In 1991, the UK Medical Research Council demonstrated that periconceptional supplementation with folic acid could significantly reduce the incidence of neural tube defects (NTD)(1). NTD are a group of congenital malformations resulting from incomplete closure of the neural tube, the embryonic precursor of the brain and spinal cord(Reference Gilbert2). Because human neural tube closure takes place 3 weeks after conception, before most women are even aware of their pregnancy(Reference Crider, Bailey and Berry3), adequate folate levels should be provided to all fertile women to be effective at preventing NTD. This situation is particularly relevant in regions such as Latin America, where 69 % of pregnancies are unintended(Reference Bearak, Popinchalk and Alkema4).
Since the early 1990s, different strategies to increase blood folate concentration in women of reproductive age have been considered. Interventions designed to increase the intake of folate-rich foods were rapidly discarded, as they were ineffective in improving folate status(Reference Cuskelly, McNulty and Scott5). In most populations, folic acid supplement use is also ineffective because of low awareness of patients and healthcare providers to the use of folic acid before pregnancy(Reference Medawar, Wehbe and Jaoude6,Reference Williams, Abelman and Fassett7) , together with low adherence to the use of pills and with the existence of socio-economic disparities (high costs and low availability) of vitamins in specific locations(Reference Liu, Chen and Liu8,Reference Kinnunen, Sletner and Sommer9) . Folic acid mandatory fortification of food is considered an effective measure to provide sufficient folate to women before they get pregnant. Enrichment of cereal grain products with folic acid was first implemented in the USA in 1998(Reference Crider, Bailey and Berry3). Since then, folic acid fortification of wheat, maize and/or rice flour has been adopted as a cost-effective public health policy in more than eighty countries(Reference Blencowe, Cousens and Modell10,Reference Hoddinott11) , and its implementation has proven to reduce the incidence of NTD significantly(Reference Arth, Kancherla and Pachon12,Reference Castillo-Lancellotti, Tur and Uauy13) .
In Chile, mandatory fortification of wheat flour with 2·2 mg folic acid/kg was first implemented in 2000. This policy was designed to increase the average intake of folic acid by 400 µg/d in women of reproductive age. In 2003, Hertrampf et al.(Reference Hertrampf, Cortes and Erickson14) showed that serum folate levels had risen from 4·3 to 16·4 ng/ml in a cohort of 605 young women from low socio-economic households. The policy showed to be effective in decreasing the incidence of NTD by 43 % from pre-fortification (1999–2000) to post-fortification (2001–2002)(Reference Lopez-Camelo, Orioli and da Graca Dutra15). The cost-effectiveness of this intervention was later demonstrated by Llanos et al.(Reference Llanos, Hertrampf and Cortes16), who showed that the overall cost of surgery and long-term rehabilitation for patients with NTD ‘significantly exceeded the investment incurred by the milling industry’.
Evidence worldwide and from Chile suggested that specific population subgroups or individuals might suffer adverse effects if exposed to high levels of folic acid(Reference Smith, Kim and Refsum17–Reference Castillo-Lancellotti, Margozzini and Valdivia21). Positive associations between high serum folate with anaemia and enhanced cognitive decline in the elderly with vitamin B12 deficiency, as well as increased risk or progression of some types of cancer, were shown both internationally and in Chile(Reference Hirsch, Sanchez and Albala20–Reference Sanchez, Hossain and Lera24). High folic acid intake was also shown to interfere with the action of certain drugs, for example, those used to treat epilepsy, some autoimmune diseases and malaria(Reference Smith, Kim and Refsum17). Those potential harmful effects of high folic acid intake, together with international concerns about the increased supply of foods fortified with folic acid, led the Chilean government to adjust the fortification policy in 2009 to 1·8 mg/kg wheat flour (range 1·0–2·6 mg/kg).
In this work, we analysed the current folate status of a subgroup of Chilean women of childbearing age for the first time after the implementation of the policy adjustment. The current study, aimed at providing evidence to assess the need for adjustment of the public policy of flour fortification with folic acid, was part of the 2016–2017 National Health Survey (Encuesta Nacional de Salud, ENS 2016–2017).
Methods
Participants and data collection
All women of childbearing age participating in the ENS 2016–2017 and living in the Metropolitan Region (MR) were selected for folate determinations, because of budget and logistic constraints (further information in the last paragraph of this section). The MR is one of the sixteen administrative and political regions from Chile. Although it is one of the smallest regions, it is the most densely populated and contains the capital city (Santiago) and its urban suburbs.
The ENS 2016–2017 is a national cross-sectional study that used stratified, multistage and clustered random sampling of households. The strata included fifteen administrative regions and urban/rural areas, from where counties were selected according to the proportion of people aged 15 years or more. Households and one person per home were selected at random in each county. The ENS was designed to estimate, at the national and regional levels, the prevalence of common chronic conditions (e.g., expected 6 and 30 % of diabetes and hypertension, respectively) and associated risk factors by sex and age groups. The Kish method was used to select one participant per household randomly(Reference Wiegand25). The number of selected households was 9303: 23·2 % were not present at home (not reached) or were non-eligible (i.e., pregnant women or persons showing aggressive behaviour, with potential risk for the interviewer) and 9·8 % refused to participate. The household national survey response rate (participants interviewed/participants sampled) was 67 %(Reference Mindell, Giampaoli and Goesswald26). No replacements were performed. The participants included in the ENS 2016–2017 survey were 6233 non-institutionalised men and women aged 15 years or more (Fig. 1). In the MR, the response rate at the first visit was 62 % (31 and 7 % non-contact and refusal, respectively). The levels of response both nationwide and in the MR are comparable to those achieved by other national health examination surveys(Reference Mindell, Giampaoli and Goesswald26).
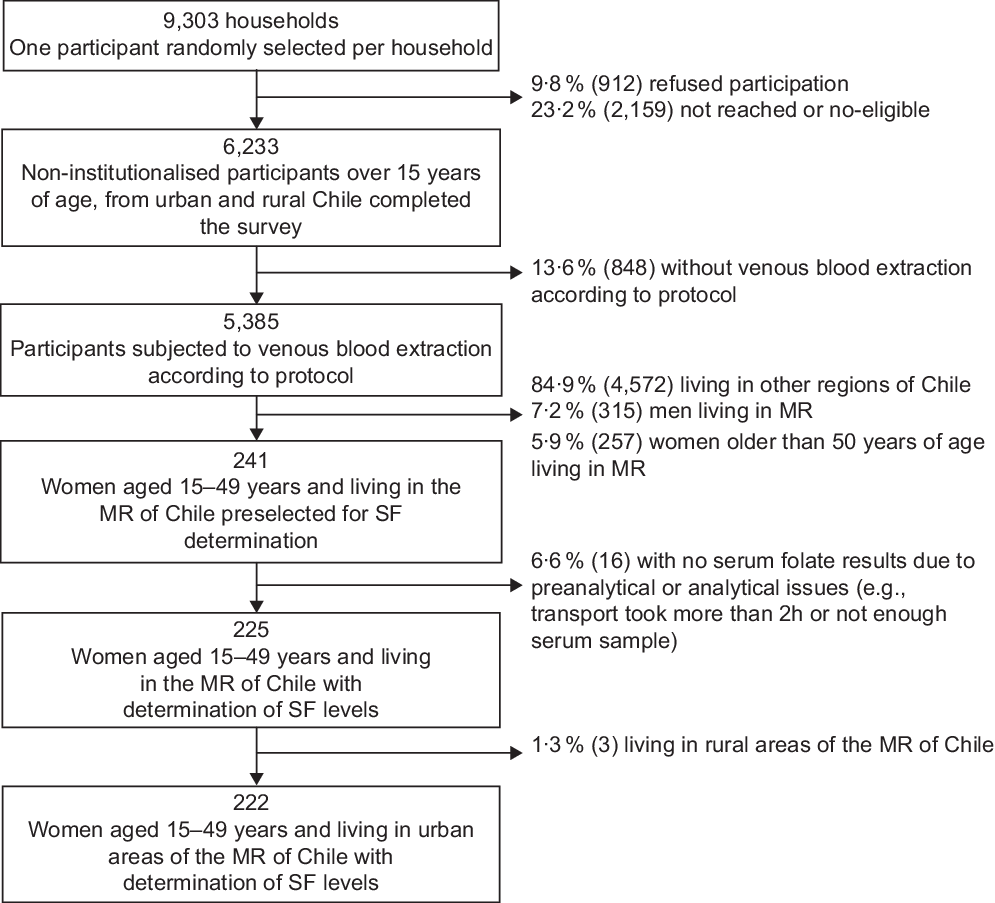
Fig. 1 Flow diagram of the subsample selected from Encuesta Nacional de Salud 2016–2017 for serum folate (SF) determinations: women of childbearing age living in the urban Metropolitan Region (MR) of Chile
Data were collected in two visits, lasting nearly 1 h each. During the first home visit, a lay interviewer applied health questionnaires using electronic data capture devices. Eighty-nine percentage of the interviewed participants received a second visit by a trained nurse, who applied questionnaires, measured blood pressure, conducted anthropometric measurements and collected fasting blood and urine samples. The nurse also recorded the medications participants were currently using (prescribed or not) via a detailed inventory. Medications were classified using the anatomical therapeutic chemical (ATC) classification system, in which supplements of folate and derivatives are given the code ATC B03BB (i.e., Ref. (27)). Similar response rates to the nurse visit were registered in the MR and the rest of the country.
Weight assessment was done with the HN289 OMRON digital scale (OMRON Healthcare Co. Ltd, accuracy 5–150 kg: ± (1 % + 0·1 kg)). Height was measured with a set square and a chrome-plated tape measure against a wall, as reported previously(Reference Mindell, Moody and Vecino-Ortiz28,Reference Mujica-Coopman, Navarro-Rosenblatt and López-Arana29) . The maximum period of stability of folate samples protected from light, if refrigerated, is 2 d(Reference Bailey, Stover and McNulty30). Among 241 women in childbearing age in the MR, 225 blood samples were processed to obtain serum within 1 h of their collection and transported in <2 d at 4°C to the Clinical Laboratory Red Salud UC CHRISTUS from Pontificia Universidad Católica de Chile in Santiago. Women subjected to serum folate analyses from the MR were mainly from urban areas (n 222), and only three women lived in rural areas, so the latter were excluded from the analyses.
Determination of serum folate concentrations
Serum folate concentrations were measured by electrochemiluminescence immunoassay, using the Roche Diagnostics ‘Cobas 8000-E602 Immunoassay Analyzer’. The assay coefficients of variation were 10·6 and 7·3 % at mean iodine concentrations of 2·5 and 6·7 ng/ml, respectively.
The serum folate levels were classified into deficient (<4·4 ng/ml), normal (4·41–20 ng/ml) or supraphysiological (>20 ng/ml) according to the WHO’s guidelines(31). The supraphysiological category was subdivided into two sub-categories: high (>20–26 ng/ml) and very high (>26 ng/ml), according to the reported cut-offs at which free folic acid is detected and cognitive deficits are reported, respectively(Reference Morris, Jacques and Rosenberg22,Reference Pfeiffer, Caudill and Gunter32) .
Socio-demographic, lifestyle and nutritional status population variables
The following variables were considered in the analysis: age, educational level (low, <8 years v. medium, 8–12 years v. high, 13 or more years of schooling), marital status (married or cohabiting v. single or divorced or widowed), parity (number of births), smoking status (smoker v. non-smoker) and nutritional status (defined as underweight (BMI < 18·5 kg/m2), normal (BMI 18·5 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2) and obese (BMI ≥ 30 kg/m2))(33,Reference de Onis, Onyango and Borghi34) . BMI (kg/m2) was derived from height and weight.
Statistical methods
Prevalence rates, means and medians were calculated with multistage sampling and adjusted following a post-stratification procedure using Chile’s 2017 census estimates. se and 95 % CI were calculated using the Taylor series expansion method(Reference Ren, Zhang and Qiao35).
Analyses were based on complete cases. P-values <0·05 were classed as statistically significant (two-tailed). Associations between serum folate levels and all variables included in the study (socio-demographic, lifestyle and nutritional status) were established by univariate and multivariate linear regression analyses, using SPSS software (version 17.0). The multivariate linear regression model for serum folate levels was adjusted for age (years) and nutritional status (BMI (kg/m2) (continuous); educational level, marital status and parity (categorical); physical activity during leisure, current smoking status and folic acid supplementation (dichotomic). Median and percentiles were calculated using SAS 9.4 (2013) statistical software. All analyses were adjusted for the complex survey design of the ENS.
Results
Socio-demographic characteristics
The socio-demographic characteristics of the weighted sample are shown in Table 1. The current study involved 222 women between the ages of 15 and 49 years (mean age 31·2 ± 0·9 years old). Almost 91 % of women had medium or high educational levels (more than 8 years of schooling). Regarding their reproductive history, around one-third of the sample was nulliparous, one-third had given birth once or twice and the remainder had given birth more than three times. Thirty-two percentage of the women declared they were smokers. Eighty-eight percentage were classified as sedentary, and almost 70 % of the participants were either overweight or obese (mean BMI 27·9 ± 0·5 kg/m2). Only 1·2 % of the participants were taking folic acid supplements.
Table 1 Socio-demographic characteristics of women aged 15–49 years living in urban areas of the Metropolitan Region of Chile, Encuesta Nacional de Salud 2016–2017
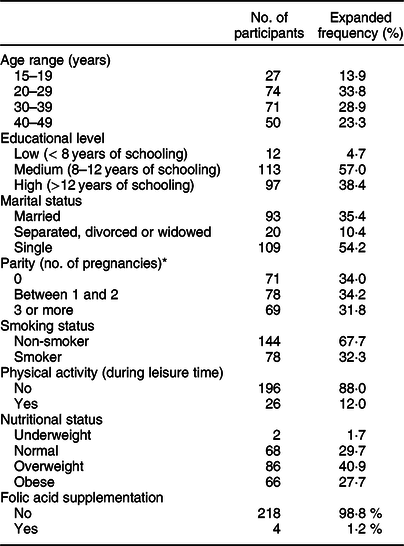
* Data from parity of four women were not reported.
Plasma folate levels
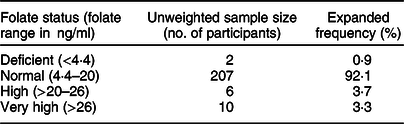
Results indicating the prevalence of folate deficiency and excess are shown in Table 2. Only 0·9 % of the women presented serum folate deficiency with levels below 4·4 ng/ml. Most participants (92·1 %) showed serum folate levels that were in the physiological range (4·4–20 ng/ml). On the other hand, 7·0 % of women exhibited supraphysiological serum folate concentrations. In this group, 3·7 % exhibited high folate levels (>20 ng/ml), and 3·3 % were in the very high or highest folate (>26 ng/ml) category.
Table 2 Serum folate levels in women of childbearing age living in urban areas of the Metropolitan Region of Chile, Encuesta Nacional de Salud 2016–2017
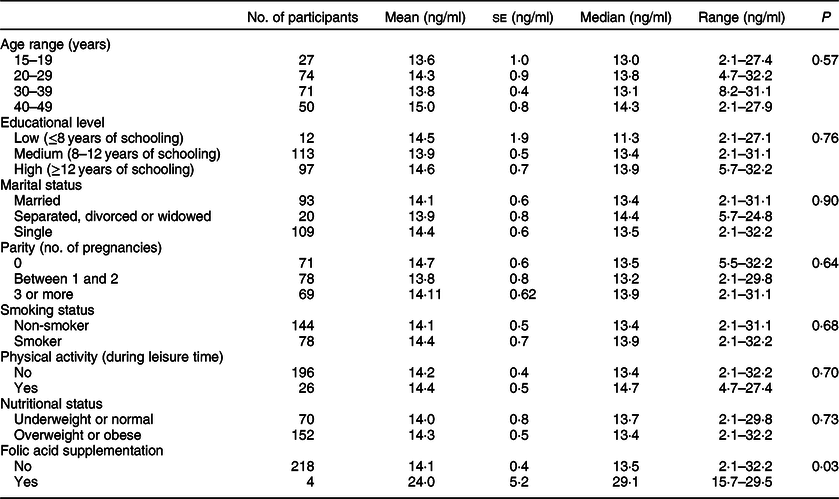
The mean serum folate level of women of childbearing age living in the urban MR was 14·2 ± 0·4 ng/ml, and the median was 13·5 ng/ml (range 2·1–32·2 ng/ml). Table 3 shows the percentile distribution in those women. Mean and median serum folate concentrations were similar across all socio-demographic variables measured (Table 4). A significant difference in serum folate levels was only found by folic acid supplementation (P = 0·028), where women with supplementation had, on average, 70 % higher levels of serum folate than women without folic acid supplementation (24·0 ± 5·2 v. 14·01 ± 0·4 ng/ml, respectively).
Table 3 Percentile distribution of serum folate in women of childbearing age living in urban areas of the Metropolitan Region of Chile

Table 4 Serum folate levels by socio-demographic characteristics in women of childbearing age living in urban areas of the Metropolitan Region of Chile
Linear regression was performed to examine the relationship between serum folate concentrations with age, educational level, marital status, parity, smoking status, nutritional status and folic acid supplement use (Table 5). The only variable that showed a significant association with folate levels after adjusting for other variables was folic acid supplement use. Women who did not use folic acid supplements presented 9·9 ng/ml lower mean serum folate levels than those taking supplements. However, only 1·2 % of women in our study used supplements.
Table 5 Adjusted linear regression model for associations between serum folate levels with socio-demographic variables and nutritional status in women of childbearing age living in urban areas of the Metropolitan Region of Chile
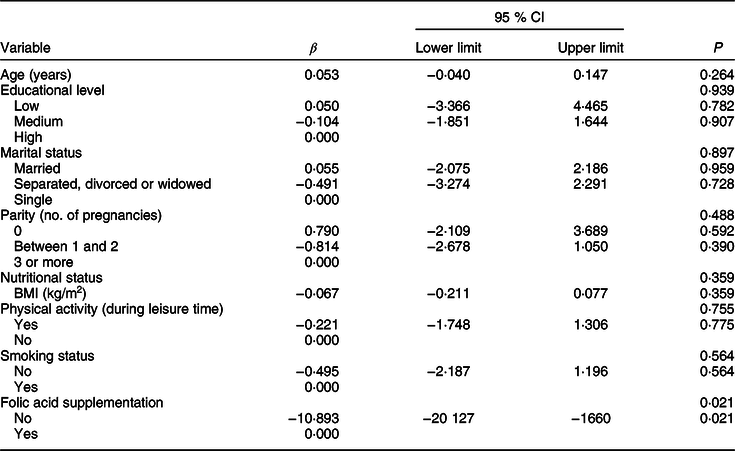
Multivariate linear regression model for serum folate levels adjusted for age (years, continuous), educational level (low, medium and high), marital status (married; separated, divorced or widowed; single), parity (0, 1–2, ≥3 pregnancies), nutritional status (BMI (kg/m2), continuous), physical activity during leisure time (yes, no), smoking status (non-smoker, current smoker) and folic acid supplementation (yes, no).
Discussion
In 2000, the implementation of mandatory folic acid fortification of wheat flour in Chile increased circulating folate levels in women of childbearing age(Reference Hertrampf, Cortes and Erickson14) and concomitantly reduced the incidence of NTD by 43 %(Reference Lopez-Camelo, Orioli and da Graca Dutra15). The folate status of women of reproductive age in Chile was last analysed a few years after, in 2003, in women of low socio-economic households(Reference Hertrampf, Cortes and Erickson14).
The aim of this paper was to determine the current folate status in Chilean women of reproductive age belonging to different socio-demographic groups for the first time after the last adjustment to the folic acid fortification policy in 2009. The results show that folate levels were mostly adequate in women included in the current study. This information, together with current data on NTD prevalence in Chile, suggests that the current folic acid fortification policy is effective and equitable.
The mean serum folate concentration in Chilean women of reproductive age from the MR of Chile was 14·2 ng/ml, lower than the 16·3 ng/ml determined in Chilean women in 2003(Reference Hertrampf, Cortes and Erickson14). Serum folate levels were adequate in 89·1 % of women, and no disparities were detected according to socio-demographic variables previously shown to affect folate status, such as educational level, smoking status or BMI(Reference Piyathilake, Eom and Hyun36). As expected, folic acid supplement intake was associated with higher serum folate levels, but only 1·2 % of the population took supplements. Despite this low level of supplement use, 7 % of women (including supplement users and non-users) exhibited supraphysiological serum folate levels.
Folate concentrations detected by the ENS 2016–2017 were lower to those in the ENS 2009–2010: the mean shifted from 21·2 to 14·2 ng/ml, and the p95 from 38·6 to 24·1 ng/ml. However, a direct comparison between serum folate levels from both surveys is inadequate because different laboratory techniques were used and different subpopulations were included in the ENS 2009–2010 and ENS 2016–2017. The former used direct competitive chemiluminescence immunoassays and included elderly participants, and the latter used competitive electrochemiluminescence-based immunoassay and analysed women of reproductive age, respectively. Indeed, existing evidence shows that higher levels of serum folate are usually detected in participants older than 60 years due to higher intake and/or lower catabolism of folic acid(Reference Pfeiffer, Sternberg and Fazili37). This difference may explain the lower level in serum folate in 2016–2017 v. 2009–2010 surveys. Future evaluation of folate status in Chileans will require the analysis of the target population (young women) in parallel to other populations potentially affected by the fortification policy, for example, children and elderly.
The WHO recommends the microbiological assay as the most reliable method to obtain comparable estimates of population folate status across different countries(Reference Cordero, Crider and Rogers38). However, this method requires standardisation and adjustment of threshold levels to obtain reproducible and comparable results within and among laboratories(Reference Cordero, Crider and Rogers38). In our country, the microbiological assay method was not available when the ENS 2016–2017 was undertaken, so we used electrochemiluminescence immunoassay, a commercial protein-based immunoassay used in clinical and research settings, to determine serum folate levels.
The current study shows that serum folate levels are mostly within the normal range in young women living in urban areas of the MR of Chile and suggests that the current folic acid fortification policy is adequate to keep NTD within folate-preventable ranges. In a recent publication, the mean reported NTD prevalence in Chile between 2001 and 2013 was 5·5:10 000(Reference Castillo-Lancellotti, Tur and Uauy13). The prevalence of NTD in 2018 was 6·4: 10 000 live births (C Mellado and RA Pardo, unpublished results). These proportions are in the range of 4–9:10 000 live births expected in countries that have reached adequate folate status(Reference Cordero, Crider and Rogers38,Reference Ray, Singh and Burrows39) .
Our study has several limitations. Firstly, we were unable to determine folate insufficiency on the basis of an increased risk of NTD because of a mismatch between the assay used to establish this folate insufficiency cut-off (microbiological assay) and the assay we used to determine folate levels in our study (electrochemiluminescence immunoassay)(Reference Cordero, Crider and Rogers38). Secondly, the current study only measured the folate levels in women of childbearing age living in the urban MR of Chile because folate integrity could not be ensured for samples transported to the clinical laboratory from other regions. Hence, our study is only representative of women residing in the MR, not in Chile as a whole. Nevertheless, it is important to note that approximately 40 % of Chileans live in the MR. Thirdly, we measured serum folate instead of erythrocyte folate, the latter being a better indicator of long-term folate levels(Reference Piyathilake, Eom and Hyun36). Finally, folate intake was not assessed in the ENS by means of dietary questionnaires (e.g., 24-h recall, food diary or FFQ). This would have enabled us to verify if women of childbearing age are meeting folate intake recommendations. Furthermore, the correlation between serum biomarker levels and women’s reported intake might have served as an indirect measure of flour manufacturers’ compliance with regulations regarding folic acid fortification in Chile.
Our analysis highlights the importance of performing additional research in order to monitor folate status in different age and sex population subgroups at a national, using the gold standard methods for measuring long-term folate status (microbiological assay in erythrocytes), before considering any further adjustments to the national policy. Technical calculations of the burden of disease due to deficit v. excess of folate may also be required to assess the benefit v. risk of folic acid fortification in our country objectively. Also, it is fundamental to ensure strict surveillance of the flour and food industries to prevent excessive fortification and addition with folic acid.
Altogether, the results and discussion described above support maintaining the current fortification policy with folic acid as a measure to maintain folate-preventable ranges of NTD in Chile. The low awareness/adherence and socio-economic disparities in the use of vitamin supplements(Reference Liu, Chen and Liu8,Reference Kinnunen, Sletner and Sommer9,Reference Ray, Singh and Burrows39,Reference Pardo, Lay-Son and Aranda Ch40) and the high proportion of unplanned pregnancies in our region(Reference Bearak, Popinchalk and Alkema4) further support this view.
Acknowledgements
Acknowledgements: The authors are grateful to the Ministry of Health from Chile for the free access to their database. All the results obtained from the current study are the responsibility of the author and in no way compromise that institution. The authors thank Luis Villarroel (Department of Public Health, School of Medicine, Pontificia Universidad Católica de Chile) for his support in analyses involving the use of SAS software. Financial support: ENS 2016–2017 was financed by the Ministry of Health from Chile (Ministerio de Salud, Subsecretaría de Salud Pública). Conflict of interest: There are no conflicts of interest. Authorship: D.B., G.E., F.M., M.F. and P.M formulated the research question, designed the study and approved the last version of this manuscript. P.M. was the Director of the ENS 2016–2017, G.E., A.P. and D.B. analysed the data and D.B., G.E., A.P. and P.M wrote the article. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving research study participants were approved by the Ethics Committee from the Pontificia Universidad Católica de Chile and the Chilean Ministry of Health. Written informed consent was obtained from all patients.









