Diabetes mellitus is characterised by hyperglycaemia, which has been strongly linked to diabetic complications such as neuropathy, retinopathy and nephropathy. Above all, diabetics are at augmented risk for end-stage renal disease consequent to diabetic nephropathy(Reference Selby, FitzSimmons, Newman, Katz, Sepe and Showstack1, Reference Held, Port, Webb, Wolfe, Garcia, Blagg and Agodoa2). Stringent control of the hyperglycaemia by insulin treatment has been shown to avert hypertrophy and hyperfiltration and the subsequent rise in urinary protein excretion(Reference Rasch3). Clinical studies suggest that there is yet no completely effective treatment for diabetic nephropathy(4). Hyperglycaemia is the principal factor responsible for structural alterations at the renal level and is directly linked to diabetic microvascular complications, particularly in the kidney(4); therefore, glycaemic control remains the main target of treatment. Prevention of nephropathy is a very important concern and many studies have been focused on traditional and herbal medicines to find novel therapeutic agents for diabetic nephropathy.
Green tea (Camellia sinensis; GT) is a rich source of polyphenols, particularly flavonoids, which have been shown to have numerous pharmacological effects. Studies using animal models show that GT catechins could be beneficial in suppressing high-fat diet-induced obesity by modulating lipid metabolism and providing some protection against lipid and glucose metabolism disorders implicated in type 2 diabetes, and could reduce the risk of CVD(Reference Matsumoto, Ishigaki and Ishigaki5–Reference Crespy and Williamson8). Administration of GT catechins in streptozotocin (STZ) diabetic animals drastically improved kidney function as a result of its anti-thrombogenic action, which in turn controls the arachidonic acid cascade system(Reference Yang, Choi and Rhee9). These studies also demonstrated an improvement in the glomerular filtration rate(Reference Yang, Choi and Rhee9–Reference Rhee, Choi and Park11). Yokozawa et al. (Reference Yokozawa, Nakagawa, Lee, Cho, Terasawa and Takeuchi12) examined variables of glomerular filtration in cisplatin (a nephropathy inducer)-treated rats and demonstrated that GT significantly decreased the blood N level, serum creatinine, serum malondialdehyde and kidney excretion of glucose and proteins and oxidative stress in the kidney(Reference Yokozawa, Nakagawa, Lee, Cho, Terasawa and Takeuchi12). Another study has shown that GT reduced serum glucose and creatinine levels and serum lipid peroxidation and increased serum superoxide dismutase, suggesting that catechins influence glucose metabolism and improve kidney function by reducing oxidative stress in alloxan-treated diabetic rats(Reference Sabu, Smitha and Kuttan13). Moreover, GT catechins decreased plasma insulin levels but did not affect plasma glucose levels in an oral glucose tolerance test in normal rats(Reference Wu, Juan, Ho, Hsu and Hwang14). In contrast, Mustata et al. have shown that GT drinking had a marginal effect on nephropathy parameters through improving renal mitochondrial defects; however, neither glycaemia nor urinary albumin were affected in GT-drinking diabetic animals(Reference Mustata, Rosca and Biemel15). These GT paradoxical effects on diabetic nephropathy animal models necessitate further studies to clarify the GT role on kidney function in the diabetic state. Accordingly, we tested the hypothesis that GT displays anti-diabetic properties on long-term follow up in the hypofiltration stage of STZ-treated diabetic animals. The effect of GT treatment on long-term (12 weeks) STZ-induced diabetic nephropathy was investigated by assessing serum and urine parameters indicative of nephropathy such as blood urea, N and serum creatinine, creatinine clearance (Ccr), urinary protein excretion and glycogen accumulation in kidney tubules.
Materials and methods
Animals
Male Sprague–Dawley rats (Kuwait Animal Laboratory Center Colony) weighing 200–230 g were cared for as outlined in the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioural Research (Institute for Laboratory Animal Research 2003). Rats were housed (four or five animals per cage) in standard plastic cages with wood chip bedding. Bedding was changed daily for all animals to maintain sanitary conditions. The animals were kept in well-ventilated rooms with adjustable light–dark cycle and temperature regulation systems. The rooms and animal cages were cleaned daily, and all animals were provided with fresh food and water on a daily basis. They were also inspected daily for any possible signs of inflammation and respiratory or gastrointestinal infection. If such signs were present, the animal was excluded from the study.
Green tea extract preparation
GT extract was made as described previously(Reference Renno, Saleh, Klepacek, Al-Khaledi, Ismael and Asfar16). Briefly, dried GT leaves were obtained from and packed in Sri Lanka (Swan brand, pure Ceylon tea; TC/E/PR/07/82; George Payne & Co. (Ceylon) Ltd, Colombo, Sri Lanka). The same quantity of GT was used in every preparation of the tea solution. Dried GT leaves (16 g) were added to 1 litre of deionised boiled water cooled to 80°C. The solution was kept to stand for 10 min before being filtered, cooled to room temperature, and dispensed in clean drinking bottles(Reference Jankun, Selman, Swiercz and Skrzypczak-Jankun17).
Experimental protocols and group treatments
Experimental rats (n 50) were injected intraperitoneally with STZ (75 mg/kg) (Sigma, St Louis, MO, USA) dissolved in 0·9 % saline(Reference Renno, Saleh, Klepacek, Al-Khaledi, Ismael and Asfar16). STZ solutions were freshly prepared due to the limited stability of the compound(Reference Rakieten, Rakieten and Moreshwar18). Non-diabetic rats were studied of the same age as an onset control, to provide a starting value against which to judge any diabetes-induced damage.
Blood glucose levels were determined in all animals using an Encore Glucometer (Bayar, Elkhart, IN, USA) from blood samples obtained by tail vein bleeds. The presence of diabetes was verified at 24 h by the presence of hyperglycaemia and glucosuria (Visidex II and Diastix; Ames, Slough, Berks, UK). After 2 weeks of uncontrolled diabetes, rats with blood glucose levels ≥ 2500 mg/l ( ≥ 14 mm) were randomly divided into different diabetic groups as detailed in Table 1: diabetic groups (2 weeks, 6 weeks, 8 weeks and 12 weeks) (ten rats per group) were given water to drink; 12 weeks diabetic+GT group (ten rats) were given GT to drink for 12 weeks instead of water as described previously(Reference Renno, Saleh, Klepacek, Al-Khaledi, Ismael and Asfar16); control normal groups (ten rats per group) were age-matched control animals and were injected (intraperitoneally) with an equal volume of 0·9 % saline (control vehicle) and given only water to drink. Water and GT bottles were made available to the animals ad libitum. The mean volume of GT extract intake was 122 (sem 10·22) ml/d. At the end of the experiments, plasma glucose was estimated (GOD–Perid method; Boehringer Mannheim, Mannheim, Germany) on samples taken from the tail vein.
Table 1 Changes in body weight†
(Mean values with their standard errors)

*** Mean value was significantly different from that of normal control rats of comparable age (P < 0·001).
† Initial body weight for all animals was 210·0 (sem 10·3) g.
At the end of the experiments, blood samples were taken by cardiac puncture and used for the determination of blood urea N, serum creatinine levels and glucose (GOD–Perid method; Boehringer Mannheim). Serum glycosylated protein level was measured using the method of McFarland et al. (Reference McFarland, Catalano, Day, Thorpe and Baynes19). The 24 h (before killing) urine was collected while rats were housed in metabolism cages and filtered for determination of urea N, glucose, protein and creatinine. Protein levels in freshly voided urine samples were assessed by the sulfosalicylic acid method(Reference Sakagishi, Saito, Kitamura and Niwa20). Urea N and creatinine were determined using the commercial test kits BUN Kainos and CRE-EN Kainos (Kainos Laboratory, Tokyo, Japan) and glucose by the Momose method(Reference Momose, Yano and Ohashi21). The Ccr was calculated on the basis of urinary creatinine, serum creatinine, urine volume and body weight using the following equation as described previously(Reference Yokozawa, Nakagawa, Oya, Okubo and Juneja22):
Ccr (ml/min per kg body weight) = (urinary creatinine (mg/l) × urinary volume (ml/d)/serum creatinine (mg/l) × (1000/body weight (g)) × (1/1440 (min)).
Specimen collection and histological staining
Respective groups (age-matched) of animals were killed after 2, 6, 8 and 12 weeks of the induction of diabetes(Reference Holson23). The kidneys were gently removed after opening the abdominal cavity. The fresh kidneys were gently bisected longitudinally and immediately immersed (fixed) in labelled vials containing 10 % of buffered formalin and kept overnight. The segments were washed with distilled water. Then, the vials were filled with 70 % alcohol (Riedel-de Haen AG, Seelze, Germany) and kept at 4°C overnight. On the next day, the specimens were dehydrated by a graded series of alcohol and then treated with xylene (Univar; Ajax Chemicals, Auburn, NSW, Australia) for 1 h. The tissues were then placed in cassettes and placed in a hot paraffin wax container at 60°C overnight. On the next day, the tissues were embedded in paraffin using paraffin embedding apparatus (Jung Histoembedder; Leica, Wetzlar, Germany). The paraffin blocks were then sectioned transversely (4 μm thick) using a microtome (Jung Histocut; Leica). Sections were stained with haematoxylin and eosin stains. Some sections from diabetic animals were stained with periodic acid-Schiff (PAS) and PAS-diastase special stains in order to verify that the areas of the tubules that showed clear cytoplasm were due to the accumulation of glycogen. The PAS stain was strongly positive and the PAS-diastase was also positive for cells with clear cytoplasm.
Quantification of glycogen-filled proximal tubules
For morphometric analysis, ten sections from each block (two blocks (right and left kidneys) from each rat) were subjected to quantitative analysis. Sections from the eight to ten rats in each group were quantified. Random light microscopic fields were digitally photographed from the kidney tissues to count the clear tubules using objective lens power 40 × .
Data analysis
A one-way ANOVA was applied to assess significant differences between the mean values of the number of clear cytoplasm tubules found in controls, untreated and GT-treated diabetic rat groups. The post hoc Scheffe F and LSD tests were performed to determine individual probability values for comparisons between groups using SPSS software (SPSS, Inc., Chicago, IL, USA). The results are presented as mean values with their standard errors and the graph drawn using SPSS software. Data collected from the blood and urine samples were analysed by one-way ANOVA followed by the post hoc Scheffe F test using SPSS software to determine the significant differences between the groups. All data are presented as mean values with their standard errors. A difference among the means at P < 0·05 was considered statistically significant.
Results
Changes in body weight
The average body weight of animals with diabetes was significantly lower than that of the normal control rats of comparable age (Table 1). Likewise, the body weights of the diabetic rats treated with GT were significantly (P < 0·001) lower than the normal control group. There was no significant difference between the mean body weights of the untreated diabetic group compared with GT-treated groups. The average gains in body weight were similar in untreated and GT-treated animals after 12 weeks of diabetes. These weight gains were 36·2 (sem 2·8) and 34·6 (sem 4·93) %, respectively.
The effect of green tea on glucose and glycosylated protein levels in serum
Table 2 shows the effect of GT consumption on the levels of serum glucose and glycosylated protein. In all diabetic treated and untreated animals, the serum glucose and glycosylated protein levels were significantly (P < 0·001) elevated compared with normal control rats. However, diabetic rats treated with GT showed a significant reduction in both serum glucose (P < 0·01) and glycosylated protein (P < 0·05) when compared with other diabetic groups. The level of serum glucose was lower in the GT-treated rats. This reduction was approximately 29·6 (sem 3·7) %. Likewise, serum glycosylated protein levels were lower in GT-treated rats. The serum glycosylated protein level was 22·7 (sem 5·2) % lower than in the diabetic group of comparable age.
Table 2 The effect of green tea (GT) on glucose and glycosylated protein in serum
(Mean values with their standard errors)

*** Mean value was significantly different from that of the control group (P < 0·001).
Mean value was significantly different from those of the diabetic groups:
† P < 0·05,
†† P < 0·01.
The effect of green tea on blood urea nitrogen, serum creatinine and creatinine clearance
Table 3 shows the effects of GT treatment on serum parameters under conditions of diabetes. The blood urea N level was significantly higher (P < 0·001) in 12-week diabetic animals than control rats of the same age (148 (sem 19) mg/l); whereas it was significantly lower (P < 0·01) in the GT rats. This reduction was 41·7 (sem 1·9) %. The serum creatinine level was significantly (P < 0·001) higher in rats with diabetes in comparison with normal control rats of the same age (Table 3). Those diabetic animals given GT showed a non-significant difference in creatinine level compared with the 12-week diabetic rats (P < 0·10). This reduction was 38·9 (sem 10) %. On the other hand, all diabetic groups, treated or untreated, showed significantly (P < 0·001) lower Ccr than the normal control animals. The GT-treated group showed a significant (P < 0·05) higher Ccr than that in untreated diabetic rats. This increase was 44 (sem 10·8) %.
Table 3 The effect of green tea (GT) on serum and urine kidney functional parameters
(Mean values with their standard errors)

BW, body weight.
Mean value was significantly different from that of the control group:
* P < 0·05,
*** P < 0·001.
Mean value was significantly different from those of the diabetic groups:
† P < 0·05,
†† P < 0·01·
‡ Mean value was marginally significantly different from those of the diabetic groups (P < 0·10).
The effect of green tea on glucose, protein, urea nitrogen and creatinine excreted in urine
Table 3 shows the effects of GT on the urinary function of diabetic animals. The glucose, protein, urea N and creatinine excretion rates were increased significantly (P < 0·001) (1·70-, 3·29-, 1·95- and 1·59-fold, respectively) in 12-week diabetic compared with normal control rats. After oral administration of GT, urinary urea N excretion was significantly lower (P < 0·01) compared with that in the 12-week diabetic group. Urea N decreased 30 (sem 7·6) % in the 12 weeks diabetic rats compared with GT-treated animals. Likewise, creatinine, glucose and protein excretion declined 35·4 (sem 5·3), 34·0 (sem 5·3) and 46·0 (sem 13·0) % in GT-treated animals compared with 12-week diabetic rats (Table 3).
Histopathological changes
Histological examination of the normal control kidney tissues showed normal kidney histology (Fig. 1 (A)). Haematoxylin and eosin sections examined under light microscopy showed that all the kidneys of the diabetic rats had multifocal areas of tubules with clear cytoplasm (Fig. 1 ((B)–(D)). Special PAS (Fig. 1 (E)) and PAS followed by diastase staining showed that the clear cytoplasm corresponded to glycogen accumulation. The PAS stain was strongly positive in 6-week- and 12-week-long diabetes and the PAS diastase effectively cleared the PAS stain indicating glycogen accumulation in the tubules of diabetic kidneys. Examples of 2, 6 and 12 weeks diabetic kidney tissues clearly show a gradual increase in the number of glycogen-filled tubules which was significantly decreased compared with normal control values in the GT-treated diabetic nephropathy group (Fig. 1). The number of tubules filled with glycogen per field in the diabetic kidneys was increased with time from zero to 25·5 (sem 2·01) at 12 weeks (Fig. 2). This increase was significantly (P < 0·001) higher compared with normal control animals. The number of glycogen-filled tubules (P < 0·001) decreased almost completely in GT-treated kidneys (Fig. 2). This decrease was 99·98 (sem 0·27) %.

Fig. 1 Representative light microscopic photographs of proximal tubules showing the effect of green tea (GT) extract treatment on diabetic nephropathy. (A) Control normal kidney section showing normal proximal tubule epithelium with eosinophilic and granular cytoplasm (haematoxylin and eosin (H&E); × 100). (B), (C) and (D) Kidney sections from 2-, 6- and 12-week diabetic nephropathy rats showing a gradual increase in the number of glycogen-filled proximal tubules ( → ). The 12-week diabetic section shows the highest level of glycogen accumulation in the proximal tubules (H&E; B and C: × 100; D: × 200). (E) Tissue section from 12-week diabetic kidney stained by special periodic acid-Schiff stain for the glycoprotein showing strong positive staining ( → ) in the lining of epithelium of the proximal tubules ( × 40). (F) GT extract treatment of diabetic animals for 12 weeks significantly decreased the number of glycogen-filled proximal tubules to zero level which was seen in the control normal animals of comparable age (H&E; × 100).
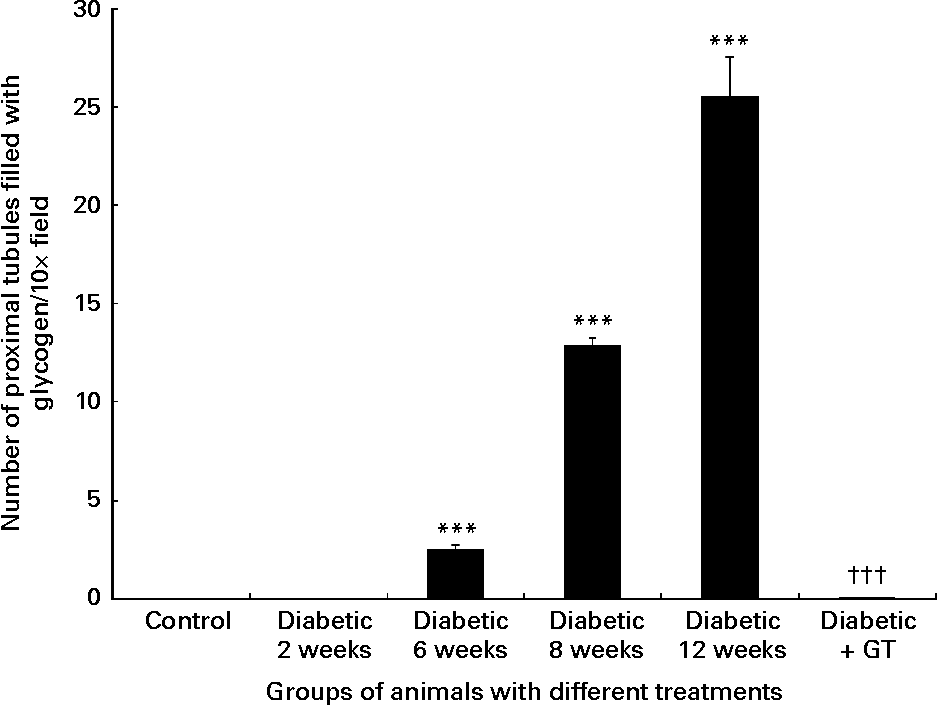
Fig. 2 Quantitative analysis of the number of proximal tubules filled with glycogen/field in the kidney sections. Note the significant steady increase in the number of the proximal tubules filled with glycogen in 6-, 8- and 12-week diabetic kidneys compared with the normal control group. The green tea (GT) treatment of diabetic rats significantly decreased the number of proximal tubules filled with glycogen to zero level compared with diabetic untreated animals. Values are means, with standard errors of difference indicated by vertical bars. *** Mean value was significantly different from that of normal control rats (P < 0·001). ††† Mean value was significantly different from that of 6-, 8- and 12-week diabetic groups (P < 0·001).
Discussion
The present results indicate that GT decreases glucose levels and renal injury associated with abnormal glucose-related oxidative stress in diabetic nephropathy. Furthermore, the present study shows a beneficial effect of GT on renal histochemical parameters, as it significantly prevented the accumulation of glycogen in the kidney tubules. It also reduced the serum levels of glucose, glycosylated proteins and creatinine and blood urea N levels compared with those in the untreated diabetic group of comparable age. In addition, the GT-treated group showed a significant increase in Ccr value and reduced creatinine and protein excretion. A progressive reduction in the glomerular filtration rate, reflected in increased serum creatinine and reduced Ccr levels, is the most common characteristic in the development of diabetic nephropathy, which in addition causes proteinuria and leads to histological changes in the kidney. This decrease in GFR is believed to be a result of changes in glomerular haemodynamic instigated by glomerulosclerosis(Reference Yokozawa, Nakagawa, Wakaki and Koizumi24, Reference Brenner, Meyer and Hostetter25). The results of the present study demonstrate that rats with diabetic nephropathy showed significant increases in the blood urea N, serum creatinine, and urinary protein excretion rate, whereas the Ccr level decreased compared with that in normal rats, representing a decline in renal function. However, the GT treatment significantly improved these parameters.
Hyperglycaemia is the principal factor responsible for structural alterations at the renal level, and The Diabetes Control and Complications Trial Research Group(4) has made it clear that hyperglycaemia is directly associated with diabetic microvascular complications, particularly in the kidney. Consequently, glycaemic control remains one of the main targets of therapy. In the present study, the serum and urine glucose levels of diabetic rats showed approximately 3- and 1·6-fold increases, respectively, and GT consumption reduced these increases by 30 and 35 %, respectively. In support of the present results, recent reports have shown that ( − )-epigallocatechin 3-O-gallate (EGCG) reduced the level of mRNA for gluconeogenesis enzymes(Reference Koyama, Abe, Sano, Ishizaki, Njelekela, Shoji, Hara and Isemura26) and caused many similar effects to insulin, including repression of glucose production and phosphoenolpyruvate carboxykinase and glucose-6-phosphatase gene expression in cells(Reference Waltner-Law, Wang, Law, Hall and Nawano27). Likewise, Yokozowa et al. (Reference Yokozawa, Nakagawa, Oya, Okubo and Juneja22) have recently shown that GT polyphenols (GTP) and partially hydrolysed guar gum (PHGG) decreased blood glucose levels and attenuated the urinary protein excretion and morphological changes characteristic of diabetic nephropathy when renal dysfunction was already evident. Furthermore, the combination of GTP and PHGG reduced kidney weight, levels of blood urea N and serum creatinine and increased Ccr in diabetic animals. Hyperglycaemia, as assessed by blood glucose and glycosylated protein levels, was reduced by administration of GTP plus PHGG(Reference Yokozawa, Nakagawa, Oya, Okubo and Juneja22). In line with the present study, several studies have also shown that the control of postprandial hyperglycaemia by GT can help reduce the risk of type 2 diabetes and provide evidence that GT promotes glucose metabolism in healthy humans, and produces an antihyperglycaemic effect in diabetic mice(Reference Tsuneki, Ishizuka, Terasawa, Wu, Sasaoka and Kimura28–Reference Yamabe, Yokozawa, Oya and Kim30). Indeed, Wu et al. (Reference Wu, Juan, Hwang, Hsu, Ho and Ho29) have shown that GT supplementation for 12 weeks improves insulin resistance and increases GLUT IV content in a fructose-fed rat model, resembling human type 2 diabetes mellitus(Reference Wu, Juan, Hwang, Hsu, Ho and Ho29). In contrast, Mustata et al. (Reference Mustata, Rosca and Biemel15) have recently shown that GT had marginal effects on nephropathy parameters but suppressed renal mitochondrial NADH-linked, ADP-dependent and dinitrophenol-dependent respiration and complex III activity in the STZ diabetic Lewis rat. In addition, GT did not induce any significant decrease in glomerular collagen IV, glomerular staining for redox active Fe and tubular Fe staining(Reference Mustata, Rosca and Biemel15). The failure to develop dramatic alterations in renal parameters may be related to the moderate hyperglycaemia and urinary albumin of these rats. These contradictory reports could result from differences in: (1) diabetic models, (2) animal strains, (3) STZ dosages resulting in mild v. severe hyperglycaemia and proteinuria and/or (4) insulin treatment used by different investigators.
Proteinuria is quantitatively related to the degree of nephropathy in patients with diabetes. The increased urinary protein excretion results from lesions in the glomerular filtration barrier. The present study shows that in comparison with normal rats, animals with diabetic nephropathy show increased urinary excretion of protein and that administration of GT reduced the degree of proteinuria which is in agreement with previously reported results that showed GTP, PHGG and GTP plus PHGG(Reference Yokozawa, Nakagawa, Oya, Okubo and Juneja22) and EGCG treatment(Reference Yamabe, Yokozawa, Oya and Kim30) significantly reduced the extent of proteinuria. Likewise, the present study reveals that GT consumption significantly decreased the serum glycosylated protein levels in diabetic animals. These data also suggest that GT minimises the development of glomerular and tubulointerstitial injuries. In addition, diabetic animals used in the present study confirmed the presence of glomerular hypertrophy and diffuse and exudative lesions (data not shown) reported by Yamabe et al. (Reference Yamabe, Yokozawa, Oya and Kim30) which also correlate with proteinuria. In addition, the decrease in total protein as a result of their excessive excretion via urine, and also an increase in lipids, whose abnormal metabolism has been proven to play a role in the pathogenesis of diabetic nephropathy and to enhance lipid peroxidation, were all improved by administration of the EGCG(Reference Yamabe, Yokozawa, Oya and Kim30). As a result, we contemplate that EGCG(Reference Yamabe, Yokozawa, Oya and Kim30), GTP(Reference Yokozawa, Nakagawa, Oya, Okubo and Juneja22) or GT consumption (in the present study) has a positive effect on blood glucose and lipid metabolic abnormalities.
Recently, it has been shown that a key morphological change associated with sustained hyperglycaemia was accumulation of glycogen granules in about half of the distal tubules and thin segment, starting at 1 month after alloxan induction of diabetes in experimental rats, which was extended to about half of the proximal tubules at 6 months(Reference Kang, Dai, Yu, Wen and Yang31). Abnormal glycogen deposits were first observed in the collecting ducts and the descending limb of Henle's loop in diabetic patients by Armanni and Ebstein(Reference Ebstein32), respectively. However, since then little attention has been paid to the accumulation of glycogen granules in renal tubules in humans with diabetes. In agreement with our observations, Nannipeiri et al. (Reference Nannipeiri, Lanfranchi, Santerini, Catalaneo, Van de Wrve and Ferrannini33) observed glycogen granules in both proximal and distal renal tubules in 9-month diabetic rats. Glycogen accumulation in the distal renal tubules was also observed by means of PAS staining on paraffin sections, electron microscopy, biochemical assays or enzyme-gold cytochemistry(Reference Rasch and Gotzsche34–Reference Rasch, Torffvit, Bachmann, Jensen and Jacobsen37). It appears that in chronic untreated diabetes, prolonged hyperglycaemia is the sole driving force for glycogen accumulation. Thus we speculate that the longer diabetes is prolonged, the more glycogen might accumulate and spread into the renal tubules, and GT reverses this pathological phenomenon. Nevertheless, it is not yet clear whether glycogen accumulation in renal tubules is an inevitable change in the diabetic condition in humans, whether it is an early-phase pathogenesis that will contribute to the end-stage diabetic nephropathy, whether it is always associated with the end-stage nephropathy, or whether it plays a role in inducing a pathway leading to the pathophysiological changes of diabetic nephropathy(Reference Raptis and Viberti38, Reference Caramori and Mauer39).
Glucose is reabsorbed almost completely by the proximal tubules and begins to appear in the urine when the renal threshold is exceeded. The present study has shown that STZ-induced diabetic nephropathy leads to the accumulation of glycogen in the renal tubules indicating an abnormal reabsorption process of glucose or malfunctioning in transporting the reabsorbed glucose back into the blood capillaries which in turn leads to the appearance of glucose in the urine. However, GT treatment of diabetic rats prevented glucose accumulation to zero level, indicating that GT may be able to restore the normal function of the proximal tubules in reabsorbing glucose from the urine back into the blood circulation. Likewise, EGCG was shown to protect Madin–Darby canine kidney tubular cells from cellular injury and apoptosis caused by oxidative stress(Reference Itoh, Yasui, Okada, Tozawa, Hayashi and Kohri40). Recently, GT extract and its constituent polyphenols have also been shown to suppress cell death in a porcine renal proximal tubular cell line (LLC-PK1 cells) caused by the addition of nephrotoxic immunosuppressant FK506(Reference Hisamura, Kojima-Yuasa, Kennedy and Matsui-Yuasa41). These results suggested that GT extract polyphenols and its constituents affect cell viability synergistically. It appears that the nephrotoxicity of drugs and their metabolites is often manifested as proximal tubule disorders, which result in the release of enzymes held in the proximal tubules. Moreover, GT reduced the urinary activity of renal tubular epithelial-cell enzymes, which are an index of renal tubular injury(Reference Jeong, Kim, Kim and Kim42).
In conclusion, the present study demonstrated that GT extract provides a beneficial effect on long-term diabetic nephropathy via suppressing hyperglycaemia and preventing glycogen accumulation in the proximal tubules. The therapeutic property of GT seems propitious in improving kidney nephropathy by significantly improving serum and urine parameters. These findings support the importance of controlling blood glucose levels and maybe slowing or even reversing some of the early pathologies of diabetic nephropathy. However, further studies should be done in order to understand the exact cellular and molecular mechanisms which mediate such effects of GT action on proximal tubules and how GT reduces the accumulation of glycogen in the proximal tubules as well as its potential therapeutic implications against renal damage associated with diabetic nephropathy.
Acknowledgements
The authors are immensely grateful to Dr Mario Nieto and Dr Ghanim Al-Khaledi for critically reviewing our manuscript. We also would like to thank Mrs Preethi Tobin, Shatoba Arpita and Solly Alex for their excellent technical assistance.
The authors declare that there is no conflict of interest pertaining to this manuscript or the publication thereof. Authors' contribution: W. M. R. laid down the original design of the experiments, supervised experimental work, designed the statistical analysis and wrote the manuscript. S. A. was responsible for the histopathological study; M. A. participated in experimental design, statistical analysis and writing the manuscript; S. A. participated in the experimental design.







