Marine fish farming is mostly based on diets containing high levels of n-3 highly unsaturated fatty acids (HUFA), particularly EPA (20 : 5n-3) and DHA (22 : 6n-3). However, the continuous expansion of aquaculture and the decreasing global availability of marine oil and fishmeal force the industry to explore alternative and sustainable lipid sources(Reference Sargent and Tacon1, Reference Watanabe2). In salmonids, the use of vegetable oils to replace the majority of dietary fish oil (FO) is now feasible in practical aquafeeds without loss of growth performance(Reference Bell, McGhee, Campbell and Sargent3–Reference Torstensen, Froyland, Ørnsrud and Lie5). Nevertheless, essential fatty acid (EFA) requirements differ between species. Thus, linoleic acid (18 : 2n-6) and α-linolenic acid (18 : 3n-3) can satisfy the EFA requirements of freshwater fish, whereas marine fish require longer-chain n-3 and n-6 PUFA for optimal growth and health(Reference Watanabe6). Supporting this, fatty acid desaturation and elongation of linoleic acid and α-linolenic acid are well established in freshwater and anadromous fish species(Reference Sargent, Tocher, Bell, Halver and Hardy7), but marine fish including European sea bass(Reference Mourente, Dick, Bell and Tocher8) and gilthead sea bream (Sparus aurata L.)(Reference Mourente and Tocher9, Reference Seiliez, Panserat, Corraze, Kaushik and Bergot10) do not show rates for bioconversion of C18 PUFA into C20 and C22 HUFA that would allow n-3 HUFA requirements to be met.
Signs of EFA deficiencies in fish include skin lesions and several neurological alterations linked to reduced growth and survival rates during larval and juvenile on-growing phases(Reference Sargent, McEvoy, Estevez, Bell, Bell, Henderson and Tocher11). Lipoid liver disease and intense accumulation of intestinal lipid droplets are also documented as metabolic disorders arising from defective supplies of phospholipids (PL)(Reference Fontagné, Geurden, Escaffre and Bergot12–Reference Olsen, Dragnes, Myklebust and Ringo14) and n-3 HUFA(Reference Caballero, Izquierdo, Kjørsvik, Montero, Socorro, Fernández and Rosenlund15). Additionally, fatty acids modulate immune responses and eicosanoids produced from arachidonic acid (20 : 4n-6) are recognised as inflammatory agents, whereas DHA, and especially EPA-derived eicosanoids, exert anti-inflammatory effects in a wide variety of experimental models(Reference Abeywardena and Head16, Reference Calder17). However, factors other than dietary ones may influence lipid metabolism, and relative rates of fat deposition and mobilisation vary greatly as a result of environmental factors including parr-smolt transformation in salmonids(Reference Fonseca-Madrigal, Karalazos, Campbell, Bell and Tocher18, Reference Fonseca-Madrigal, Bell and Tocher19). Likewise, gonadal maturation and spawning have a significant impact in the muscle fatty acid profile of gilthead sea bream females(Reference Almansa, Martín, Cejas, Badía, Jerez and Lorenzo20). Deposition rates and fatty acid profiles also vary seasonally in wild gilthead sea bream(Reference Özyurt, Polat and Özkütük21), but the feeding regimen is a major influence and most of these changes can be overridden by full rations given under intensive aquaculture. Indeed, monitoring studies in various Greek fish farms failed to show a seasonal impact in the muscle fat deposition and profiling of gilthead sea bream(Reference Grigorakis22).
Gilthead sea bream is a major cultured finfish in the Mediterranean area, and extensive research to sustain further growth has proved that vegetable oils can replace up to 60 % of the added FO, in fishmeal-based diets, without adverse effects on growth, feed efficiency and survival rates(Reference Mourente, Dick, Bell and Tocher8, Reference Izquierdo, Obach, Arantzamendi, Montero, Robaina and Rosenlund23, Reference Izquierdo, Montero, Robaina, Caballero, Rosenlund and Ginés24). Additional studies have addressed the extensive replacement of fishmeal by plant proteins(Reference Gómez-Requeni, Mingarro, Calduch-Giner, Médale, Martin, Houlihan, Kaushik and Pérez-Sánchez25, Reference De Francesco, Parisi, Pérez-Sánchez, Gómez-Requeni, Médale, Kaushik, Mecatti and Poli26), and recently growth-compensatory mechanisms of the somatotropic axis have been evidenced in short-term trials when juvenile fish were fed during the summer growth spurt with plant protein-based diets and graded levels of vegetable oils(Reference Benedito-Palos, Saera-Vila, Calduch-Giner, Kaushik and Pérez-Sánchez27). Indeed, with the total replacement of dietary FO some growth reduction occurred, and it was accompanied by decreased production of hepatic insulin-like growth factor-I not compensated by the local expression (skeletal muscle) of insulin-like growth factor and/or growth hormone receptors. In humans and other animal models, there is also increasing evidence linking endocrine and metabolic dysfunctions resulting in obesity and insulin resistance with steatosic livers and altered fatty acid profiles of PL and stored TAG(Reference Kelley28). In this sense, three major goals were addressed in the present paper in a gilthead sea bream trial conducted over a growth trial of 8 months' duration: (a) the relationship between dietary and muscle fatty acid profiles; (b) the robustness of the PL fatty acid profile when EFA requirements are theoretically covered in the diet; (c) histological alterations of liver and intestine as sensitive target tissues of lipid-metabolism dysregulation.
Materials and methods
Diets
Four isoproteic, isolipidic and isoenergetic plant protein-based diets were made with a low inclusion level (20 %) of fishmeal and fish soluble protein concentrates (Tables 1 and 2). All diets were supplemented with l-lysine (0·55 %) and contained soya lecithin (1 %). Added oil was either Scandinavian FO (control diet) or a blend of vegetable oils, replacing 33 % (33VO diet), 66 % (66VO diet) and 100 % (VO diet) of the FO. The blend of vegetable oils (rapeseed oil–linseed oil–palm oil, 2·5:28·8:3·6, by wt) provided a similar balance of saturates, monoenes and PUFA to that found in FO, but without HUFA(Reference Torstensen, Froyland, Ørnsrud and Lie29, Reference Mourente, Good, Thompson and Bell30). All diets were manufactured using a twin-screw extruder (Clextral, BC 45) at the INRA experimental research station of Donzacq (Landes, France), dried under hot air, sealed and kept in air-tight bags until use.
Table 1 Ingredients and chemical composition of experimental diets

33VO diet, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; VO diet, diet in which vegetable oil replaces 100 % of fish oil; CP, crude protein.
* Fishmeal (Scandinavian LT; Norsildmel, Fyllingsdalen, Norway).
† Fish soluble protein concentrate (Sopropêche, Boulogne-Sur-Mer, France).
‡ 3 Fish oil (Sopropêche).
§ Supplied the following (mg/kg diet, except as noted): calcium carbonate (40 % Ca), 2·15 g; magnesium hydroxide (60 % Mg), 1·24 g; potassium chloride, 0·9 g; ferric citrate, 0·2 g; potassium iodide, 4; sodium chloride, 0·4 g; calcium hydrogen phosphate, 50 g; copper sulfate, 0·3; zinc sulfate, 40; cobalt sulfate, 2; manganese sulfate, 30; sodium selenite, 0·3.
∥ Supplied the following (mg/kg diet): retinyl acetate, 2·58; dl-cholecalciferol, 0·037; dl-α-tocopheryl acetate, 30; menadione sodium bisulfite, 2·5; thiamin, 7·5; riboflavin, 15; pyridoxine, 7·5; nicotinic acid, 87·5; folic acid, 2·5; calcium pantothenate, 2·5; vitamin B12, 0·025; ascorbic acid, 250; inositol, 500; biotin, 1·25; choline chloride, 500.
Table 2 Fatty acid composition of experimental diets (% of total fatty acid methyl esters)
(Mean values of two determinations)

33VO diet, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; VO diet, diet in which vegetable oil replaces 100 % of fish oil; tr, trace values < 0·05; HUFA, highly unsaturated fatty acids.
* Calculated excluding 18 C atoms of the n -3 series.
† Calculated excluding 18 C atoms of the n -6 series.
Growth trial and tissue sampling
Juvenile gilthead sea bream of Atlantic origin (Ferme Marine de Douhet, Ile d'Oléron, France) were acclimatised to laboratory conditions at the Institute of Aquaculture Torre de la Sal (IATS) for 20 d before the start of the growth study. Fish of 16 g initial mean body weight were distributed into twelve fibreglass tanks (500 litres) in groups of sixty fish per tank. Water flow was 20 litres/min, and O2 content of outlet water remained higher than 85 % saturation. The growth study was undertaken over 8 months (23 May to 18 January), and day-length and water temperature (11–27°C) varied over the course of the trial following natural changes at IATS latitude (40°5′N; 0°10′E).
Each diet was randomly allocated to triplicate groups of fish, and feed was offered by hand to apparent visual satiety twice per day (09.00 and 14.00 hours) from May to September, and once per day (12.00 hours) from October to January. No mortality was registered, and feed intake was recorded daily. At regular intervals, fish were counted and group-weighed under moderate anaesthesia (3-aminobenzoic acid ethyl ester, MS 222; 100 μg/ml). At critical step windows over the growth trial (midsummer, 5 August; early autumn, 27 September; early winter, 18 January), randomly selected fish (four fish per tank; twelve fish per treatment) were killed by a blow on the head before tissue sampling. Portions of dorsal muscle (white muscle) were extracted and rapidly excised, frozen in liquid N2, and stored at − 80°C until fatty acid analyses of lipid extracts. Liver and intestine samples for fat content determinations and histological samples were taken only in September (20 h after the last feeding) when fish still show an active feeding behaviour. All procedures were carried out according to national and institutional regulations (Consejo Superior de Investigaciones Científicas, Institute of Aquaculture Torre de la Sal Review Board) and the current European Union legislation on handling experimental animals.
Histology and tissue lipid content determinations
Tissue fragments of liver and hindgut were fixed in 10 % buffered formalin, embedded in Technovit-7100 resin (Kulzer, Heraeus, Germany), and stained with toluidine blue or haematoxylin-eosin after thin sectioning (1–3 μm). Liver and muscle lipids were extracted according to Folch et al. (Reference Folch, Lees and Sloane Stanley31), and determined gravimetrically after the evaporation of the organic solvent under a stream of N2 and overnight desiccation.
Fatty acid analyses
Muscle total lipids (TL) for fatty acid analyses were extracted by the method of Folch et al. (Reference Folch, Lees and Sloane Stanley31), using chloroform–methanol (2:1, v/v) containing 0·01 % butylated hydroxytoluene as antioxidant. PL from muscle lipid extracts were isolated by TLC (Silica gel G 60, 20 × 20 cm glass plates; Merck, Darmstadt, Germany) using hexane–diethyl ether–acetic acid (85:15:1·5, by vol.) as a solvent system. PL bands at the bottom of plates were scraped and extracted with chloroform–methanol (2:1, v/v) containing 0·01 % butylated hydroxytoluene.
After the addition of nonadecaenoic acid (Sigma, Poole, Dorset, UK) as internal standard, muscle PL and TL extracts were subjected to acid-catalysed transmethylation for 16 h at 50°C using 1 ml toluene and 2 ml 1 % (v/v) sulfuric acid in methanol(Reference Christie and Christie32). Fatty acid methyl esters were extracted with hexane–diethyl ether (1:1, v/v), and those derived from TL were purified by TLC using hexane–diethyl ether–acetic acid (85:15:1·5, by vol.) as a solvent system. Fatty acid methyl esters were then analysed with a gas chromatograph (Fisons Instruments GC 8000 Series; Rodano, Italy) equipped with a fused silica 30 m × 0·25 mm open tubular column (Tracer, TR-WAX; film thickness: 0·25 μm; Teknokroma, Spain) and a cold on-column injection system. The carrier gas used was He, and temperature programming was from 50 to 180°C at 40°C/min and then to 220°C at 3°C/min. Peaks were recorded in a personal computer using the Azur software package (version 4.0.2.0; Datalys, Saint-Martin-d'Hères, France). Individual fatty acid methyl esters were identified by reference to well-characterised FO standards, and the relative amount of each fatty acid was expressed as a percentage of the total amount of fatty acids in the analysed sample.
Statistical analysis
Growth parameters (tank average values) and the relative amount of fatty acids were checked for normal distribution and homogeneity of variances, and when necessary arcsin transformation was performed. Data were analysed by one-way ANOVA followed by Student–Newman–Keuls test at a significance level of 5 %. Also, the percentages of each fatty acid were chemometrically analysed by including them as variables in a multivariate principal components analysis (MPCA) model. With such a parsimonious approach, the dataset of variables (fatty acids) is reduced into a smaller set of factors or components. Parsimony is achieved by explaining the maximum amount of common variance in a correlation matrix using the smallest number of explanatory concepts. Factors are statistical entities that can be visualised as classification axes along which measurement variables can be plotted, giving an idea of their correlation with the corresponding factor (loading). Score plots are a graphical representation of individual (dietary groups) scores in the new subset of measurement variables (factors). They illustrate the relationship among individual cases (dietary groups), and the variables, and help in the analysis of data by showing graphical associations, or through new statistical analyses. In the present study, factor scores were subsequently analysed by one-way ANOVA and Student–Newman–Keuls multiple-comparison tests. All analyses were made using the SPSS package version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Growth performance
Fish grew from 16 to 240–270 g over a growth trial of 8 months' duration under natural light and temperature conditions (Fig. 1). The final body weight of fish fed the control diet did not differ from that of fish fed 33VO and 66VO diets, with overall specific growth rates ranging between 1·12 and 1·16 (see Table 3). By contrast, the total replacement of FO dictated a slight but significant reduction (10 %) of final body weight in fish fed the VO diet. A concurrent and significant decrease of voluntary feed intake (g DM intake) was found in fish fed the VO diet. Feed efficiency (0·97–1·01) remained high and unchanged irrespective of dietary treatment.

Fig. 1 (A) Seasonal changes of temperature (—) and day length (- - -). (B) Body weight over the course of the trial of fish fed the experimental diets. Jun, June; Jul, July; Aug, August; Sep, September; Oct, October; Nov, November; Dec, December; Jan, January; Feb, February; (-●-), control diet; (-○-), diet in which vegetable oil replaces 33 % of fish oil; (-▾-), diet in which vegetable oil replaces 66 % of fish oil; (-△-), diet in which vegetable oil replaces 100 % of fish oil; ↑ , tissue sampling times. Values are the means of triplicate tanks, with standard errors represented by vertical bars.
Table 3 Growth performance of fish fed the four experimental diets during 8 months
(Mean values and standard deviations of triplicate tanks)
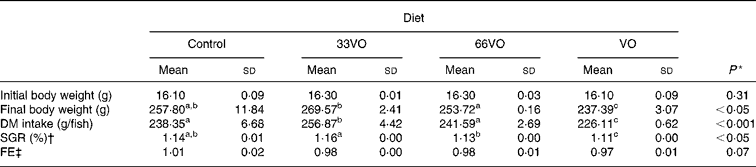
33VO diet, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; VO diet, diet in which vegetable oil replaces 100 % of fish oil; SGR, specific growth ratio; FE, feed efficiency.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05; Student–Newman–Keuls).
* P values result from one-way ANOVA.
† SGR = (100 × (ln final fish wt − ln initial fish wt))/d.
‡ FE = wet wt gain/dry feed intake.
Tissue fat deposition and histological alterations
After the summer replenishment of energy stores, lipid content of dorsal white muscle (6–8 %) was not affected by the dietary treatment. Hepatic fat content in fish fed control and 33VO diets was high and of the same order of magnitude (15 % on wet-matter basis; 0·23–0·25 g/100 g body weight). A progressive and significant increase (up to 25 %; 0·44 g/100 g body weight) was found with the graded replacement of FO in fish fed 66VO and VO diets (Fig. 2 (C)). However, signs of initial and localised lipoid liver disease were only found with the total replacement of FO with vegetable oils (Fig. 2 (A) and (B)). None of the FO-replaced diets produced apparent signs of histological damage in the intestine. Only one fish fed the VO diet had a moderate accumulation of lipid droplets in the intestinal epithelium that was not considered pathological.

Fig. 2 Representative histological sections of livers of fish sampled in September after 18 weeks of feeding the experimental diets: (A) control (CTRL) diet; (B) diet in which vegetable oil replaces 100 % of fish oil (VO diet) (staining by toluidine blue; scale bars = 50 μm). Notice the lipoid liver degeneration with breakdown of hepatocyte membranes ( → ). Liver fat content (C) of fish fed the four experimental diets (18 weeks). BW, body weight; 33VO diet, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil. Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05; Student–Newman–Keuls).
Muscle fatty acid profile
The effects of dietary treatment upon muscle fatty acid profiles of TL are shown on a time-course basis (Table 4). Overall, fish fed the control diet contained 28 % saturates (mainly 16 : 0 and 14 : 0), almost 32 % monoenes (over half of which were 18 : 1n-9), 12 % n-6 fatty acids (predominantly 18 : 2n-6) and 18–20 % n-3 HUFA (predominantly EPA and DHA). Increased amounts of 18 : 1n-9, 18 : 2n-6 and 18 : 3n-3, in combination with reduced proportions of n-3 HUFA and saturated fatty acids, were found with the progressive replacement of FO by vegetable oils. The two first components of MPCA accounted for the 78 % of variation of this dataset, although 67·9 % of variation was explained by component 1 itself (Fig. 3 (A)). Thus, no grouping was recognised on the basis of sampling time (second factor score), whereas four groups were significantly separated (Student–Newman–Keuls; P < 0·05) and identified as the VO, 66VO, 33VO and control diets in the first factor score (Fig. 3 (B)).
Table 4 Effects of the feeding regimen on the muscle fatty acid profile of total lipids (% of total fatty acid methyl esters) in fish sampled in August, September and January
(Mean values and standard deviations of ten fish)

33VO diet, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; VO diet, diet in which vegetable oil replaces 100 % of fish oil; tr, trace value < 0·05; HUFA, highly unsaturated fatty acids.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05; Student–Newman–Keuls).
* Calculated excluding 18 C atoms of the n -3 series.
† Calculated excluding 18 C atoms of the n -6 series.

Fig. 3 Component plot (A) and factor score plot (B) of the multivariate principal components analysis for the muscle fatty acid profile of total lipids (TL) in fish sampled in August (Aug), September (Sep) and January (Jan). VO, diet in which vegetable oil replaces 100 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; 33VO, diet in which vegetable oil replaces 33 % of fish oil; CTRL, control diet. Mean values are shown in the factor score plot to simplify the graph representation. Ovals stand for different clusters in the factor score 1 (P < 0·05; Student–Newman–Keuls).
The fatty acid profile of muscle PL of fish sampled at the end of the trial (January) is shown in Table 5. All experimental groups retained high amounts of SFA, predominantly 16 : 0 (>13 %) and 18 : 0 (>8 %), but the relative amount of 18 : 2n-6 increased up to 23 % in fish fed the VO diet. A concurrent reduction in n-3 HUFA was also found, decreasing the EPA plus DHA content from 36 to 28 % (fish fed control, 33VO and 66VO diets) to 16 % (fish fed the VO diet). Thus, when data of PL and TL fractions were analysed by MPCA, the two principal components accounted for 67 % of variation (Fig. 4 (A)). Component 1 explained 39·6 % of variation and separated fatty acids that predominate in TL (on the left) from those characteristic of more unsaturated PL (on the right). Component 2 accounted for 27·8 % of variation, and separated fatty acids representative of FO (above the zero line) from those characteristic of vegetable oils (below the zero line). The factor score plot separated TL and PL in the abscise axis, whereas grouping in the ordinate axis was based on the different effects of dietary intervention upon each lipid class. Accordingly, three major clusters were significantly separated (Student–Newman–Keuls; P < 0·05) and identified in the first factor score plot as: (a) TL group, (b) PL of fish fed the VO diet and (c) a homogeneous group corresponding to PL of fish fed the control, 33VO and 66VO diets (Fig. 4 (B)).
Table 5 Effects of the feeding regimen on the muscle fatty acid profile of phospholipids (% of total fatty acid methyl esters) in fish sampled at the end of the trial (January)
(Mean values and standard deviations of ten fish)

33VO diet, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; VO diet, diet in which vegetable oil replaces 100 % of fish oil; HUFA, highly unsaturated fatty acids.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05; Student–Newman–Keuls).
* Calculated excluding 18 C atoms of the n -3 series.
† Calculated excluding 18 C atoms of the n -6 series.
‡ Calculated taking into account all n -3 and n -6 fatty acid series.
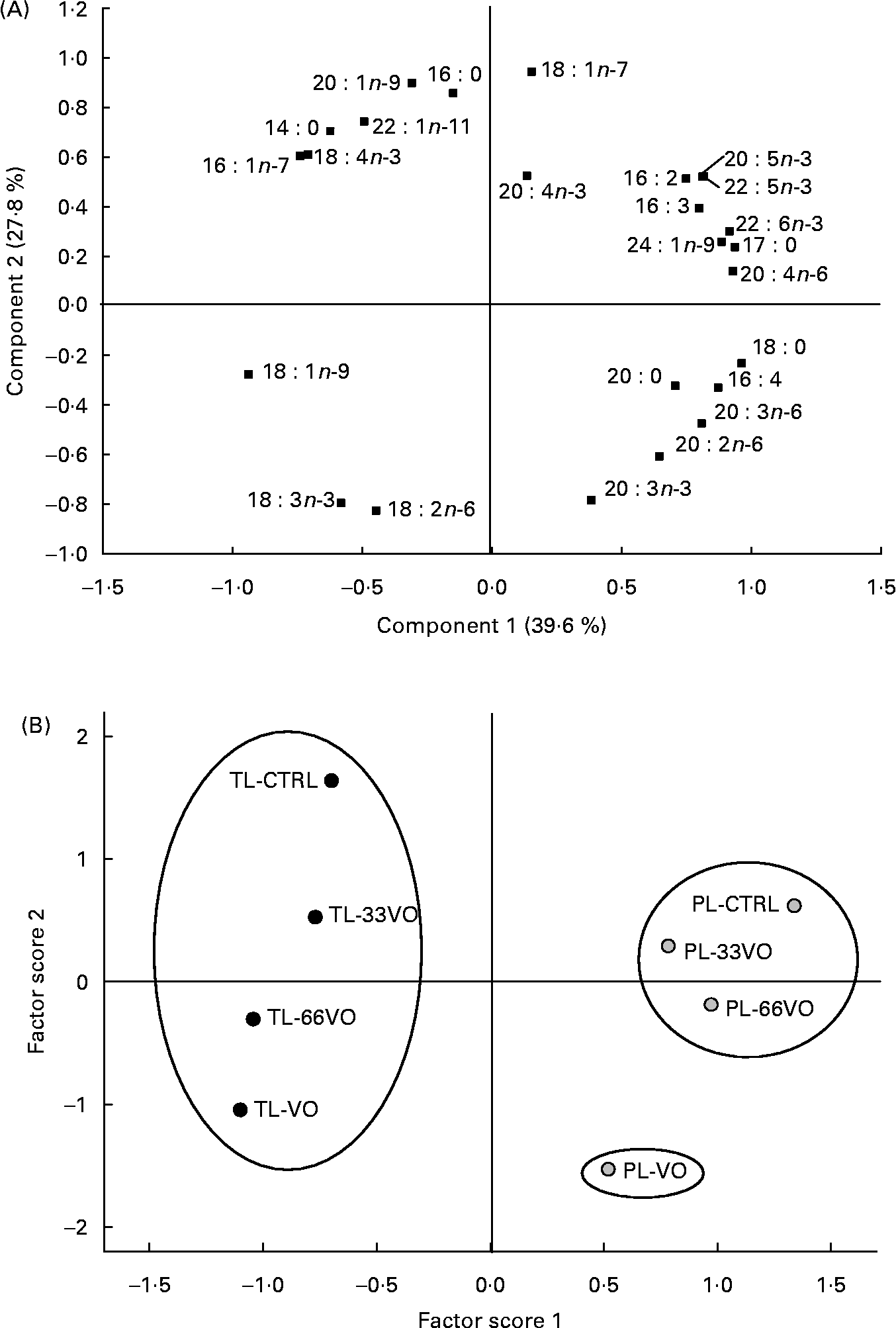
Fig. 4 Component plot (A) and factor score plot (B) of the multivariate principal components analysis for the muscle fatty acid profile of total lipids (TL; ●) and phospholipids (PL; ![]() ) (January-sampled fish). CTRL, control diet; 33VO, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; VO, diet in which vegetable oil replaces 100 % of fish oil. Mean values are shown in the factor score plot to simplify the graph representation. Ovals stand for different clusters in the factor score 1 (P < 0·05; Student–Newman–Keuls).
) (January-sampled fish). CTRL, control diet; 33VO, diet in which vegetable oil replaces 33 % of fish oil; 66VO, diet in which vegetable oil replaces 66 % of fish oil; VO, diet in which vegetable oil replaces 100 % of fish oil. Mean values are shown in the factor score plot to simplify the graph representation. Ovals stand for different clusters in the factor score 1 (P < 0·05; Student–Newman–Keuls).
Discussion
The demand for feed in intensive aquaculture has increased over recent years and extensive research has been done on alternative raw materials of vegetable origin. However, the main constraint for the use of vegetable oils in marine fish feeds is the lack of n-3 long-chain PUFA, particularly EPA and DHA. Moreover, quantitative requirements depend on species and growth rates, and the biological demand for n-3 HUFA was at least 1·6 % of DM for flatfish larvae(Reference Le Milinaire, Gatesoupe and Stephan33) decreasing to 0·8–0·6 % in juvenile(Reference Gatesoupe, Leger, Boudon, Metailler and Luquet34, Reference Lee, Lee and Kim35) and grower fish(Reference Leger, Gatesoupe, Metailler, Luquet and Fremont36). Similar requirements were reported for juvenile European sea bass(Reference Skalli and Robin37) and gilthead sea bream(Reference Kalogeropoulos, Alexis and Henderson38). In the present study the theoretical requirements of EFA were met by 33VO (1·6 % EPA+DHA) and 66VO (0·9 % EPA+DHA) diets, but not by the VO diet (0·3 % EPA+DHA). Thereby, in this and in a previous short-term trial(Reference Benedito-Palos, Saera-Vila, Calduch-Giner, Kaushik and Pérez-Sánchez27), no detrimental effects on growth performance were found with the replacement of up to 66 % of the added FO, whereas a slight but significant reduction in feed intake and weight gain was found with the total FO replacement, indicating that a dietary supply of 0·3 % of EPA+DHA was not sufficient for normal growth and development of gilthead sea bream. However, fishmeal itself contains appreciable amounts of FO, and trials conducted in our experimental facilities show that the total replacement of the added FO is feasible without adverse effects on growth in gilthead sea bream diets with a 30–35 % fishmeal inclusion (L Benedito-Palos, JC Navarro, A Sitjá-Bobadilla, JG Bell, S Kaushik and J Pérez-Sánchez, unpublished results). Regost et al. (Reference Regost, Arzel, Robin, Rosenlund and Kaushik39) also reported the feasibility of the total replacement of FO by vegetable oils in turbot fed fishmeal-based diets. Similar results were reported in sharpsnout sea bream by Piedecausa et al. (Reference Piedecausa, Mazon, Garcia Garcia and Hernandez40) However, in the present study, we report for the first time, over the production cycle of a marine fish, the use of well-balanced plant protein diets with a low inclusion of marine raw materials ( < 20 %) just to cover EFA needs.
It is noteworthy that growth rates in the trial conducted in the present study were excellent and even improved upon the values reported for fish of the same size class under similar experimental conditions(Reference Gómez-Requeni, Mingarro, Calduch-Giner, Médale, Martin, Houlihan, Kaushik and Pérez-Sánchez25, Reference De Francesco, Parisi, Pérez-Sánchez, Gómez-Requeni, Médale, Kaushik, Mecatti and Poli26, Reference Mingarro, Vega-Rubín de Celis, Astola, Pendón, Martínez Valdivia and Pérez-Sánchez41, Reference Gómez-Requeni, Mingarro and Kirchner42). This fact can be attributed to the genetic improvement of fish strains but also to better fish management, culture conditions and dietary formulation. Since fishmeal is also a source of PL, the plant protein mixture in the present study was adequately supplemented with amino acids and PL supplied in the form of soya lecithin. This added component is rich in phosphatidylcholine, a polar lipid molecule that is a natural component of lipoproteins and cellular membranes, adding fluidity and rigidity to cells as well as being required for lipoprotein synthesis, lipid mobilisation and digestibility. Our experimental design does not delineate unequivocally the beneficial effects of soya lecithin, but it must be noted that signs of intestine damage and transport dysfunction (massive accumulation of lipid droplets) were not found in any experimental group. By contrast, intense accumulation of lipid droplets was reported earlier in the hindgut of juvenile gilthead sea bream fed plant protein and FO-based diets without PL supplementation(Reference Sitjà-Bobadilla, Peña-Llopis, Gómez-Requeni, Médale, Kaushik and Pérez-Sánchez43). Similar histological alterations have been reported by other authors using transmission electron microscopy(Reference Caballero, Izquierdo, Kjørsvik, Montero, Socorro, Fernández and Rosenlund15) and, interestingly, earlier studies in young larvae demonstrated that dietary lecithin increases the appearance of lipoproteins and enhances the lipid transport through the gut(Reference Fontagné, Geurden, Escaffre and Bergot12, Reference Salhi, Hernández-Cruz, Bessonart, Izquierdo and Fernández-Palacios44, Reference Liu, Caballero, Izquierdo, El-Sayed Ali, Hernández-Cruz, Valencia and Fernández-Palacios45). Likewise, intense accumulation of lipid droplets was seen in the gastrointestinal tract of salmonids fed with plant oils, but this condition was reversed by PL supplementation(Reference Olsen, Myklebust, Kaino and Ringo13, Reference Olsen, Dragnes, Myklebust and Ringo14).
Defects in fatty acid storage and oxidation are a central initiating factor for metabolic and endocrine alterations, resulting in enhanced fatty acid flux from adipose tissue towards liver and muscle(Reference Browning and Horton46, Reference Avramoglu, Basciano and Adeli47). Ration size by itself is also a major disrupting factor, and long-term feeding close to satiation increases hepatic fat deposition in gilthead sea bream juveniles, leading to lipoid liver disease and enterocyte desquamation in fish fed commercial diets(Reference Sitjà-Bobadilla, Mingarro, Pujalte, Garay, Alvarez-Pellitero and Pérez-Sánchez48). Dietary inclusion of vegetable oils(Reference Caballero, Izquierdo, Kjørsvik, Fernández and Rosenlund49, Reference Wassef, Wahby and Sakr50) and plant proteins(Reference Sitjà-Bobadilla, Peña-Llopis, Gómez-Requeni, Médale, Kaushik and Pérez-Sánchez43) also induces lipoid liver disease, and the role of TNFα and lipoprotein lipase as lipolytic cytokines and rate-limiting enzymes in tissue fatty acid uptake has been reported in gilthead sea bream(Reference Saera-Vila, Calduch-Giner, Gómez-Requeni, Médale, Kaushik and Pérez-Sánchez51, Reference Saera-Vila, Calduch-Giner, Navarro and Pérez-Sánchez52). Precise effects of nutrients on the dysregulation of lipid metabolic pathways still remain largely unknown, but several studies indicate that soyabean phosphatidylcholine may alleviate signs of liver diseases, promoting a healthy lipid metabolism(Reference Fontagné, Geurden, Escaffre and Bergot12, Reference Canty and Zeisel53, Reference Patova, Prozorovskaia, Torkhovskaia, Baranova and Guseva54). This notion is supported in the present study by the observation that hepatic fat deposition varied between 15 and 25 % of wet weight, though signs of initial and focal lipoid liver disease were only found with the total FO replacement. By contrast, clear signs of liver disease have been reported with a liver fat deposition below 15 % in fish fed 16 % lipid diets(Reference Sitjà-Bobadilla, Peña-Llopis, Gómez-Requeni, Médale, Kaushik and Pérez-Sánchez43) (22 % lipid diets were used in the present study). This finding suggests that the fat threshold level for liver damage was significantly increased in the present study. However, the extent to which this condition is due to PL supplementation with soya lecithin rather than to other poorly defined dietary factors merits more specific research.
The gilthead sea bream, as other poikilotherms, utilises favourable conditions in summer for rapid growth and replenishment of energy stores, but analyses of fatty acid profiles in this and other fish species including Atlantic salmon(Reference Bell, McEvoy, Tocher, McGhee, Campbell and Sargent55, Reference Moya-Falcon, Hvattum, Tran, Thomassen, Skorve and Ruyter56), rainbow trout(Reference Caballero, Obach, Rosenlund, Montero, Gisvold and Izquierdo57), turbot(Reference Regost, Arzel, Robin, Rosenlund and Kaushik39) and European sea bass(Reference Richard, Mourente, Kaushik and Corraze58, Reference Mourente and Bell59) suggest a selective incorporation of n-3 PUFA in polar lipids and perhaps increased oxidation rates of other more easily utilisable fatty acids. Moreover, the seasonal cycling increases in fat storage alter the ratio of polar and neutral lipids, driving the well-reported changes in the muscle fatty acid profile seen in wild gilthead sea bream(Reference Özyurt, Polat and Özkütük21). In addition, there is experimental evidence linking fatty acid profiles of wild brown trout with the trophic level of the species, the location of the catch, and the size and physiological status of the animal(Reference Kaushik, Corraze, Radunz-Neto, Larroquet and Dumas60). However, feeding regimens under intensive aquaculture production apparently override the impact of the season on the fatty acid profile of farmed gilthead sea bream(Reference Grigorakis22). This notion is supported by data from the present study, and the MPCA analysis revealed that the 68 % of the total variation in the muscle fatty acid profile of TL is explained by the dietary component. Likewise, alterations in the muscle fatty acid profile of cultured Chinook salmon are viewed as a direct consequence of changes in body weight, fat deposition and ration size(Reference Kiessling, Pickova, Eales, Dosanjh and Higgs61). This information is of relevance and highlights important nutritional and quality traits, in particular for meeting human requirements for n-3 PUFA and HUFA, which needs to be considered for a proper timing and use of FO finishing diets for the recovery of a marine fatty acid profile in fish fed vegetable oils through most of the production cycle(Reference Torstensen, Froyland, Ørnsrud and Lie29, Reference Mourente, Good, Thompson and Bell30, Reference Regost, Arzel, Robin, Rosenlund and Kaushik39).
The degree of unsaturation of fatty acids mediates the fluidity and structural integrity of cell membranes, which may exacerbate signs of EFA deficiency during fish overwintering(Reference Sargent and Tacon1, Reference Jobling and Bendiksen62, Reference Skalli, Robin, Le Bayon, Le Delliou and Person-Le Ruyet63). This is the reason why the analysis of PL fatty acid profiles was focused in the present study on the cold season. At this time, the factor score plot showed two major clusters corresponding to PL and TL subgroups. In addition, the PL branch of fish fed the control, 33VO and 66VO diets appeared as a high homogeneous group, which evidenced the robustness of the PL fatty acid profile when EFA requirements were theoretically covered. However, fish fed the VO diet were deficient in EFA, and PL-VO appeared as an outlier group in the MPCA analysis. More detailed analyses revealed the relative enrichment of these fish in 20 : 2n-6, 20 : 3n-6 and 20 : 3n-3. Since vegetable oils are devoid of these fatty acids and they are part of the biosynthetic routes of n-6 and n-3 HUFA, this finding highlights adaptive attempts to alleviate EFA deficiencies. The accumulation of 20 : 3n-6 indicates increased Δ-6 desaturation and elongation of dietary 18 : 2n-6 that is driven by increased dietary and tissue levels of this fatty acid, derived from vegetable oils, as well as reduced tissue levels of n-3 HUFA(Reference Mourente, Dick, Bell and Tocher8). The increased levels of 20 : 2n-6 and 20 : 3n-3, which are ‘dead-end’ elongation products of 18 : 2n-6 and 18 : 3n-3, respectively, reflect increased levels of dietary C18 PUFA although increased levels of 20 : 3n-9, a marker of EFA deficiency, were not observed. In gilthead sea bream, the expression of Δ-6 desaturase is highly induced in fish fed a HUFA-free diet(Reference Seiliez, Panserat, Corraze, Kaushik and Bergot10). There is also now evidence for a regulatory role of conjugated linoleic acid upon the hepatic and intestine expression of fatty acyl elongase and Δ-6 fatty acyl desaturase(Reference Diez, Menoyo and Pérez-Benavente64). However, a low activity of Δ-5 fatty acyl desaturase activity has been reported either in vitro (Reference Tocher and Ghioni65) or in vivo (Reference Mourente and Tocher9), which may act as a major constraining factor for bioconversion of C18 PUFA into C20 and C22 HUFA at appreciable rates.
In summary, data on growth performance, tissue histology and fatty acid analysis prompted us to use practical diets with a low inclusion of marine raw materials through most of the production cycle of gilthead sea bream, linking the robustness of the PL fatty acid profile with endocrine, metabolic and somatotropic factors. Precise effects at different developmental stages need to be further evaluated, and, interestingly, muscle fatty acid profiles and MPCA emerge not only as powerful tools to understand foraging ecology and food webs, but also to evaluate alternative and sustainable aquafeeds in a global change scenario.
Acknowledgements
This research was funded by the European Union (FOOD-CT-2006-16 249: Sustainable Aquafeeds to Maximise the Health Benefits of Farmed Fish for Consumers, AQUAMAX) and Spanish (AGL2004-06 319-CO2) projects. The authors declare that there are no conflicts of interest perceived to bias the study. J. G. B. and S. K. have contributed to the experimental design of the diets. A. S.-B. carried out the histology part of the study. J.-C. N. and L. B.-P. performed the fatty acid analyses and data process, and J. P.-S. coordinated the work.











