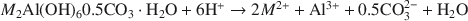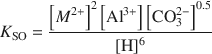Volume 51 - Issue 1 - February 2003
Research Article
Hydrotalcite-Like Minerals (M2Al(OH)6(CO3)0.5.XH2O, Where M = Mg, Zn, Co, Ni) in the Environment: Synthesis, Characterization and Thermodynamic Stability
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 1-8
-
- Article
-
- You have access
- Export citation
Diffusion of H2O in Smectite Gels: Obstruction Effects of Bound H2O Layers
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 9-22
-
- Article
-
- You have access
- Export citation
Laser Shadow Analysis of Particle-Size Distribution of Montmorillonites in Aqueous Suspensions
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 23-32
-
- Article
-
- You have access
- Export citation
Exchangeable Ion and Thermal Treatment Effects on Basal Spacings of Al-Hydroxy Pillared Montmorillonites
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 33-40
-
- Article
-
- You have access
- Export citation
Preparation and Characterization of Ti-Pillared Clays Using Ti Alkoxides. Influence of the Synthesis Parameters
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 41-51
-
- Article
-
- You have access
- Export citation
Pretreatment Effects on the Catalytic Activity of Jordanian Bentonite
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 52-57
-
- Article
-
- You have access
- Export citation
Adsorption of Cu Ions onto a 1.10 Phenanthroline-Grafted Brazilian Bentonite
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 58-64
-
- Article
-
- You have access
- Export citation
Wettability of Montmorillonite Clays in Humic Acid Solutions
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 65-74
-
- Article
-
- You have access
- Export citation
Loss of K-Bearing Clay Minerals in Flood-Irrigated, Rice-Growing Soils in Jiangxi Province, China
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 75-82
-
- Article
-
- You have access
- Export citation
Biogeochemical and Environmental Factors in Fe Biomineralization: Magnetite and Siderite Formation
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 83-95
-
- Article
-
- You have access
- Export citation
Characterization and a Fast Method for Synthesis of Sub-Micron Lithiophorite
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 96-101
-
- Article
-
- You have access
- Export citation
High-Grade Diagenetic Dickite and 2M1 Illite From the Middle Proterozoic Kombolgie Formation (Northern Territory, Australia)
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 102-116
-
- Article
-
- You have access
- Export citation
Book Review
Teaching clay science, edited by A. C. Rule and S. Guggenheim. CMS Workshop Lectures, 11, (2002), viii + 223 pp. [ISBN 1-881208-12-5]. Price $26.00
-
- Published online by Cambridge University Press:
- 01 January 2024, pp. 117-118
-
- Article
-
- You have access
- Export citation
Announcement
Forthcoming Papers
-
- Published online by Cambridge University Press:
- 01 January 2024, p. 119
-
- Article
-
- You have access
- Export citation