook provided to all expecting mothers by the authorities, which includes all measures done for their children. Anthropometric measures for newborns are completed first at hospitals and after that in health check-ups covering virtually all Japanese children. The measurement protocol until 1 year of age included measures of length, weight, HC and CC, and was performed by public health nurses. HC was measured as the largest occipitofrontal circumference using a plastic tape and CC by placing the tape under the arm in the axillae when the child was inhaling normally. Length was rounded to the nearest centimeter, weight to the nearest gram and CC and HC to the nearest 0.1 cm. Information on zygosity was based on validated questions on physical similarity and confusion of identity by others (Ooki & Asaka, Reference Ooki and Asaka2004). The twins were 7 years old or older at the time of survey, thus decreasing the likelihood of misclassification of zygosity, which is a problem when studying infant twins (Forget-Dubois et al., Reference Forget-Dubois, Pérusse, Turecki, Girard, Billette, Rouleau and Tremblay2003). Information on the age at reaching eight motor developmental milestones (maintain head, roll over, sit alone, stand holding on, walk holding on, walk independently and running) was reported in full months.
We tested the associations between the growth trajectories of anthropometric measures and the motor developmental milestones using linear mixed models in the package lme4 of R 2.15.2 statistical software (www.r-project.org). We first fitted a model having an anthropometric trait as a dependent and age as an independent variable. Thus, linear growth over infancy is seen as a positive effect of age. We then included age squared into the model to test whether growth slows down during the infancy, indicated by a negative effect of age squared. Age at birth was calculated from standard gestation of 38 weeks. Thus, earlier or later gestational age was taken into account in the analyses as days before or after age zero. After that we tested the interaction effects between age and age squared with the motor developmental milestones. This indicates whether the growth trajectories are different depending on the motor development. The effect of sample design, that is, sampling families with twin pairs rather than independent individuals on the standard errors and subsequently two-sided p values and 95% confidence intervals, was taken into account by estimating robust standard errors in all individual level analyses.
We continued the analyses by studying the associations within twin pairs. Because the age at achieving the motor development milestones was reported in full months, we were able to identify only twin pairs discordant for at least 1 month difference in the motor development. We identified twin pairs discordant for one or several milestones (236 pairs, 64% of all twin pairs). As the data was not large enough to analyze twin pairs discordant for each milestone separately, we classified the discordance based on whether a co-twin was ahead in the majority of the milestones. The correlations between the motor development indicators varied from modest to high (r = .17–.83). However, within-pair similarity in these different milestones was higher. Only 31 pairs showed difference in the discordance status that was dependent on the used milestone; in the majority of these cases only one indicator was different. We then calculated mean values of the anthropometric indicators for the delayed and advanced co-twins and tested the statistical significance of the difference by using the t test of paired samples. The Stata/SE 11.2 statistical software (StataCorp, College Station, Texas, USA) was used in these analyses. Finally, we tested the similarity of the trajectories of anthropometric measures within the discordant twin pairs (R 2.15.2, the package lme4). Differences in the level of anthropometric measures between discordant co-twins would be seen as the main effect of age and possible catch-up growth as the effect of age squared.
Results
Table 1 presents the basic characteristics of twin individuals by sex and zygosity. No systematic differences in the motor development milestones or anthropometric measures were found between MZ and dizygotic (DZ) twins or between boys and girls, and thus we used the pooled data in the further analyses. The average ages at achieving the milestones varied from 3.6 months for maintaining head to 22.2 months for running. Growth was most rapid after birth and then declined until 1 year of age. From birth to 1–3 months of age, growth in length was 5.3 cm, in weight 1.3 kg, in HC 3.7 cm and in CC 5.1 cm. From 9–11 to 11–13 months of age, the growth was 2.9 cm, 0.5 kg, 0.9 cm and 0.9 cm, respectively.
TABLE 1 Physical and Motor Development By Sex and Zygosity Among Twin Individuals

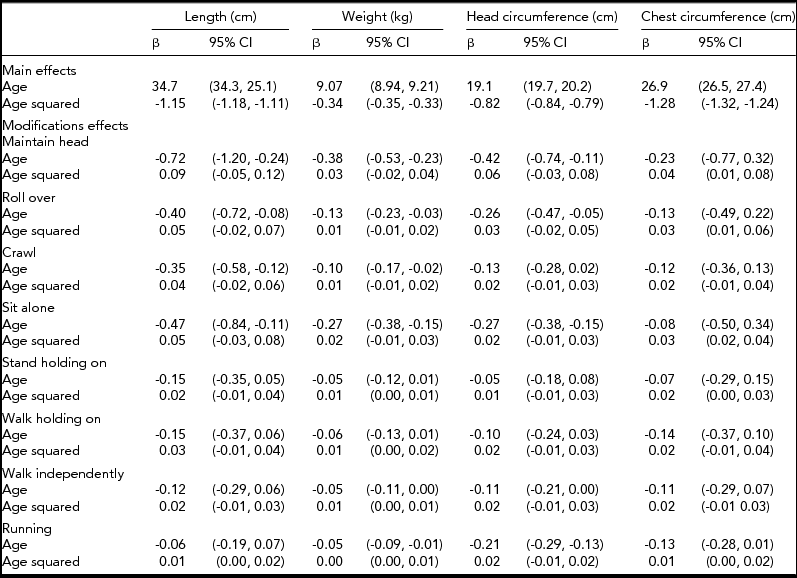
We then fitted growth models to these data (Table 2). As expected, a positive effect of age and negative effect of age squared was found for all anthropometric traits showing curvilinear growth trajectories. We then analyzed how motor development modified these trajectories. We found that for all motor developmental milestones and anthropometric traits, the modification effect with the linear effect of age was negative and with age squared, positive. The negative linear effect of age indicates that those children whose motor developmental milestones are delayed are smaller over infancy. Further, the positive effect of age squared indicates that these children catch up with other children over infancy in the growth of anthropometric traits. However, most of these modification effects with age squared reflecting catch-up growth were not statistically significant. Generally, the age interaction effects were stronger with earlier than later milestones. However, statistically significant associations were found with weight and HC also with the last milestone — that is, running.
TABLE 2 Parameters for Infant Growth Per 10 Months and Motor Development Milestone Modifications of These Parameters
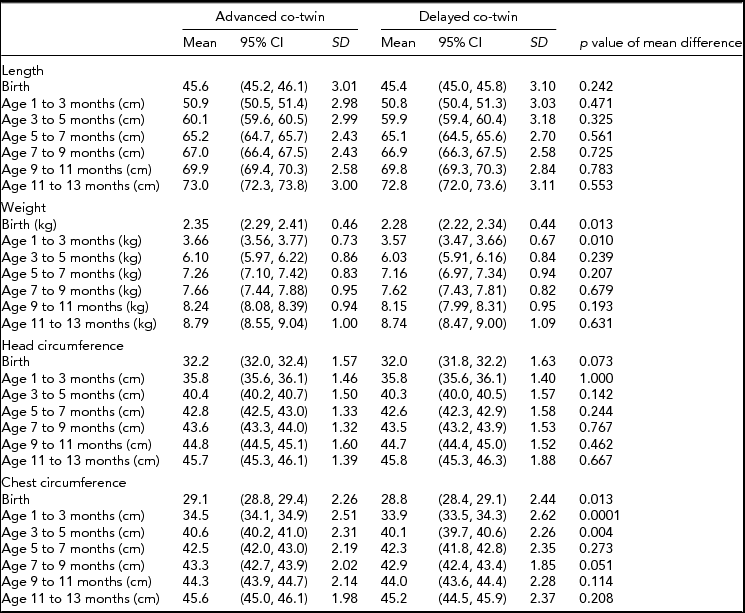
We continued the analyses by studying more detailed growth in twin pairs discordant for the age at achievement of motor developmental milestones (Table 3). The most consistent association was found for CC where the co-twin who was delayed in the motor development had lower CC from birth until 3–5 months of age compared to the advantaged co-twin; at 7–9 months of age the difference was marginally statistically significant. For weight, a difference was found from birth until 1–3 months of age. For the other anthropometric indicators, the differences were not statistically significant, but they were generally to the same direction such that poorer growth was associated with slower motor development. Finally, we conducted the growth model for the discordant twin pairs. However, the age (p = .38–.68) or age-squared effects (p = .47–.57) did not differ statistically significantly, suggesting that the growth trajectories were at a similar level and showed the same shape over infancy within discordant twin pairs.
TABLE 3 Physical Development in Twin Pairs Discordant for Motor Development
Discussion
Our results showed that length, weight, HC and CC in infancy are all associated with motor development among twin individuals; greater size at any time point was associated with earlier achievement of milestones, that is, faster development. It is well-known from previous studies that low birth weight, short birth length and small HC are associated with delayed cognitive development (Broekman et al., Reference Broekman, Chan, Chong, Quek, Fung, Low and Saw2009; de Kieviet et al., Reference de Kieviet, Piek, Aarnoudse-Moens and Oosterlaan2009; Fattal-Valevski et al., Reference Fattal-Valevski, Toledano-Alhadef, Leitner, Geva, Eshel and Harel2009, Heinonen et al., Reference Heinonen, Räikkönen, Pesonen, Kajantie, Andersson, Eriksson and Lano2008; Lira et al., Reference Lira, Eickmann, Lima, Amorim, Emond and Ashworth2010; Wheeler et al., Reference Wheeler, Bresnahan, Shephard, Lau and Balk2004). However, our study differs in two important ways from these previous studies. First, while in most studies neurodevelopment was measured using IQ, we found the corresponding associations for multiple indicators of motor development as well. In our study the physical development in infancy was most strongly associated with the earliest motor development milestone — that is, maintaining head — and showed mostly weaker association for the later milestones. However, we found clear associations also for the last milestone (i.e., running). This shows that early physical development is widely associated with different indicators of motor development. Second, while most of the previous studies have been conducted in Caucasian populations, we demonstrated this association in an Asian population. Thus, even though height and weight are generally lower in Asian populations compared to Caucasian populations from birth until adulthood (Eveleth & Tanner, Reference Eveleth and Tanner2003), corresponding associations between anthropometric indicators and motor development can still be seen, suggesting a universal nature of this effect independent of ancestry group.
By including twins our study design gave us an opportunity to obtain more information on the background of these associations. Studying co-twin differences within twin pairs allows one to adjust for gestational age as well as many maternal and postnatal environmental factors and is thus more informative than the population level associations alone. When studying twins discordant for motor development, CC showed the strongest association. Thus, CC seems to be more strongly associated with motor development than the other anthropometric indicators, independently of family and other factors shared by the twins. CC is used in developing countries as an indicator of nutritional status of the newborn (Goto, Reference Goto2011), but it is not widely used in developed countries. In our previous study, we found that birth weight was correlated with CC from birth until 1 year of age and these correlations were explained by environmental rather than genetic factors (Silventoinen et al., Reference Silventoinen, Kaprio, Dunkel and Yokoyama2012). Thus, birth weight and development of CC seem to capture the same intrauterine environmental variation, which is also relevant for further motor development.
When studying developmental trajectories, we found evidence that delayed motor development was associated with catch-up growth of all anthropometric indicators over infancy. Many of these effects were not statistically significant, but they were all in the same direction in providing more evidence that these effects are not merely due to sampling error. This is in contrast to previous studies which have reported that higher IQ in mid-childhood is associated with rapid growth in height and HC and is not associated with weight gain (Beyerlein et al., Reference Beyerlein, Ness, Streuling, Hadders-Algra and von Kries2010; Fattal-Valevski et al., Reference Fattal-Valevski, Toledano-Alhadef, Leitner, Geva, Eshel and Harel2009; Heinonen et al., Reference Heinonen, Räikkönen, Pesonen, Kajantie, Andersson, Eriksson and Lano2008; Lira et al., Reference Lira, Eickmann, Lima, Amorim, Emond and Ashworth2010). A likely reason for this is that in these previous studies the children had normal birth weight whereas our study included twins who have generally lower birth weight than singletons. Similar results to our study have been found in a Dutch twin study suggesting that catch up-growth in length is associated with lower IQ (Estourgie-van Burk et al., Reference Estourgie-van Burk, Bartels, Hoekstra, Polderman, Delemarre-van de Waal and Boomsma2009). On the other hand, we did not find evidence that growth trajectories differed within twin pairs. This is partly because of lack of power in these analyses, but can also indicate that gestational age and other birth-related factors shared by co-twins explain the association between physical development trajectories in infancy and motor development over early childhood.
Our data have strengths but also limitations. The strength of our data is that we have longitudinal measures of anthropometric indicators, including HC and CC, and information on the age of achievement of several key motor developmental milestones. Especially CC is rarely available in studies on cognitive and motor development. Further, because our participants are twins, we were able to optimally take into account gestational age and many maternal and postnatal environmental factors. Even when technically reported by mothers, the measurements of weight, length, HC and CC were performed by trained public health nurses using the standardized measurement protocol, and thus the reliability of the measures is expected to be good. However, measurement error, especially for length, HC and CC in infancy, cannot be avoided and may have weakened the found associations. A limitation of our data is that as our sample size was only moderate, we did not have power to analyze whether the associations were similar in MZ and DZ twin pairs. This would have given more information on the role of genetic factors behind of these associations. Also probably because of lack of power, the catching-up effects, even when systematically found, were mostly statistically non-significant. Further, age at the achievement of the motor development milestones was reported in full months, and thus we were able to identify only the most discordant pairs. Differences of less than 1 month are unlikely to be clinically relevant. On the other hand, larger difference may reflect pathological cases not in the main focus of this study. Reporting motor development in full months also made it impossible to estimate the role of genetic and environmental factors behind of these associations. Finally, the response rate was only moderate (53%). However, this was partly compensated by our sample design where mothers reported retrospectively information for both of the co-twins. Thus, we did not have incomplete twin pairs or attrition over the follow-up in our data, which may have biased the results. It is also noteworthy that the mothers reported the achievements of the milestones retrospectively based on the information recorded in Maternal and Child Health Handbook when the children were 7 years old or older. Thus, responses are not likely to be related to early growth or milestone achievement as it happened some years after the achievement of the last milestone. Also, the MZ/DZ ratio in our data (1.33) was similar to this ratio in the general Japanese population in largely overlapping birth cohorts (1.18) suggesting that our data is representative (Imaizumi, Reference Imaizumi2003).
In conclusion, length, weight, HC and CC from birth until 1 year of age are associated with motor development until mid-childhood. When studying discordant twin pairs and thus taking into account gestational age and many maternal and post-natal environmental factors, CC showed the most robust association with motor development. CC may offer additional benefits for surveillance of childhood development as compared to other anthropometric measures. CC should be evaluated further to see whether it might be of value in the healthcare of infants also in developed countries.
Acknowledgments
This study is supported by the Academy of Finland (grants 265240 and 263278 for JK and 266592 for KS).





