In humans, fat of ruminant origin represents a high proportion of the dietary intake of saturated fatty acids (SFA) (Demeyer & Doreau, Reference Demeyer and Doreau1999), which have been implicated in the onset of CVD (Department of Health, 1994). However, ruminant fats also contain higher concentrations of conjugated linoleic acid (CLA) than other food fat sources and are the main sources of CLA in the human diet (Chin et al. Reference Chin, Liu, Storkson, Ha and Pariza1992). CLA refers to a mixture of positional and geometric isomers of linoleic acid. The cis-9, trans-11 isomer is the most common natural isomer with biological activity, representing 75–90 % of total CLA in meat depending on the diet of the animal, but biological activity has been proposed for other isomers, in particular trans-10, cis-12 (Pariza et al. Reference Pariza, Park and Cook2001).
The anticancer effect of CLA was initially discovered in animal models using lipid extracted from beef (Ha et al. Reference Ha, Grimm and Pariza1987) and an anticancer effect of beef CLA has been confirmed in vitro by De la Torre et al. (Reference De la Torre, Debiton, Juaneda, Durand, Chardigny, Baerthomeuf, Bauchart and Gruffat2006). A range of other positive isomer-specific effects on human health has been proposed, including a reduction in atherosclerosis, decreased inflammation and improved cardiovascular health (e.g. Pariza et al. Reference Pariza, Park and Cook2001). Consequently, there is considerable interest in increasing the concentration of CLA in ruminant fat.
CLA is produced following incomplete ruminal biohydrogenation of dietary 18 : 2 (Kepler & Tove, Reference Kepler and Tove1967) and by tissue desaturation of trans-11 18 : 1, another product of incomplete ruminal biohydrogenation of dietary fatty acids (Griinari et al. Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000). An increase in the concentration of CLA in ruminant muscle fat has been achieved primarily by dietary addition of plant oils or seeds rich in PUFA (Enser et al. Reference Enser, Scollan, Choi, Kurt, Hallett and Wood1999; Mir et al. Reference Mir, Mir and Huber2002; Raes et al. Reference Raes, De Smet and Demeyer2004; Noci et al. Reference Noci, O'Kiely, Monahan, Stanton and Moloney2005b). Thus, supplementation with sunflower oil (SFO), a rich source of 18 : 2, has been shown to increase the concentration of CLA and trans-11, 18 : 1 by providing substrate for ruminal biohydrogenation (Mir et al. Reference Mir, Mir and Huber2002; Noci et al. Reference Noci, O'Kiely, Monahan, Stanton and Moloney2005b). Inclusion of fish oil (FO) has been shown to increase the concentration of CLA in milk (reviewed by Chilliard et al. Reference Chilliard, Ferlay and Doreau2001; Offer et al. Reference Offer, Speake, Dixon and Marsden2001; Shingfield et al. Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003), and a combination of a source of 18 : 2 and FO increased the concentration of CLA in milk still further (AbuGhazaleh et al. Reference AbuGhazaleh, Schingoethe, Hippen and Whitlock2002, Reference AbuGhazaleh, Schingoethe, Hippen and Kalscheur2003). The first hypothesis tested in this study was that bovine muscle and subcutaneous adipose tissue concentrations of CLA and long-chain n-3 PUFA would respond in a dose-dependent manner to supplementation of diets with FO and that FO could be used to optimise CLA accretion in ruminants fed a PUFA-rich ration.
Grazed and conserved grass are the major sources of n-3 PUFA, particularly 18 : 3, in ruminant diets. This makes fat from ruminants fed grass-based diets an important source of n-3 PUFA for humans. On a laboratory scale, wilting grass prior to ensiling decreased the content of total fatty acids and 18 : 3n-3 in silage (Dewhurst & King, Reference Dewhurst and King1998). While wilting grass prior to ensiling is environmentally beneficial, it may result in animals fed wilted silage having lower concentrations of n-3 PUFA in tissue than animals fed unwilted silage. The second hypothesis tested was that the effects of the wilting process are reflected in the fatty acid composition of muscle and subcutaneous adipose tissue of cattle consuming the resulting silage.
Materials and methods
Experimental design and animal management
Eighty Friesian steers (mean initial body weight 565 kg, sd 37·24) were blocked on initial body weight and assigned to one of eight dietary treatments (n 10/treatment) in a randomised complete block (initial body weight) design. Animals were assigned at random to pens in a slatted-floor shed that accommodated five or six animals/pen and were fed individually through Calan gates. The initial silage allowances were 15 kg of wilted silage and 30 kg of unwilted silage, and were increased, as the experiment progressed, to 20 and 35 kg, respectively. The silages were offered once daily, and refusals (which were rare) were recorded daily. The silages were prepared from a sward of predominantly perennial ryegrass in mid May. Grass for unwilted silage was ensiled directly with 3 litres of Addsafer (Interchem Ltd., Dublin, Ireland) (48 % formic acid and 16 % ammonium tetraformate)/tonne grass. The remaining grass was wilted for an average of 32 h (maximum temperature 20·2°C, minimum temperature 5·7°C, relative humidity 68·1 %), turned once and then ensiled without an additive. Concentrate rations were formulated to have 120 g fat/kg, which included 80 g of SFO/kg and increasing levels of FO (0, 10, 20 or 40 g/kg concentrate) derived from a mix of mackerel and herring oil (Fish Industries, Killybegs, Co. Donegal, Ireland), balanced with decreasing amounts of lard (Table 1). Animals were offered one of the four concentrate rations, at an initial daily allowance of 4·5 kg/head, which increased with increasing body weight to average 5 kg/head over the duration of the 108 d experiment. The concentrates were offered daily in a separate container, in two equal meals, and no refusals were observed. On the day of slaughter, animals were weighed without fasting and transported 120 km to a commercial facility (Meadow Meats, Rathdowney, Co. Laois, Ireland) within 3 h from Grange Research Centre and slaughtered within 60 min of arrival.
Table 1 Ingredients and formulation of the concentrate rations
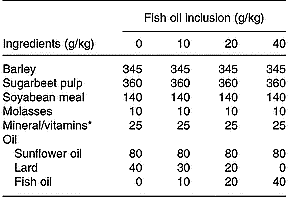
* The mineral and vitamin mix contained Ca (28·5 %), P (1·6 %), Na (5·6 %), vitamin A (150 mg/kg), vitamin D3 (3·1 mg/kg), vitamin E (16,7-50), cobalt carbonate (42 mg/kg), cupric sulphate (500 mg/kg), calcium iodate (910 mg/kg), iron sulphate (1000 mg/kg), manganese sulphate (5800 mg/kg), sodium selenite (16 mg/kg) and zinc sulphate (7500 mg/kg) on an as-fed basis.
Post-slaughter measurement and sampling procedure
Carcass and perirenal fat weights were recorded immediately after slaughter. The carcasses were hung from the Achilles tendon and chilled in the abattoir for 24 h. The M. longissimus dorsi and associated muscles and adipose tissue were excised from the right side of the carcass and transported to the Teagasc National Food Centre (Castleknock, Dublin, Ireland) where they were held at 4°C for a further 24 h. The procedures for tissue sampling, lipid extraction and fatty acid analysis of the neutral lipid (NL) and polar lipid (PL) fractions of intramuscular fat and total lipid (TL) fraction of subcutaneous adipose tissue were as described by Noci et al. (Reference Noci, Monahan, French and Moloney2005a).
In brief, extracted lipid was separated into NL and PL fractions using solid-phase extraction cartridges with 500 mg of aminopropyl packing (Bone-Elut 500 mg, 3 ml reservoir; Varian Instruments, Palo Alto, CA). The separated lipid classes were dried, weighed, dissolved in toluene and initially methylated with NaOCH3, which was followed with a 4 % solution of HCl in methanol. Both methylation procedures were carried out at 50°C for 20 min, and tricosanoic acid (C23 : 0) methyl ester was used as an internal standard for fatty acid quantification.
The fatty acid methylesters were separated by gas chromatography (GC) using a Varian 3800 gas chromatograph (Varian Instruments) equipped with a CP-Sil 88 capillary column (100 m × 0·25 mm i.d., 0·2 μm film thickness; Chrompack, The Netherlands) and a Varian 8400 autosampler. The injector and the flame ionization detector were kept at constant temperatures of 250 and 260°C, respectively. The column oven temperature was held at 40°C for 2 min, increased at 20°C/min to 80°C and held for 2 min, increased to 160°C at 20°C/min, to 220°C at 4°C/min, and to 240°C at 2°C/min and held for 8 min. The total run time was 43 min, and the carrier gas used was H2. For peak identification, a standard mix of 37 fatty acid methylesters (Supelco Inc., Bellefonte, PA) was used, and individual standards from Matreya (Matreya Inc., Pleasant Gap, PA) were used for identification of those fatty acid methylesters not contained in the mix.
Feed chemical analysis
The dry matter content of the concentrates and silages was determined as described by Moloney et al. (Reference Moloney, Read and Keane1996) and Porter & Murray (Reference Porter and Murray2001), respectively. Concentrates were analysed for crude protein concentration as described by Association of Analytical Chemists (1990), for ash concentration as described by Moloney et al. (Reference Moloney, Read and Keane1996) and for oil Procedure B (extraction after acid hydrolysis) as described in European Communities (1984). The fatty acid composition of lipid sources and feedstuffs was determined as described by Sukhija & Palmquist (Reference Sukhija and Palmquist1988). The fatty acid methylesters were dissolved in toluene and analysed by GC following the GC conditions described above.
Statistical analysis
Data were subjected to the analysis of variance procedures of Genstat (Release 3.2, Lawes Agricultural Trust, Rothamstead Experimental Station, UK). The model used had block, type of silage, level of FO inclusion and the interaction between the main effects as sources of variation. Linear and quadratic effects of increasing levels of inclusion of FO were partitioned using orthogonal polynomials. The concentration of fatty acids in intramuscular TL was calculated from the concentrations of fatty acids in PL and NL.
Results
Feed composition
The fatty acid compositions of the dietary fat sources are summarised in Table 2 and the chemical compositions of the silages and concentrates are summarised in Table 3. Wilted silage had a lower total fat and fatty acid content than unwilted silage mainly as a result of the lower 18 : 3n-3 and total PUFA content. The concentrates had similar total oil, crude protein and ash contents. The concentrate containing 40 g FO/kg had the lowest SFA and MUFA contents and the highest n-3 PUFA and total PUFA contents.
Table 2 Fatty acid composition of oil supplements (Mean and standard deviation)

SFA, saturated fatty acids.
† Total n-6 PUFA = sum of 18 : 2, 20 : 4 and 22 : 2.
‡ Total n-3 PUFA = sum of 18 : 3n-3, 20 : 5, 22 : 5 and 22 : 6.
Table 3 Chemical and fatty acid composition of grass silages and concentrate rations (Mean and standard deviation)

DM, dry matter; SFA, saturated fatty acids.
*Ether extract after acid hydrolysis.
† Total n-6 PUFA = sum of 18 : 2 and 20 : 4 (18 : 3n-6, 20 : 2, 20 : 3n-6 and 22 : 2, present in trace amounts).
‡ Total n-3 PUFA = sum of 18 : 3n-3, 2 ;5, 22 : 5 and 22 : 6.
Animal production
Concentrate intake was similar for all treatments, but consumption of wilted silage was higher than of unwilted silage, resulting in a higher total dry matter intake for the wilted silage-based rations (Table 4). Neither the type of silage nor the level of inclusion of FO in the diet affected average daily gain or pre-slaughter weight of the steers. However, the interaction between the two main effects was significant for carcass weight. Thus, when unwilted silage was offered, steers receiving 0 or 10 g FO/kg concentrate had the highest carcass weight, which decreased for steers fed 20 g FO/kg concentrate, but increased again for those receiving 40 g FO/kg concentrate. With wilted silage, however, steers fed 0 or 10 g FO/kg concentrate had the lowest carcass weight, which increased when animals were fed the concentrates containing 20 and 40 g FO/kg.
Table 4 Daily feed intake and animal performance

DM, dry matter. * and *** refer to significance levels P < 0.05 and P < 0.001, respectively.
Total intramuscular lipids
Feeding wilted instead of unwilted silage led to an increase in the concentration of trans-9 18 : 1, trans-11 18 : 1 (P = 0·07), cis-9, trans-11 CLA and trans-10, cis-12 CLA and to a decrease in the n-6:n-3 PUFA ratio (P = 0·06) (Table 5).
Table 5 Fatty acid concentration in the total lipid fraction of intramuscular fat from M. longissimus dorsi

CLA, conjugated linoleic acid; SFA, saturated fatty acids; L and Q, significant (P < 0·05) linear and quadratic effects of level of inclusion of fish oil; P:S, PUFA:SFA.
*, ** and *** refer to significance levels P < 0·05, P < 0·01 and P < 0·001, respectively.
† n-6 fatty acids = sum of 18 : 2, 18 : 3n-6, 20 : 2, 20 : 3n-6, 20 : 4 and 22 : 2.
‡ n-3 fatty acids = sum of 18 : 3n-3, 20 : 3n-3, 20 : 5, 22 : 5 and 22 : 6.
Increasing the level of inclusion of FO in the concentrates led to a significant linear increase in the concentration of trans-9 18 : 1 (quadratic term also significant), trans-11 18 : 1, trans-10, cis-12 CLA, cis-9, trans-11 CLA, 20 : 0, 20 : 1, 20 : 2n-6, 20 : 5n-3, 22 : 0, 22 : 1, 22 : 2n-6 and 22 : 6n-3, and to a linear decrease in the n-6:n-3 PUFA ratio (Table 5).
There was an interaction between the effects of type of silage and increasing levels of inclusion of FO on the total concentration of intramuscular fatty acids. As the level of inclusion of FO increased, there was an increasing quadratic response in muscle from steers offered unwilted silage, but a linear decrease in muscle from those offered wilted silage. A similar interaction was found for the concentration of 12 : 0, 16 : 0, 17 : 0, 17 : 1, 18 : 0, cis-9 18 : 1 and 18 : 3n-3, total SFA and MUFA.
Intramuscular neutral lipids
Feeding wilted instead of unwilted silage lead to an increase in the proportion of trans-9 18 : 1, cis-9, trans-11 CLA and trans-10, cis-12 CLA and MUFA, and to a decrease in the proportions of 18: 0 (P = 0·07), SFA (P = 0·05) and n-6 PUFA (P = 0·06) (Table 6).
Table 6 The proportion of individual fatty acids in the neutral lipid fraction of intramuscular fat from M. longissimus dorsi

CLA, conjugated linoleic acid; SFA, saturated fatty acids; L and Q, significant (P < 0·05) linear and quadratic effects of level of inclusion of fish oil.
*, ** and *** refer to significance levels P < 0·05, P < 0·01 and P < 0·001, respectively.
† n-6 fatty acids = sum of 18 : 2, 18 : 3n-6, 20 : 2, 20 : 3n-6, 20 : 4 and 22 : 2
‡ n-3 fatty acids = sum of 18 : 3n-3, 20 : 3n-3, 20 : 5, 22 : 5 and 22 : 6.
Increasing the level of inclusion of FO in the concentrates led to a significant linear increase in the proportion of trans-11 18 : 1, cis-9, trans-11 CLA, 18 : 3n-6, 20 : 0, 20 : 1, 22 : 0, 22 : 1 and 22 : 2n-6, and to a linear decrease in the proportion of cis-9 18 : 1, 18 : 2n-6, 18 : 3n-3 (quadratic term also significant), 20 : 3n-6, MUFA (P = 0·07) and n-6 PUFA and in the n-6:n-3 PUFA ratio (Table 6).
There was an interaction between the effects of type of silage and increasing level of FO inclusion on the proportion of 20 : 4n-6 which decreased following a quadratic trend as the level of FO increased when unwilted silage was fed, but was unchanged when wilted silage was offered.
Intramuscular polar lipids
Feeding wilted instead of unwilted silage led to a decrease in the proportion of 20 : 4n-6 in muscle PL (Table 7). Increasing the level of inclusion of FO in the concentrates led to a significant linear increase in the proportion of 17 : 1, 20 : 1, 20 : 5n-3, 22 : 2n-6, 22 : 6n-3 and n-3 PUFA and to a linear decrease in the proportion of 20 : 4n-6, SFA (quadratic) and n-6 PUFA and the n-6:n-3 PUFA ratio (Table 7).
Table 7 The proportion of individual fatty acids in the polar lipid faction of intramuscular fat from M. longissimus dorsi

CLA, conjugated linoleic acid; SFA, saturated fatty acids; L and Q, significant (P < 0·05) linear and quadratic effects of level of inclusion of fish oil.
*, ** and *** refer to significance levels P < 0·05, P < 0·01 and P < 0·001, respectively.
† n-6 fatty acids = sum of 18 : 2, 18 : 3n-6, 20 : 2, 20 : 3n-6, 20 : 4 and 22:2
‡ n-3 fatty acids = sum of 18 : 3n-3, 20 : 3n-3, 20:5, 22:5 and 22 : 6.
Total subcutaneous adipose tissue lipids
Feeding wilted instead of unwilted silage led to an increase in the concentration of cis-9, trans-11 CLA and total PUFA and in the PUFA:SFA (P:S) ratio, and to a decrease in the concentration of 18 : 0 (P = 0·07) (Table 8).
Table 8 Fatty acid concentration in subcutaneous adipose tissue

CLA, conjugated linoleic acid; SFA, saturated fatty acids; L and Q, significant (P < 0·05) linear and quadratic effects of level of inclusion of fish oil; P:S, PUFA:SFA.
*, ** and *** refer to significance levels P < 0·05, P < 0·01 and P < 0·001, respectively.
† n-6 fatty acids = sum of 18 : 2, 18 : 3n-6, 20 : 2, 20 : 3n-6, 20 : 4 and 22:2
‡ n-3 fatty acids = sum of 18 : 3n-3, 20 : 3n-3, 20:5, 22:5 and 22 : 6.
Increasing the level of inclusion of FO in the concentrates led to a significant linear increase in the concentration of cis-9 18: 1 (P = 0·06), cis-9, trans-11 CLA, 20 : 1, 20 : 0 (P = 0·07), 20 : 5n-3 (P = 0·06) and 22 : 6n-3, and a linear decrease in the concentration of 18 : 2n-6, 18 : 3 n-3 and total n-6 PUFA.
Discussion
The myriad putative health benefits of CLA, and in particular the cis-9, trans-11 isomer, have stimulated interest in increasing its concentration in beef. Since CLA is formed either directly or indirectly during ruminal biohydrogenation of dietary lipids, the most common strategy examined has been to increase/modify the supply of lipids for cattle. Recent studies have indicated an influence of dietary long-chain PUFA on pathways of biohydrogenation in the rumen, either by direct action of the PUFA or via the action of intermediate products of PUFA biohydrogenation (Scollan et al. Reference Scollan, Dhanoa, Choi, Maeng, Enser and Wood2001b; Shingfield et al. Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003). Specifically, these studies showed that dietary long-chain PUFA increased trans-11 18 : 1 as a product of rumen biohydrogenation of dietary PUFA. An increase in the outflow of trans-11 18 : 1 from the rumen may then induce an increase in the concentration of CLA in tissues, via the action of Δ9-desaturase (Griinari et al. Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000). The demonstration that trans-11 18 : 1 is also converted to CLA in humans and augments the CLA potential of ruminant products (Kuhnt et al. Reference Kuhnt, Kraft, Moeckel and Jahreis2006) has focused attention on increasing the concentration of this fatty acid per se. The strategy used in this experiment was, first, to supply significant amounts of 18 : 2n-6 to the rumen by feeding SFO, because increased dietary 18 : 2n-6 per se has been shown to increase the concentration of CLA in intramuscular fat (Mir et al. Reference Mir, Mir and Huber2002; Noci et al. Reference Noci, O'Kiely, Monahan, Stanton and Moloney2005b). Secondly, in contrast to other studies in which one concentration of FO was examined (Enser et al. Reference Enser, Scollan, Choi, Kurt, Hallett and Wood1999; Shingfield et al. Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003), increasing levels of dietary FO were used to determine the response pattern of an increase in CLA and in the nutritionally important n-6:n-3 PUFA ratio.
With the objective of enhancing the nutritional attributes of bovine muscle fat and subcutaneous adipose tissue, the contribution of forage to the diet is important because the lipids of grass-based forages are rich in 18 : 3n-3. Since grass silage is the major feed ingredient in housed cattle rations in Western Europe, the concentrates were offered in a conserved grass-based ration.
Effects of type of silage
Dewhurst & King (Reference Dewhurst and King1998) found that wilting perennial ryegrass before ensiling decreased the total fatty acid concentration, particularly the 18 : 3n-3 concentration. A similar observation was made by Boufaïed et al. (Reference Boufaïed, Chouinard, Tremblay, Petit, Michaud and Belanger2003), who reported a significant decrease in fatty acids of increasing chain length from 14 : 0 to 18 : 3n-3 when wilted grass was compared with fresh grass. The lower concentrations of 16 : 0, 18 : 0, 18 : 1, 18 : 2, 18 : 3n-3 and total fatty acids in wilted silage in the present study confirm the loss of fatty acids (mainly 18 : 3n-3) due to the wilting process, possibly due to oxidative loss, as suggested by Dewhurst & King (Reference Dewhurst and King1998).
While there was an interaction between type of silage and level of FO inclusion for the concentration of total and several individual fatty acids in the NL (data not shown) and TL of muscle, the fact that the interaction was generally not detected when data were analysed as proportions of the total fatty acids confirmed that the interaction was mainly due to the small differences in fatness across the eight treatments.
With respect to the silages, daily consumption of 18 : 3n-3 averaged 95 g for steers fed unwilted silage compared with the 83 g for steers fed wilted silage. Ruminal biohydrogenation of dietary 18 : 3n-3 apparently eliminated this difference in intake since the proportions of intramuscular 18 : 3n-3 were 0·37 and 0·36 for unwilted and wilted silage-fed steers, respectively, similar to the proportion reported by Scollan et al. (Reference Scollan, Choi, Kurt, Fisher, Enser and Wood2001a) for cattle fed grass silage and concentrates.
The increase in the concentration of cis-9, trans-11 CLA in both TL and subcutaneous adipose tissue as a result of feeding wilted silage indicates a possible effect of the type of silage on rumen biohydrogenation pathways. Since the muscle concentration of CLA is linked to the outflow of trans-11 18: 1 from the rumen (Griinari et al. Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000), the results suggest that the conditions created by feeding wilted silage instead of unwilted silage were more effective in modifying microbial flora action towards an incomplete biohydrogenation of the dietary PUFA. The higher proportion of trans-9 18 : 1 and trans-11 18 : 1 in the NL (and TL) lends support to the hypothesis of an increased outflow of trans-11 18 : 1 as a precursor for desaturation in the muscle. An effect of concentration and type of substrate, dilution rate and rumen pH on the production of long-chain fatty acids, including trans 18 : 1 isomers, by mixed ruminal bacteria in continuous culture was shown by Martin & Jenkins (Reference Martin and Jenkins2002).
Effect of level of FO inclusion
The muscle and subcutaneous adipose tissue fatty acid data in the present study generally support the findings of Enser et al. (Reference Enser, Scollan, Choi, Kurt, Hallett and Wood1999) and Scollan et al. (Reference Scollan, Choi, Kurt, Fisher, Enser and Wood2001a) with regard to FO supplementation of beef cattle. Of particular interest from a human nutrition perspective is the increase in cis-9, trans-11 CLA (and its precursor trans-11 18 : 1), 20 : 5n-3 and 22 : 6n-3 proportions in muscle and subcutaneous adipose tissue, and the decrease in the n-6:n-3 PUFA ratio in muscle with FO consumption. It has been established that dietary FO increases production of trans-11 18 : 1 in the rumen, and subsequently its concentration in milk fat or in the intramuscular adipose tissue (Chilliard et al. Reference Chilliard, Ferlay and Doreau2001; Scollan et al. Reference Scollan, Choi, Kurt, Fisher, Enser and Wood2001a, Reference Scollan, Dhanoa, Choi, Maeng, Enser and Woodb). The results of the present study indicate that this effect of FO, via the supply of long-chain PUFA to the rumen (or via intermediates of their biohydrogenation in the rumen), is dose-dependent. This was confirmed in a parallel study in which the concentrates containing 0, 10 or 40 g FO/kg were offered to duodenally fistulated steers (Lee et al. Reference Lee, Tweed, Moloney and Scollan2005), i.e. a linear increase in the outflow of trans-11 18 : 1 from the rumen was observed. In contrast, there was a quadratic increase in cis-9, trans-11 CLA outflow from the rumen such that the ratio trans-11 18 : 1:cis-9, trans-11 CLA was greater for the 40 g FO/kg concentrate than for the 10 g FO/kg concentrate. Despite this, the trans-11 18 : 1:cis-9, trans-11 CLA ratio in muscle and subcutaneous adipose tissue was not influenced by FO inclusion. These data suggest a greater efficiency of absorption of cis-9, trans-11 CLA and/or a lower efficiency of absorption of trans-11 18 : 1 from the intestine of cattle fed the high FO concentrates or differences in metabolism post-absorption. The lack of an effect of FO inclusion on the trans-11 18 : 1:cis-9, trans-11 CLA ratio does not support the hypothesis that long-chain PUFA inhibit tissue desaturase activity, at least at the concentration of long-chain PUFA observed in the present study. While there were analytical differences in the present study and that of Lee et al. (Reference Lee, Tweed, Moloney and Scollan2005), the influence of FO or long-chain PUFA consumption per se on post-ruminal trans-11 18 : 1 and cis-9,trans-11 CLA transformations merit investigation.
Enser et al. (Reference Enser, Scollan, Choi, Kurt, Hallett and Wood1999) reported a cis-9, trans-11 CLA concentration of 24·3 mg/100 g muscle from Charolais steers fed a 20 g FO/kg concentrate diet compared with an average of 57·5 g/100 g muscle for cattle offered the 40 g FO/kg concentrate in the present study. The higher concentration of this CLA isomer achieved in the present experiment may be attributed to the 3-fold and 1·5-fold higher daily intake of 18 : 2n-6 and 18 : 3n-3, respectively (steers consuming the 40 g FO/kg concentrate had a total intake from silage and concentrate of 227 g of 18 : 2n-6 and 89 g of 18 : 3n-3), leading to higher trans-11 1 8:1 (and CLA) production through biohydrogenation (Lee et al. Reference Lee, Tweed, Moloney and Scollan2005). It should be noted that the animals in the present experiment had a higher concentration of total fatty acids in the muscle than those reported by Enser et al. (Reference Enser, Scollan, Choi, Kurt, Hallett and Wood1999). Since CLA was found predominantly in the NL fraction, which increases as animals accrete lipid, this will account for some of the difference in CLA concentrations between the studies.
While earlier in vitro and laboratory animal studies convincingly demonstrated an anticancer effect of the cis-9, trans-11 isomer of CLA, this effect has not been demonstrated in humans. This relates at least in part to the aetiology of cancer and the difficulty in conducting studies on the prevention of cancer in humans. Long-term studies with appropriate end points such as incidence of cancer remain to be conducted so an anticancer effect of CLA in humans cannot be excluded. As a range of other positive isomer-specific effects on human health have been proposed, including a reduction in atherosclerosis, decreased inflammation and improved cardiovascular health (e.g. Pariza et al. Reference Pariza, Park and Cook2001), the efficacy of CLA in the treatment and prevention of several of these conditions has also been examined. The findings from human studies to date have been equivocal (reviewed by Yaqoob et al. Reference Yaqoob, Tricon, Burdge, Calder, Williams and Buttriss2006). This review suggests that much of the variability in the human studies conducted to date is due to the use of mixtures of CLA isomers (e.g. trans-10, cis-12 CLA appears to have a negative effect on blood lipids while the cis-9, trans-11 isomer does not) and short duration, to which could be added insufficient statistical power to detect differences of biological significance. Considerably more information is required in this regard.
Epidemiological associations between the risk of coronary heart disease and the consumption of trans PUFA has also focused attention on the concentration of these fatty acids in food. The trans fatty acid profile in ruminant fat tends be enriched with the trans-11 isomer of 18 : 1, as was seen in the present study. Industrially derived oils such as hydrogenated vegetable oils, in contrast, have a broader spectrum of trans 18 : 1 isomers and have a considerably higher concentration of trans-9 and trans-10 18 : 1 isomers (Scollan et al. Reference Scollan, Hocquette, Nuernberg, Dannenberger, Richardson and Moloney2006). The relative risk to human health of consuming the individual isomers remains to be elucidated, but current epidemiological evidence suggests that consumption of ruminally derived trans PUFA is not a risk factor for heart disease (Jakobsen, Reference Jakobsen2006).
Data in the literature are unclear with respect to ruminal biohydrogenation of long-chain PUFA of FO. In an in vitro study, Gulati et al. (Reference Gulati, Ashes and Scott1999) suggested that the ability of rumen micro-organisms to hydrogenate long-chain PUFA is dependent on the concentration of those fatty acids in the rumen environment. Scollan et al. (Reference Scollan, Dhanoa, Choi, Maeng, Enser and Wood2001b) reported biohydrogenation of 92 and 91 % for 20 : 5n-3 and 22 : 6n-3, respectively, in beef cattle fed 30 g FO/kg dry matter intake. In our parallel study, Lee et al. (Reference Lee, Tweed, Moloney and Scollan2005) reported corresponding values of 79 and 80 % for beef cattle fed the 40 g FO/kg concentrate. In the present study, the incorporation of long-chain PUFA was relatively low in the NL fraction and in the subcutaneous adipose tissue, in agreement with Ashes et al. (Reference Ashes, Siebert, Gulati, Cuthbertson and Scott1992) and Mitchell et al. (Reference Mitchell, Reed and Rogers1991), respectively, while the greatest effect of dietary treatments was observed in the PL where PUFA are present in higher concentrations. While the highest level of inclusion of FO resulted in the highest incorporation of long chain n-3 PUFA in the muscle PL, the proportion of long-chain PUFA was lower than that found by Scollan et al. (Reference Scollan, Choi, Kurt, Fisher, Enser and Wood2001a). Differences could be attributed to the different proportions of long-chain n-3 PUFA in the FO used, to the different proportion of oil included in the diets used (1·9 and 2·4 % of dietary dry matter in our study and that of Scollan et al. (Reference Scollan, Choi, Kurt, Fisher, Enser and Wood2001a), respectively) and to the different levels of fatness of the animals in the two experiments. This may also relate to the higher 18 : 2n-6 intake in the present study as n-6 PUFA compete for deposition with the n-3 PUFA and are preferentially deposited.
In the present study, a decrease in 20 : 4n-6 in the PL fraction with a concomitant increase in long-chain n-3 PUFA suggests that the latter were incorporated at the expense of the former to maintain the structural integrity of the membrane phospholipid fraction. Such a decrease in long-chain n-6 PUFA in the PL has also been observed in other studies with both ruminants and non-ruminants in which fishmeal and FO were included as dietary supplements (Morgan et al. Reference Morgan, Noble, Cocchi and McCartney1992; Mandell et al. Reference Mandell, Buchanan-Smith, Holub and Campbell1997).
The considerable proportion of 20 : 1 and 22 : 1 contained in the FO used in this experiment (Table 2) could explain the linear increase in very-long-chain MUFA and SFA in the NL fraction. Shingfield et al. (Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003) also found increased amounts of some long-chain fatty acids (20 : 1, 20 : 2, 22 : 0 and 22 : 1) entering the omasal canal and being incorporated into milk fat following the inclusion of FO in the diet. Overall, however, the addition of FO had virtually no impact on the total SFA, MUFA or PUFA content of the intramuscular adipose tissue or of the subcutaneous adipose tissue. Consequently, the P:S ratio was similar across the dietary treatments and it was also considerably lower than the recommended value of 0·45 suggested by the Department of Health (1994). Our results are consistent with those of Mandell et al. (Reference Mandell, Buchanan-Smith, Holub and Campbell1997), Choi et al. (Reference Choi, Enser, Wood and Scollan2000) and Scollan et al. (Reference Scollan, Choi, Kurt, Fisher, Enser and Wood2001a). Scollan et al. (Reference Scollan, Enser, Gulati, Richardson and Wood2003) suggested the existence of a negative exponential relationship between the amount of intramuscular fat and the P:S ratio. When animals have intramuscular fat concentrations ranging between 2000 and 4000 mg/10 0 g, P:S ratio values, calculated as (18 : 2n-6+18 : 3n-3)/(Σ(12 : 0, 14 : 0, 16 : 0, 18 : 0)), generally range between 0·05–0·11 (Choi et al. Reference Choi, Enser, Wood and Scollan2000; Scollan et al. Reference Scollan, Choi, Kurt, Fisher, Enser and Wood2001a). The steers in the present study had a high muscle fat content (6600 g fatty acids/100 g muscle), and so the low P:S ratio results were consistent with this negative exponential relationship.
Replacing lard with FO in the diets of finishing cattle had a beneficial effect on the n-6:n-3 PUFA ratio of intramuscular fat. The ratio observed was below 4:1, a value regarded as compliant with the guidelines from the Department of Health (1994). In the present study, the addition of FO contributed to a modification of the proportion of long-chain n-3 and n-6 fatty acids, without affecting 18 : 2n-6 and 18 : 3n-3, leading to a reduction in the n-6:n-3 PUFA ratio to 2·95:1 (in steers fed 40 g FO/kg). Based on the data from this study, and assuming an average beef consumption of 100 g/day (Enser et al. Reference Enser, Hallett, Hewett, Fursey and Wood1996), beef produced using the 40 g FO/kg concentrate (averaged across the type of silage) would supply 67 mg of n-3 PUFA (36 mg of which come from n-3 long-chain PUFA), accounting for 20–40 % of the recommended daily intake of n-3 fatty acids (Department of Health, 1994).
Conclusions
Notwithstanding the fact that emerging data on the human health benefits of CLA are not as dramatic as those from model systems and concerns about the consumption of trans PUFA, the results of this experiment are interpreted as indicating that inclusion of FO in rations used for beef cattle has a positive impact on the fatty acid profile of muscle and subcutaneous adipose tissue lipids from a human health perspective. The positive linear relationship between the level of inclusion of FO in the ration and the level of beneficial fatty acids in tissue offers opportunities for inclusion of higher levels of FO. The study also demonstrated that utilisation of wilted rather than unwilted silage will enhance the CLA content of bovine intramuscular fat and subcutaneous adipose tissue without a negative effect on the incorporation of n-3 PUFA.
Acknowledgements
This research was supported through the European Union, Fifth Framework Programme (Project QLRT-2000-31 423, ‘Healthy Beef’). The technical assistance of V. McHugh, A. McArthur, N. Blount (Grange Research Centre) and D. Donovan (Moorepark Research Centre) is gratefully acknowledged, as is the cooperation of Meadow Meats, Rathdowney, Ireland.










