Vitamin A deficiency (VAD) is of public health significance if 15 % or more of pre-school children have serum retinol concentration less than 0·7 μmol/l(Reference de Pee and Dary1, Reference Sommer and Davidson2). The 2006 Uganda Demographic and Health Survey (UDHS 2006) assessed VAD in children aged 6–59 months by testing for retinol-binding protein (RBP), a surrogate of retinol, in dried blood spots (DBS)(Reference Baingana, Matovu and Garrett3). The VAD prevalence estimate in children was 20·4 %(4). Circulating concentrations of serum retinol(Reference Filteau, Morris and Abbott5–Reference Thurnham, McCabe and Northrop-Clewes7) and RBP(Reference Baeten, Richardson and Bankson8) decline in response to the acute-phase response and may not adequately reflect vitamin A status. These changes, which are an integral part of the acute-phase response, reflect decreased synthesis of RBP and increased excretion of retinol in urine(Reference Mitra, Alvarez and Wahed9, Reference Mitra, Alvarez and Guay-Woodford10). Because levels of circulating acute-phase proteins (APP) can aid in the interpretation of low serum retinol levels, the simultaneous measurement of retinol and one or more APP is recommended(Reference Filteau, Morris and Abbott5–Reference Thurnham, McCabe and Northrop-Clewes7, Reference Paracha, Jamil and Northrop-Clewes11–Reference Wieringa, Dijkhuizen and West13).
There is currently no consensus on how to correct retinol concentrations for the influence of the acute-phase response. Paracha et al.(Reference Paracha, Jamil and Northrop-Clewes11) proposed the application of new cut-offs for low and deficient retinol concentrations for children in whom α1-antichymotrypsin and α1-acid glycoprotein concentrations were elevated. In the approach proposed by Thurnham et al.(Reference Thurnham, McCabe and Northrop-Clewes7), individuals with normal APP levels provide the reference group for whom it is assumed that diet is the main factor responsible for the concentration of retinol. The median retinol value for the reference group is divided by the respective median values for groups at different stages of the acute-phase response. Correction factors thus obtained were applied to apparently healthy HIV-1-positive adults with raised APP; the proportion with low plasma retinol concentration (<0·7 μmol retinol/l) was significantly reduced from 20 % to 16 %(Reference Thurnham, Mburu and Mwaniki14). Wieringa et al.(Reference Wieringa, Dijkhuizen and West13) recommend the application of correction factors estimated using a general linear model based on the differences in plasma retinol concentrations between individuals with normal C-reactive protein (CRP) and α1-acid glycoprotein, and those with raised CRP and α1-acid glycoprotein. An alternative approach is to exclude the individuals with raised APP from the analysis. However, excluding individuals with raised APP may result in sampling bias for variables such as age, gender and history of hospitalization(Reference Maqsood, Dancheck and Gamble15). Moreover, exclusion of individuals results in a reduced sample. Regardless of the approach, the consensus is that including measurements of APP will improve the interpretation of results when vitamin A status is assessed using retinol and RBP. The application of a correction factor is a logical approach for interpreting levels of serum retinol(Reference Thurnham, Mburu and Mwaniki12–Reference Thurnham, Mburu and Mwaniki14) and other nutritional biomarkers(Reference Mburu, Thurnham and Mwaniki16–Reference Mburu, Thurnham and Mwaniki18) that are influenced by infection, when these indicators are measured in populations with a high burden of infection.
We previously reported on the assessment of the prevalence of VAD in the UDHS 2006 using an enzyme immunoassay to measure the levels of RBP in DBS(Reference Baingana, Matovu and Garrett3). However, we did not measure any APP at that time. Given the extent of the burden of infection in Uganda(19) there is a high likelihood that many of the young children who participated in UDHS 2006 had subclinical infection/inflammation and therefore raised APP, which probably influenced the VAD prevalence estimate. We report here the effect of the acute-phase response on the VAD prevalence estimate in children in a population-level survey in Uganda using CRP as a biomarker of inflammation.
Methods
Study design and participants
The DBS samples analysed in the present study were collected as part of the UDHS 2006, which was the fourth Demographic and Health Survey to be conducted in Uganda and the first Demographic and Health Survey to cover the entire country. The study design and selection of the survey sample are described in the UDHS 2006 Report(4). Briefly, the UDHS 2006 was a household-based survey in which a representative probability sample of 9864 households was selected from 368 clusters. Vitamin A status was assessed in one-third of the selected households. All children aged 6–59 months in these households were eligible for RBP testing. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Uganda National Council for Science and Technology. Verbal informed consent for RBP testing was obtained on behalf of the children from the parent or another adult responsible for the child. Verbal consent was witnessed and formally recorded. Consent was also obtained specifically for additional tests on the DBS(4).
Sample size and selection of dried blood spots for analysis of C-reactive protein
Blood samples were collected from a total of 2460 children (aged 6–59 months) from May to October 2006(Reference Baingana, Matovu and Garrett3). The collection, transport and storage of the DBS were described previously(Reference Baingana, Matovu and Garrett3). Briefly, blood samples were collected at the household from eligible children by finger or heel prick. Blood drops were allowed to fall freely to fill 13-mm diameter pre-printed circles on filter paper cards (Whatman 903; GE Healthcare Ltd, Piscataway, NJ, USA). The filter paper cards were labelled and dried overnight in opaque boxes containing desiccant (Minipak; Multisorb Technologies, Buffalo, NY, USA), packed individually in low-gas-permeable plastic zip-lock bags containing desiccant and humidity indicator cards, and stored at −20°C until analysis. Analysis for RBP using enzyme immunoassay (SCANLISA® RBP Assay; Scimedx Corporation, Denville, NJ, USA) was carried out in 2006 and is described elsewhere(Reference Baingana, Matovu and Garrett3). In preparation for CRP analysis, all 2460 DBS were checked individually for stability (humidity status) and adequacy of spots. After elimination of unsuitable samples, the sample frame comprised 1554 DBS. The samples were arranged by region and every other sample was selected, giving a total of 775 samples selected. Analysis for CRP was carried out in May and June 2008.
Laboratory procedures
Reagents
CRP was determined using an in-house enzyme immunoassay(Reference Brindle, Fujita and Shofer20) which was optimized in our laboratory before analysis of samples. The following reagents were used: capture antibody was a mouse monoclonal antibody to CRP, clone C5 (#M86005 M, 6·5 mg/ml stock; Biodesign International, Saco, ME, USA); signal antibody was a mouse monoclonal antibody to CRP clone C6, biotin conjugated (Biodesign #M86284B, 1·5 mg/ml stock); CRP calibrator (#30-AC10, 2·7 mg/ml; Fitzgerald Industries International, Action, MA, USA); and horseradish peroxidase (HRP)-conjugated streptavidin (Invitrogen #43-8323; Life Technologies, Grand Island, NY, USA). The following solutions were prepared using reagents from AppliChem GmbH (Darmstadt, Germany): coating buffer (0·20 m-NaHCO3, pH 9·6); CRP assay buffer (0·01 m-phosphate buffer, 0·5 m-NaCl, 0·1 % (v/v) Tween 20, pH 7·2 ± 0·3); wash solution (0·15 m-NaCl, 0·05 % (v/v) Tween 20); and citrate buffer (0·05 m, pH 4·0). 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) solution was prepared by dissolving each ABTS tablet (#9941; Sigma-Aldrich, St Louis, MO, USA) in 50 ml of 0·05 m-phosphate–citrate buffer, pH 5·0. Hydrogen peroxide (30 % v/v) was added to the ABTS solution immediately before use (0·25 μl/ml).
Elution of dried blood spots for C-reactive protein
The filter paper cards with the DBS were removed from the freezer and one disc from a blood spot on each card, corresponding to an individual child, was punched out using a standard 3·2 mm (1/8 in) hole punch and placed in a micro centrifuge tube. CRP assay buffer (500 μl) was added to each tube. Each 3·2 mm disc contains ∼1·5 μl of serum(Reference Mei, Alexander and Adam21); this corresponds to a dilution factor of ∼1:333·3. The tubes were vortexed for 15 s and centrifuged at 5000 rpm for 2 min. Samples were incubated overnight at 4°C. The following day, samples were removed from refrigeration and rotated at 350 rpm at room temperature for 1 h.
C-reactive protein assay protocol
Microwell plates (Nunc™ MaxiSorp; Nalge Nunc International, Rochester, NY, USA) were coated with capture antibody (2 μg/ml) in coating buffer (100 μl/well), covered with microplate lids and incubated at 4–8°C for not more than 72 h. On the day of the assay, the plates were removed from the refrigerator, washed three times with wash buffer and blocked with CRP assay buffer (200 μl/well). The plates were incubated for at least 30 min and for not more than 2 h at room temperature after which they were washed three times with wash buffer. Then, 100 μl of calibrators (eight points ranging from 0 ng/ml to 20·2 ng/ml), eluted DBS samples and internal controls were added in duplicate to appropriate microwells. The plates were covered, rotated on a plate shaker (Stuart® SSM5 Microtitre Plate Shaker; Bibby Scientific Limited, Stone, UK) at 250 rpm for 1 min and incubated overnight at 4°C. The next day, the plates were washed three times with wash buffer and then signal antibody (500 ng/ml) in CRP assay buffer (100 μl/well) was added. The plates were incubated for 2 h at room temperature after which they were washed four times with wash buffer. HRP-conjugated streptavidin diluted 1:3000 in CRP assay buffer was added (100 μl/well). After 1 h incubation at room temperature, 100 μl of freshly prepared substrate solution was added. The plates were shaken at 250 rpm at room temperature for 60–90 min and read at 405 nm on a microplate reader (Sunrise™ Remote Control; Tecan Austria GmbH, Salzburg, Austria). Unknown concentrations were calculated from the best-fit four-parameter logistic standard curve (GraphPad Prism® version 3·02). An acceptable agreement between duplicates was defined as a CV of ≤10 % between the optical densities of duplicates according to established assay acceptance criteria. Internal DBS controls and a pooled serum control were run with each plate. The inter-assay variability was 7·4 % for serum and 6·2 % for DBS. No external quality controls were included. CRP concentrations recorded as being below the lowest detectable level of the assay or higher than the limit of detection were classified as normal and raised, respectively.
Validation of dried blood spots as a sample matrix for C-reactive protein
Fifty matching serum and DBS samples collected from volunteers in June 2008 were used to validate DBS as a sample matrix for CRP analysis. Venous blood samples (∼3 ml) were drawn into plain vacutainer tubes. Serum was separated by centrifugation on the day of sampling, separated into aliquots and frozen at –20°C until analysis, which was carried out in the same month that the samples were collected. Matching DBS samples were collected, stored and eluted as described above. Serum was first diluted 1:333·3 (1·5 μl of serum in 500 μl of CRP assay buffer). Matching DBS eluate and diluted serum were loaded onto the same coated microwell plates in order to exclude potential variations that would arise from analysing matching samples on separate plates and were analysed as described above. The validation experiment demonstrated excellent correlation between CRP values of matching serum and DBS (r = 0·84, P < 0·0001). A factor to correct DBS CRP values to serum CRP values was derived by dividing each serum CRP value by its matching DBS CRP value. The median of the values obtained was 4·24; this was then used to correct all DBS CRP values. These validation data are summarized in Table 1. A similar validation experiment for RBP had already demonstrated an excellent correlation between RBP values of matching serum and DBS samples (r = 0·79, P < 0·00001). A correction factor of 1·44 was used to correct all DBS RBP values(Reference Baingana, Matovu and Garrett3).
Table 1 Validation of DBS as a matrix for CRP analysis
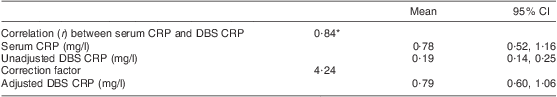
DBS, dried blood spot; CRP, C-reactive protein.
Fifty matched DBS and serum samples.
*P = 0·000.
Data analysis
Logarithmic transformation of the data was performed in order to normalize the skewed distribution. The resulting values are presented in their original units. The method of applying a correction factor(Reference Thurnham, McCabe and Northrop-Clewes7) was used to correct RBP values for the effect of the acute-phase response. First, the children were classified as ‘normal CRP’ (Group A) or ‘raised CRP’ (Group B) on the basis of a CRP cut-off of 5 mg/l(Reference Thurnham, Mburu and Mwaniki12). A correction factor of 1·127 was obtained as the ratio of the geometric means of the RBP concentrations of Group A to Group B (the difference between mean log RBP value for Group A and mean log RBP for Group B is back-transformed to give the correction factor). Group B RBP values were then multiplied by the correction factor to give the corrected RBP values. Cross-tabulation was used to obtain frequencies and proportions of children in Group A and in Group B by background characteristics and of VAD before and after correction for the influence of the acute-phase response indicated by raised CRP. Pearson's χ 2 test was used to measure associations between CRP status and background characteristics and P values less than 0·05 were considered as statistically significant. Data analysis was done using the statistical software package SPSS 13·0 for Windows.
Results
Seventy-three of the results were excluded after laboratory analysis because the children were aged ≥60 months. A further forty-one results were excluded because of invalid results (CV between duplicates >10 %), leaving 661 samples that were included in data analysis. As Table 2 shows, the background characteristics of the 661 children included in data analysis are comparable to those of the parent UDHS 2006 sample of the children from whom the sub-sample was drawn; except for the lowest wealth quintile which has a higher representation in the sub-sample compared with the parent sample (24·0 % v. 20·3 %, P = 0·04) and the second wealth quintile which has a lower representation in the sub-sample compared with the parent sample (18·6 % v. 22·5 %, P = 0·03).
Table 2 Comparison of background characteristics of children (aged 6–59 months) between the sub-sample and the parent sample, UDHS 2006

UDHS 2006, 2006 Uganda Demographic and Health Survey,
Seventy-three of the 775 results were excluded after laboratory analysis because the children were aged ≥60 months. A further forty-one results were excluded because of invalid results (CV between duplicates >10 %), leaving 661 samples.
The difference between the parent UDHS sample and the sub-sample was significant: *P < 0·05.
Mean CRP was 6·2 (95 % CI 5·5, 7·0) mg/l; the prevalence of raised CRP (>5 mg/l) was 55·7 %. Children in Group A had a mean CRP of 1·6 (95 % CI 1·5, 1·8) mg/l compared with 17·9 (95 % CI 16·4, 19·6) mg/l for the children in Group B. As Table 3 shows, there were some differences in background characteristics between children in Group A and Group B. Group B had a higher proportion of children aged 12–23 months (P = 0·05) and a lower proportion of children aged 48–59 months (P = 0·01), compared with Group A. The association between CRP status and age was significant (χ 2 = 13·6, P = 0·009). The proportion of children in Group B who live in Central 2 region was twice the proportion of children in Group A living in the same region (12·2 % (95 % CI 8·9, 15·5 %) v. 6·1 % (95 % CI 3·4, 8·9 %); P < 0·0 0 1). The association between CRP status and mother's education status was significant (χ 2 = 9·5, P = 0·009); the proportion of children in Group B whose mothers had no formal education was significantly higher than the proportion of children in Group A whose mothers had no formal education (26·9 % (95 % CI 22·4, 31·4 %) v. 18·1 % (95 % CI 13·7, 22·5 %); P < 0·0 0 1). There was no association with wealth quintile.
Table 3 Background characteristics of children (aged 6–59 months) with normal CRP (<5 mg/l, Group A) and raised CRP (≥5 mg/l, Group B), UDHS 2006

CRP, C-reactive protein; UDHS 2006, 2006 Uganda Demographic and Health Survey.
Seventy-three of the 775 results were excluded after laboratory analysis because the children were aged ≥60 months. A further forty-one results were excluded because of invalid results (CV between duplicates >10 %), leaving 661 samples.
The difference between Group A and Group B was significant: *P = 0·05, **P = 0·01, ***P = 0·001.
Figure 1 shows the mean RBP values of the children before and after correction for the acute-phase response. Geometric mean RBP of the whole group and of Group B before correction was 1·18 (95 % CI 1·14, 1·22) μmol/l and 1·12 (95 % CI 1·07, 1·17) μmol/l, respectively. Both were significantly different from geometric mean RBP of Group A (1·26 μmol/l; 95 % CI 1·20, 1·33 μmol/l). After correction, geometric mean RBP of Group B (1·26 μmol/l; 95 % CI 1·21, 1·31 μmol/l) and of the whole group (1·26 μmol/l; 95 % CI 1·22, 1·30 μmol/l) were not significantly different from geometric mean RBP of Group A.

Fig. 1 Retinol-binding protein (RBP) in children (aged 6–59 months, n 661) before (![]() $$$\raster="fx1"$$$) and after correction (
$$$\raster="fx1"$$$) and after correction (![]() $$$\raster="fx1"$$$) for the influence of the acute-phase response, 2006 Uganda Demographic and Health Survey. Values are geometric means, with 95 % confidence intervals represented by vertical bars. The difference in mean RBP of Group A (n 293) and Group B (n 368) before correction was significant: *P < 0·0001
$$$\raster="fx1"$$$) for the influence of the acute-phase response, 2006 Uganda Demographic and Health Survey. Values are geometric means, with 95 % confidence intervals represented by vertical bars. The difference in mean RBP of Group A (n 293) and Group B (n 368) before correction was significant: *P < 0·0001
The recommended cut-off for VAD of 0·7 μmol/l of serum retinol(Reference Sommer and Davidson2) is taken to be biologically equivalent to 0·825 μmol/l RBP(Reference Gorstein, Dary and Pongtorn22). Using this cut-off, the prevalence of VAD in Group B before correction (22·0 %; 95 % CI 17·8, 26·2 %) was higher than the prevalence of VAD in Group A (14·7 %; 95 % CI 10·6, 18·8 %). After correction, the prevalence of VAD in Group B (13·3 %; 95 % CI 9·8, 16·8 %) was comparable to the prevalence of VAD in Group A. In addition, the prevalence of VAD among all of the children after correction (13·9 %; 95 % CI 11·3, 16·5 %) was not significantly different from the prevalence of VAD in Group A. After correction the prevalence of VAD among all children was reduced from 18·4 % (95 % CI 17·2, 23·0 %) to 13·9 % (95 % CI 11·3, 16·5 %), which is just below the cut-off of 15 % at which VAD is deemed to be a public health problem. Out of 125 children with VAD and raised CRP, thirty-three changed status from VAD to vitamin A sufficiency after correction, representing a reduction of about 25 %. On the basis of these findings, we postulate that the prevalence of VAD would reduce by at least 25 % from 20·1 % to 15·4 %.
Discussion
The overall aim of the present study was to determine if the estimate of the prevalence of VAD in children of pre-school age in the 2006 UDHS was distorted by the influence of the acute-phase response on RBP and to improve this estimate by correcting RBP measurements for the effect of the acute-phase response. The results show that over half of this population-based sub-sample of children had raised CRP. The group with raised CRP had a significantly lower mean RBP and a significantly higher prevalence of VAD compared with the group with normal CRP levels. The same effect has been demonstrated in several other studies in children(Reference Filteau, Morris and Abbott5, Reference Thurnham, McCabe and Northrop-Clewes7, Reference Paracha, Jamil and Northrop-Clewes11–Reference Wieringa, Dijkhuizen and West13, Reference Kongsbak, Wahed and Friis23, Reference Filteau, Morris and Raynes24). After correction of the RBP values, the two groups had a comparable mean RBP and prevalence of VAD, and the overall prevalence of VAD of the sub-sample was significantly reduced. Given that the children with raised CRP constituted over half of the sub-sample and that the sub-sample was generally representative of the parent UDHS 2006 sample, we postulate that the prevalence of VAD reported in the UDHS 2006 could have been overestimated by as much as 25 %, which is the proportion by which VAD was overestimated in the present study. Effect sizes of similar magnitude have been reported(Reference Thurnham, McCabe and Northrop-Clewes7).
Our findings add to the body of evidence of the high rate of subclinical infection/inflammation in children who participate in population-based surveys in settings with a high burden of disease: the proportion of apparently healthy pre-school children with one or more raised APP was 45 % in the North West Frontier Province in Pakistan(Reference Paracha, Jamil and Northrop-Clewes11), 57 % in the Ghana Vitamin A Supplementation Trial(Reference Filteau, Morris and Abbott5) and 24 % in the 1996 National Nutrition Survey in Honduras(Reference Nestel, Melara and Rosado25). The data used in the meta-analysis by Thurnham and colleagues show that the rate of raised APP in apparently healthy pre-school children ranged from 17 % in the UK to 72 % in Ghana(Reference Thurnham, McCabe and Northrop-Clewes7).
There were some differences in background characteristics between children in Group A and Group B. Group B had a lower proportion of children aged 48–59 months and a higher proportion of children aged 12–23 months compared with Group A, reflecting the higher vulnerability to infection of children in this age group. These findings are substantiated by the UDHS 2006, which shows that children aged 6–11 and 12–23 months were most likely to have shown symptoms of acute respiratory infection and to have had fever and diarrhoea than children in other age groups(4). The proportion of children whose mothers had no formal education was significantly higher in Group B than it was in Group A. Again, the UDHS 2006 confirms that children whose mothers did not have any formal education were most likely to have shown symptoms of acute respiratory infection and to have had fever and diarrhoea in the two weeks preceding the survey(4). We re-emphasize the observation made by Maqsood et al.(Reference Maqsood, Dancheck and Gamble15) that excluding children with raised APP is not a suitable approach for estimating the prevalence of VAD in settings such as ours because it leads to sampling bias and to a substantially reduced sample.
A limitation of using DBS is that the composition of the eluate cannot be equated to serum or plasma and elution of the biomarker of interest may not be complete(Reference McDade, Williams and Snodgrass26), thus making interpretation of results from DBS samples a challenge. Nevertheless, the significant linear correlation between matched serum and DBS samples for many analytes(Reference Baingana, Matovu and Garrett3, Reference Brindle, Fujita and Shofer20, Reference Williams and McDade27–Reference Shirtcliff, Reavis and Overman30) implies that serum or plasma equivalents can be derived from DBS values(Reference Baingana, Matovu and Garrett3, Reference McDade, Burhop and Dohnal28, Reference Shirtcliff, Reavis and Overman30–Reference McDade and Shell-Duncan33). It is on this basis that we derived a value of 4·24 to correct the DBS CRP values to serum CRP values. However, the relationship between DBS values and plasma or serum values varies across analytic methods(Reference McDade, Williams and Snodgrass26, Reference Williams and McDade27), meaning that we are able to evaluate our correction factor relative only to Brindle et al.(Reference Brindle, Fujita and Shofer20) who used the same in-house enzyme immunoassay. The volume they used to elute their DBS ranged from 0·25 to 5 ml making their mean ratio of DBS CRP (venous or capillary) to serum CRP of 1·6 difficult to interpret relative to ours. Also to consider is haematocrit(Reference Mei, Alexander and Adam21), which has been identified as the single most important parameter that influences the spread of blood on DBS cards, ultimately affecting the reproducibility of assays especially when a sub-sample punch is taken from the DBS(Reference Timmerman, White and Globig34). The majority of the individuals in the study done by Brindle and colleagues were US adults(Reference Brindle, Fujita and Shofer20) who are reported to have higher haematocrit than Africans(Reference Karita, Ketter and Price35, Reference Zeh, Amornkul and Inzaule36). This further complicates the interpretation of their mean ratio of DBS CRP to serum CRP relative to ours and indicates that these correction factors cannot be applied universally.
A correction factor of 1·127 was obtained and used to adjust the RBP values of Group B. A meta-analysis of three studies with a total of 1797 apparently healthy pre-school children aged 1–5 years generated an adjustment factor of 1·25(Reference Thurnham, McCabe and Northrop-Clewes7). Using this factor to adjust the RBP values of Group B gives a mean RBP of 1·40 (95 % CI 1·34, 1·46) μmol/l, which is significantly higher than the mean RBP of the children with normal CRP (1·26 (95 % CI 1·20, 1·33) μmol/l). Thurnham and colleagues also found that specific correction factors derived from the data of a particular group of individuals are more effective than the meta-analysis correction factors(Reference Thurnham, Mburu and Mwaniki12, Reference Thurnham, Mburu and Mwaniki14).
Although CRP has been recommended as a reasonable choice of APP for interpreting the influence of the acute-phase response in the assessment of vitamin A status(Reference Stephensen and Gildengorin37), it is only elevated during the earlier, clinical stages of infection(Reference Young, Gleeson and Cripps38, Reference Cruickshank, Hansell and Burns39). Consequently, in measuring only CRP we may have underestimated the size of the effect of the acute-phase response on RBP. Despite the limitation of not including a marker of chronic infection in the current study, our findings demonstrate the utility of adjusting RBP levels for the effect of the acute-phase response. The results strongly suggest that by not adjusting for the acute-phase response, the prevalence of VAD in the UDHS 2006 was overestimated. Taking acute-phase response into account improves the population-level assessment of vitamin A status and allows a better comparison between populations with different rates of infection. Longitudinal studies will increase our understanding of the relationship between APP and micronutrient biomarkers. As more surveys incorporate nutritional biomarker measurement using DBS as the sample, there is a need to standardize the laboratory methods involved and to establish a proficiency testing programme.
Acknowledgements
Sources of funding: The 2006 UDHS was conducted by the Uganda Bureau of Statistics. Laboratory consumables, reagents, equipment and technical assistance were provided by the US Agency for International Development (USAID). Additional financial assistance for the UDHS 2006 was also provided by the UK Department of International Development, the USAID/Uganda Mission, the President's Emergency Plan for AIDS Relief, the Government of Uganda, the Health Partnership Fund, UNICEF, the United Nations Population Fund and the Government of Japan. Laboratory consumables, reagents, equipment and technical assistance for the supplemental CRP testing were provided by the USAID through ICF Macro International. Conflicts of interest: The authors do not have any conflict of interest to declare. Authors’ contributions: R.B. and D.G. designed the study; R.B. and D.M.-K. conducted the laboratory analyses; R.B. carried out data analysis, interpretation and drafted the manuscript; D.M.-K. and D.G. made major revisions to the manuscript and all authors approved it for submission. Acknowledgements: The authors acknowledge and are grateful to Eleanor Brindle, CSDE Biodemography Core, University of Washington, for the CRP protocol; and thank all of the women and children of Uganda who participated in the UDHS 2006. They are grateful to the following individuals and institutions: the field technicians, field supervisors, Andrew Mukulu, Helen Nviiri and Stephen Baryahirwa, all of the Uganda Bureau of Statistics; Fred Juuko of the Department of Biochemistry, Makerere University; and Joy Fishel of ICF Macro International. The authors appreciate the reviewers’ comments which enhanced the quality of the manuscript.






