The Lancet series on the development of children in developing countries reviewed the association between early childhood development and further health and cognitive development( Reference Grantham-McGregor, Cheung and Cueto 1 – Reference Walker, Wachs and Grantham-McGregor 3 ). Since the Lancet series was published, motivation for improving early childhood development has increased. In addition, previous studies of long-term follow-up of children have shown the harmful effects of growth failure in the first 2 years of life on chronic disease, lower educational attainment and adult earnings( Reference Martorell, Horta and Adair 4 ). Many intervention studies conducted in the first 2 years of life in low- and middle-income countries revealed consistent short-term benefits in the early development and growth of children. Therefore, the first 2 years of life is an important period for further physical development and cognitive function( Reference Aboud and Yousafzai 5 – Reference Yousafzai, Obradović and Rasheed 8 ).
Previous studies in healthy populations suggest that faster growth during infancy and childhood is associated with better cognitive outcomes( Reference Yang, Tilling and Martin 9 – Reference Smithers, Lynch and Yang 11 ), whereas others have found no association( Reference Belfort, Rifas-Shiman and Rich-Edwards 12 ). Not only is the empirical evidence inconclusive, but the majority of studies estimated differences in cognitive ability associated with a change in weight between only two time points, rather than with changes estimated from multiple measures of growth( Reference Yang, Tilling and Martin 9 , Reference Bhutta, Cleves and Casey 13 ). In addition, although many previous studies reported that low birth weight or preterm birth has been associated with later impairment of cognitive development, comprehensive estimation of the long-term effect of growth in early life on intellectual function and nutritional status has rarely been studied.
Cognitive development is important regarding the potential for success in life and is inversely associated with several health outcomes in later life( Reference Batty, Mortensen and Nybo Andersen 14 , Reference Li, Zeng and Wang 15 ). However, the critical periods where poor growth results in longer-term impairments that might be addressed by nutritional interventions to promote cognitive development in rural areas are unclear. Therefore, the aim of this study is to clarify the relationships between postnatal weight and height gain in the first 2 years of life and nutritional status and intellectual development in early school-aged children. The results from this study could identify an important window of opportunity to improve early growth to promote intellectual development and the prevention of childhood malnutrition in low- and middle-income countries.
Methods
Study design and participants
The children and their mothers were enrolled in a large trial that aimed to determine the effect of micronutrient supplementation during pregnancy on birth weight. The details of the double-blind, cluster-randomised, controlled trial of prenatal supplementation with various combinations of micronutrients have been previously published( Reference Li, Zeng and Wang 15 – Reference Li, Yan and Zeng 17 ). Briefly, this trial was conducted in two rural counties in Shaanxi Province of Northwest China from 2002 to 2006. Pregnant women who were residents in the studied townships or counties and who had a diagnosed pregnancy gestation age of <28 weeks were included in the trial. In addition, women who had severe diseases or pregnancies with gestational ages >28 weeks were excluded from the trial. The trial was cluster randomised with the village as the unit of randomisation. Clusters were stratified according to county and township to ensure geographic balance and random assignment to three supplementation groups. The intervention groups were (i) folic acid (400 mg/d) (control group), (ii) folic acid plus Fe (60 mg/d) and (iii) fifteen minerals or vitamins as follows: 30 mg/d Fe, 400 mg/d folate, 15·0 mg/d Zn, 2·0 mg/d Cu, 65·0 mg/d Se, 150·0 mg/d iodine, 800·0 mg/d vitamin A, 1·4 mg/d thiamine, 1·4 mg/d riboflavin, 1·9 mg/d vitamin B6, 2·6 mg/d vitamin B12, 5·0 mg/d vitamin D, 70·0 mg/d vitamin C, 10·0 mg/d vitamin E and 18·0 mg/d niacin. A total of 5828 pregnant women from 531 villages were enrolled in the study. Of these, 133 women were lost to follow up, 279 stopped taking supplements and dropped out of the study and 601 had a spontaneous or induced abortion or another medical condition resulting in fetal loss. There were 4864 births from 4815 women, and after excluding the stillbirths and multiple live births, there were 4604 single live births included in the study.
Follow-up monitoring and data collection
The baseline information was collected at ≥3 prenatal health-care visits in different stages of pregnancy. The hospital nursing staff measured birth weight within 1 h of delivery. For home deliveries, township maternal and child health staff visited the homes to measure birth weight and collect birth information within 72 h after delivery. Gestational age at birth was measured as completed days based on the 1st day of the last menstrual period (obtained at the baseline interview).
Due to limited funding, a subgroup of singleton neonates (born in 2004) was included in the postnatal surveillance, and children were assessed at 6, 12, 18 and 24 months of age. Primary caregivers were interviewed about feeding practices and the frequency of complementary foods. Infant feeding was categorised into bottle-feeding (non-breast-feeding), partial breast-feeding and exclusive breast-feeding for infants aged 0–6 months. In addition, the complementary foods that were introduced between 6 and 12 months of age, including meat, eggs and milk (including milk powder) with an intake frequency of ≥3 times a week, were categorised into a quality protein supplement group.
At all visits, anthropometric measurements and physical examinations were conducted using standardised methods( 18 ). Weight was measured to the nearest 10 g on an electronic scale (type BD 585; Tanita), and infants were measured with light clothing and without shoes. The length was measured to the nearest 1 mm using a portable measuring board with a fixed headpiece. Scales were checked by calibration with a standard weight (10 kg), and recumbent length was measured to the nearest 1 mm on a length board.
From October 2012 to September 2013, when the children were aged 7–9 years, we conducted a follow-up study to determine the long-term effects of prenatal and postnatal nutrition status on further development of children. Households with eligible children (single birth, born in 2004 and whose mother participated in the randomised controlled trial (RCT)) were invited to participate at the local hospital or school in a standardised manner. A face-to-face interview was conducted to collect information on current socio-economic background (baseline information had been collected in the original RCT) in households using a household questionnaire for parents or primary caregivers of children. For children, anthropometric measurements and the Wechsler Intelligence Scale for Children (WISC) were conducted. The anthropometric measurements of young school-aged children were collected by trained staff using standard procedures. Height (barefooted) was recorded to the nearest 0·1 cm using a calibrated stadiometer (model SZG-210; Shanghai JWFU Medical Apparatus Factory), and weight was recorded to the nearest 0·1 kg in standard school clothing (without shoes) using an electronic scale (Tanita BC-420; Tanita Corporation). Written informed consent was obtained from parents. The follow-up study was designed to estimate the intellectual developmental differences among the prenatal micronutrient supplementation groups. We estimated that a minimum of 426 children (142 children in each group) was needed to detect five Full-Scale Intelligence Quotient (FSIQ) points between groups with 80 % power and with a type I error probability of α=0·05. A difference of five FSIQ points was considered clinically significant because it is the order of magnitude associated with intelligence quotient differences in children who were exposed to high lead concentrations or were fed milk rather than formula as infants. The observed power of the linear regression model (FSIQ as a dependent variable) obtained in Stata was larger than 0·99.
All field assistants and nurses received training were supervised regularly and remained blinded to the treatment group until the end of the follow-up period. The study was approved by the Human Research Ethics Committee of the Xi’an Jiaotong University Health Science Center.
Psychological testing
Wechsler tests are among the most widely used intelligence tests in the world( Reference Wechsler 19 ). The fourth and latest edition of the WISC (WISC-IV), which can be applied to children aged 6–16 years, was used to assess the cognitive ability of children. The WISC-IV consists of ten core subtests and four supplemental subtests. The core subtests are Block Design, Similarities, Comprehension, Vocabulary, Picture Concepts, Digit Span, Letter-Number Sequence, Matrix Reasoning, Coding and Symbol Search; and the supplemental subtests are Picture Completion, Information, Cancellation and Arithmetic. In addition, the WISC-IV generates a FSIQ, which represents overall cognitive ability, and four other composite scores (Verbal Comprehension Index (VCI), Working Memory Index (WMI), Processing Speed Index (PSI) and Perceptual Reasoning Index (PRI)), which represent different domains of cognitive function( Reference Wechsler 19 ). Currently, the WISC-IV has been commercialised in China, and Chinese norms have been established. China has standardised the WISC-IV to be culturally appropriate. The reliability and validity of these norms have also been measured and were shown to be satisfactory( Reference Chen, Keith and Weiss 20 ).
Anthropometry and malnutrition definition
The growth variables (weight gain and height gain) were the standardised residuals from the linear regression models of body size. Body size at each time point was regressed on corresponding measures at all earlier time points( Reference Gale, O’Callaghan and Bredow 21 ). For example, weight gain from 6 to 12 months of age conditional on weight gain from 0 to 6 months of age was calculated by saving the residuals from the regression model of weight sd scores at 12 months of age on weight at birth and at 6 months of age. Weight-for-age z scores (WAZ), height-for-age z scores (HAZ) and BMI-for-age z scores (BAZ) of early school-aged children were derived from these observational data based on the WHO 2007 reference( Reference de Onis, Onyango and Borghi 22 ). Low body weight, stunting and thinness were defined as WAZ, HAZ and BAZ ≤–2, respectively( 23 ).
Statistical analysis
All data were double entered into the data management system for verification and checked manually for completeness. Extremum, range and logical checks were conducted for accuracy. Statistical significance was set at a P value <0·05 for all statistical tests, and testing was two sided. The distribution of household demographics and socio-economic status in study fields were described by their means, standard deviations and percentages. A wealth index was constructed from sixteen different household facilities or assets using principal component analysis( Reference Filmer and Pritchett 24 ), and the wealth index was categorised into thirds as an indicator of high-income, middle-income and low-income households. HAZ, WAZ and BAZ scores were calculated with the use of the WHO Anthro Plus Software package, which utilises the 2007 WHO reference values( Reference de Onis, Onyango and Borghi 22 ). Multilevel analyses with township as level 3, village as level 2 and individual as level 1 were used to analyse the association between the WISC-IV scores and growth in weight and height during infancy. The multilevel model was also used to analyse the effect of breast-feeding from 0 to 6 months of age on weight and height gain from 0 to 6 months and the association between protein intake (meat, eggs or milk intake ≥3 times a week) from 6 to 12 months of age and weight and height gain from 6 to 12 months of age. Because of the relatively small size of malnourished children, we used a multivariable logistic regression model to compare weight and height gain during infancy between malnourished and normal children. Estimations of the coefficients and 95 % CI were derived.
Previous studies identified that in children, age, gestational age, type of micronutrient supplement and sex can detrimentally affect the cognitive development of children( Reference Walker, Wachs and Gardner 2 , Reference Li, Yan and Zeng 17 , Reference Christian, Murray-Kolb and Khatry 25 – Reference Lundgren, Cnattingius and Jonsson 28 ). In addition, the effect of prenatal Fe supplementation, breast-feeding, poverty and social factors on intellectual functioning in early school-aged children was also reported in a previous study( Reference Christian, Murray-Kolb and Khatry 25 ). We considered the following variables as potential confounders in the multivariable-adjusted analysis: age, gestational age, sex, type of micronutrient supplement, duration of breast-feeding, household wealth index, mother’s educational level, father’s educational level, mother’s occupation, father’s occupation and the child’s school level. To achieve a better understanding of the associations between these confounders and outcomes, we used a linear regression model to estimate the crude association between these confounders and intellectual development in early school-aged children (online Supplementary Table S1). Logistic regression was also used to estimate the crude association between confounders and the malnutrition status of children (online Supplementary Table S2).
To test the reliability of the analysis, we fitted two models, with one model adding the age of the children, gestational age, sex, and type of micronutrient supplement, and the other model including the variables mentioned above plus household wealth index, mother’s educational level, father’s educational level, mother’s occupation, father’s occupation, child’s school level and duration of breast-feeding. To minimise the possibility of bias, a multivariate imputation by a chained equations approach with twenty replications was implemented to impute the missing values of weight and height at different ages during early life and other confounders. In addition, the equality of the distribution of the imputed and observed values were tested using Kolmogorov–Smirnov tests to identify whether the data were missing at random or were not missing at random( Reference Abayomi, Gelman and Levy 29 , Reference White, Royston and Wood 30 ). The details of the complete and imputed data are reported in online Supplementary Table S3.
We considered the following variables as confounders when estimating the effect of infant feeding (0–6 months) and complementary foods (6–12 months): gestational age, type of micronutrient supplement, sex of children, household wealth index, mother’s educational level, father’s educational level, mothers’ occupation, father’s occupation, primary caregiver of infant, weight at birth and height at birth. Data were analysed using Stata software, version 12.0 (Stata Corp LP).
Results
The flowchart of the participants is shown in Fig. 1. The most common reason for lost to follow-up was that the child had moved out of the study area (30·0 %) or his/her location was unknown (16·6 %). There was no difference in any enrolment measure among those who did not take part in the study and those who did participate in the study (online Supplementary Table S4).
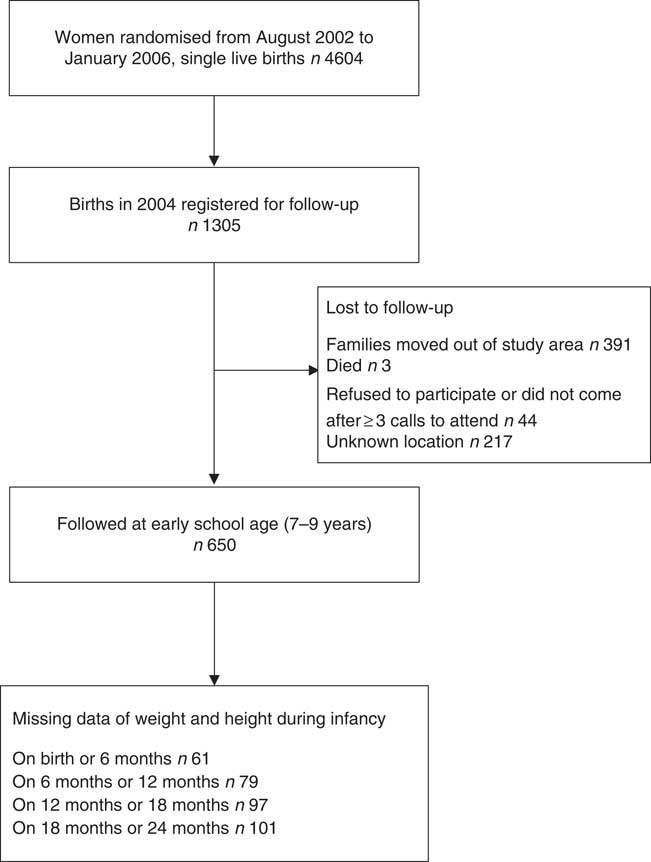
Fig. 1 Participant flowchart.
Baseline information in Changwu and Bing counties
The baseline characteristics of the households and children are shown in Table 1. The mean age of the 650 children at follow-up was 8·31 years, and the majority were boys (60·9 %). The majority of children (84·0 %) attended township or village schools, and this rate (77·6 %) was lower in Bing County. The general intellectual development of children was better in Changwu County, and the mean of the FSIQ was 91·17 (sd 12·93). In addition, the means of VCI, WMI and PSI were also higher in Changwu County, which were 88·84 (sd 15·25), 93·36 (sd 12·60) and 98·00 (sd 12·89), respectively. In Bing County, education and household economic levels of the parents were lower, and there were lower scores of intellectual development and a higher prevalence of underweight, thinness and stunting.
Table 1 Baseline characteristics of children and households by study fields (Numbers and percentages; mean values and standard deviations)
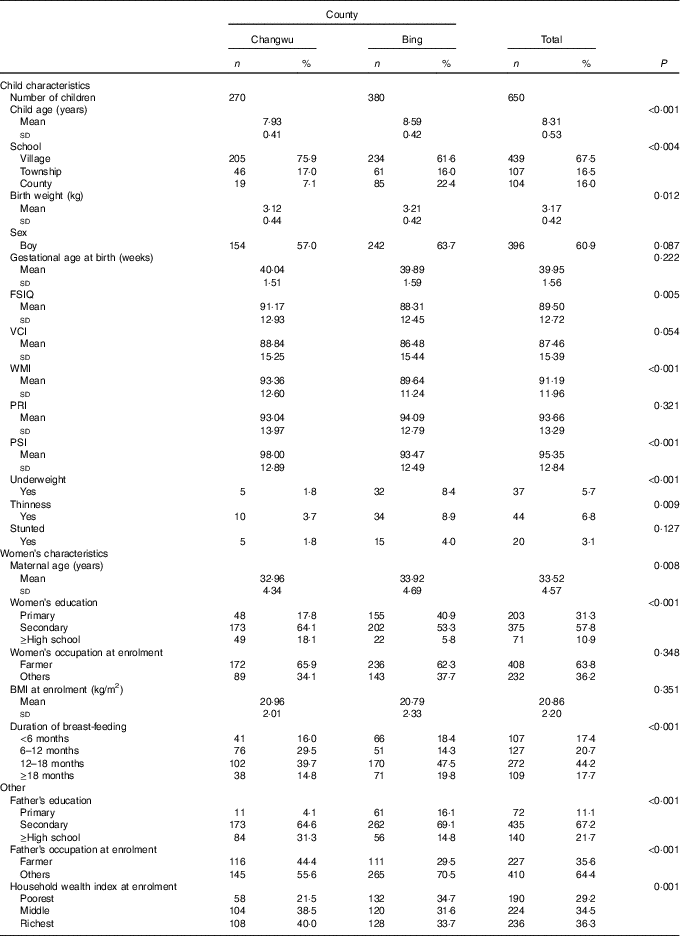
FSIQ, Full-Scale Intelligence Quotient; VCI, Verbal Comprehension Index; WMI, Working Memory Index; PRI, Perceptual Reasoning Index; PSI, Processing Speed Index.
Association between infant weight gain and intellectual and physical development
We found an association between weight gain from 6 to 12 months of age and FSIQ in model 1 (coefficient: 1·72, 95 % CI 0·70, 2·74) and model 2 (coefficient: 1·53, 95 % CI 0·48, 2·58). An impact of weight gain from 6 to 12 months of age was found on VCI, WMI and PRI both in model 1 and in model 2 (Table 2).
Table 2 Association between weight and height during different periods of postnatal life and general intellectual development and four composite scores in early school-aged childrenFootnote * (Coefficients (Coef) and 95 % confidence intervals)
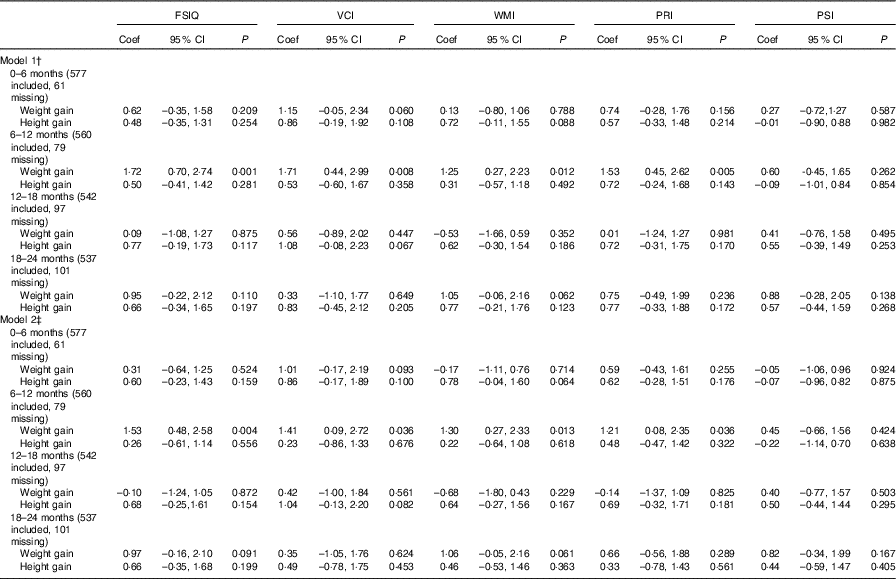
FSIQ, Full-scale Intelligence Quotient; VCI, Verbal Comprehension Index; WMI, Working Memory Index; PRI, Perceptual Reasoning Index; PSI, Processing Speed Index.
* Multilevel models were used to adjust for the effect of randomisation by villages, with township to level 3, village to level 2 and individual to level 1.
† P value adjusted for the age of children, gestational weeks, type of micronutrient supplement, sex of children.
‡ P value adjusted for the age of children, gestational weeks, type of micronutrient supplement, sex of children, household wealth index, mother’s educational level, father’s educational level, maternal occupation, father’s occupation, child school level, duration of breast-feeding.
The weight gain from 0 to 6 months of age (OR: 0·25, 95 % CI 0·15, 0·41), 6–12 months of age (OR: 0·54, 95 % CI 0·35, 0·81) and 18–24 months of age (OR: 0·40, 95 % CI 0·24, 0·65) was associated with a reduced risk of being underweight in childhood in model 1. In addition, poor weight gain from 0 to 6 months of age was also associated with thinness (OR: 0·45, 95 % CI 0·31, 0·65, model 1; OR: 0·41, 95 % CI 0·27, 0·62, model 2) and stunting (OR: 0·47, 95 % CI 0·28, 0·77, model 1; OR: 0·48, 95 % CI 0·28, 0·82, model 2).
Association between infant height gain and intellectual and physical development
Infant height gain was found to not be associated with intellectual development (WISC-IV test scores). For example, the height gain from 0 to 6 months of age was not associated with FSIQ, and the coefficients in model 1 and model 2 were 0·48 (95 % CI –0·35, 1·31) and 0·60 (95 % CI –0·23, 1·43), respectively. For physical development, we found poor height gain from 0 to 6 months of age to be associated with stunting (OR: 0·55, 95 % CI 0·32, 0·94, model 1; OR: 0·56, 95 % CI 0·32, 0·98, model 2) (Table 3). Multiple imputation was used as a sensitivity analysis to estimate the missing data, and no observable differences between the results of the original and sensitivity analyses were found (online Supplementary Tables S5 and S6).
Table 3 Association between weight and height during two different periods of postnatal life and malnutrition status in early school-aged childrenFootnote * (Odds ratios and 95 % confidence intervals)
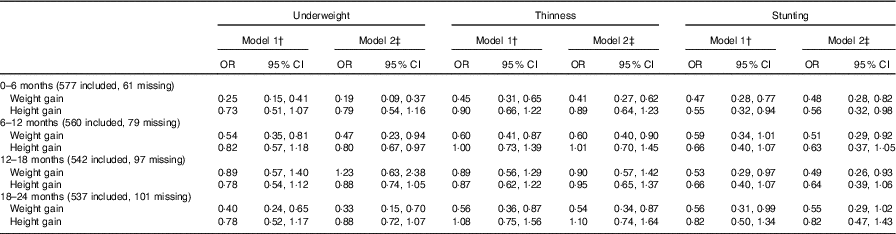
* Multi-variable logistic regression model was used.
† P value adjusted for the age of children, gestational weeks, type of micronutrient supplement, sex of children.
‡ P value adjusted for the age of children, gestational weeks, type of micronutrient supplement, sex of children, household wealth index, mother’s educational level, father’s educational level, maternal occupation, father’s occupation, child school level, duration of breast-feeding.
Association between breast-feeding, protein intake and weight gain and height gain
We used multilevel modelling to estimate the association between breast-feeding and protein intake during infancy on weight and height gain from 0 to 6 and 6 to 12 months of infancy. We found an association between breast-feeding (from 0 to 6 months of age) and weight gain (from 0 to 6 months of age). Compared to the pure ewe’s milk/milk feeding group, the breast-feeding only, partial breast-feeding and formula feeding groups gained more weight from 0 to 6 months of age. The coefficients were 0·38 (95 % CI 0·12, 0·66), 0·39 (95 % CI 0·14, 0·63) and 0·39 (95 % CI 0·07, 0·71), respectively (model 1). In model 2, the coefficients were 0·40 (95 % CI 0·11, 0·70), 0·41 (95 % CI 0·15, 0·68) and 0·38 (95 % CI 0·07, 0·70), respectively. Protein was important for weight gain from 6 to 12 months of age, and those consuming protein at least three times per week gained more weight (coefficient: 0·32, 95 % CI 0·14, 0·51, model 1; coefficient: 0·27, 95 % CI 0·09, 0·44, model 2) from 6 to 12 months of age (Table 4).
Table 4 Association between feeding practice and weight and height gain during 0–6 and 6–12 months of infancyFootnote * (Coefficients (Coef) and 95 % confidence intervals)
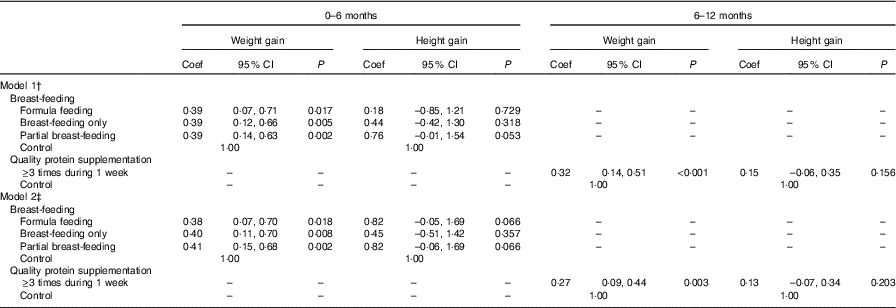
* Multilevel models were used to adjust for the effect of randomisation by villages, with township to level 3, village to level 2 and individual to level 1.
† P value adjusted for the gestational weeks, type of micronutrient supplement, sex of children, weight at birth (added when dependent variable was 0–6 months weight gain), height at birth (added when dependent variable was 0–6 months height gain), weight at 6 months (added when dependent variable was 6–12 months weight gain), height at 6 months (added when dependent variable was 6–12 months height gain).
‡ P value adjusted for the gestational weeks, type of micronutrient supplement, sex of children, household wealth index, mother’s educational level, father’s educational level, mothers’ occupation, father’s occupation, primary carer of infant, weight at birth (added when dependent variable was 0–6 months weight gain), height at birth (added when dependent variable was 0–6 months height gain), weight at 6 months (added when dependent variable was 6–12 months weight gain), height at 6 months (added when dependent variable was 6–12 months height gain).
Discussion
In this study, we found that weight gain from 6 to 12 months of age was associated with an increase in intelligence test scores (FSIQ, VCI, WMI, PRI) in young school-age children. In addition, weight gain from 0 to 6 months, 6 to 12 months and 18 to 24 months of age was associated with a decreased frequency of being underweight, thin and stunted. Promoting breast-feeding from 0 to 6 months of age and consuming protein (meat, eggs or milk intake ≥3 times a week) through complementary feeding from 6 to 12 months of age should be suggested to improve weight gain during infancy.
Studies examining the association between growth during infancy and childhood intellectual development are characterised by wide variations in the examined growth period, growth measures used and ages at which intellectual development was assessed, and most of these studies have reported small but positive associations( Reference Yang, Tilling and Martin 9 – Reference Smithers, Lynch and Yang 11 , Reference Huang, Martorell and Ren 31 ). One exception is the study conducted in the USA that reported no association between weight gain from 0 to 6 months of age and intellectual development at 3 years of age( Reference Belfort, Rifas-Shiman and Rich-Edwards 12 ). Consistent with previous studies, we found that weight gain from 6 to 12 months of age was associated with intellectual development in early school-aged children, and weight gain in the first 6 months was not associated with the intellectual development of 7- to 10-year-old children. Conversely, no association was found between height gain during each time interval and further intellectual development. A possible explanation for these results may be the relatively small size of the study, as it is likely to have been underpowered to detect a modest effect of height gain. In secondary analyses of our data, we found that children in the lowest category of weight and height gain (<–2sd WHO reference) in infancy had lower WISC-IV test scores than children in the normal range of weight and height gain (≤1sd and ≥−1sd WHO reference)( 32 ) (online Supplementary Table S7). These results emphasise the importance of height gain on further intellectual development and provide indirect evidence for the possible effect of the small sample size in the study on the ability to detect a modest effect of height gain.
For the association between growth during infancy and childhood physical development, recent studies have mainly focused on the effect of increased weight gain during the first 2 years of life on higher overweight and obesity rates in the context of the obesity epidemic( Reference Sacco, de Castro and Euclydes 33 , Reference Giles, Whitrow and Rumbold 34 ). Similar studies regarding the association between weight gain during early life and childhood undernourished status were rare. The results from the USA study showed weak positive associations between postnatal growth between 0 and 2 years of age and adult fat mass and body fat percentage( Reference Li, Stein and Barnhart 35 ). The results of our study are similar to those of the study conducted in the USA, as we identified a positive association between postnatal growth at each time interval between 0 and 2 years of age and a reduced risk of childhood undernutrition. In addition, our study area comprised the poorest area of China. Evidence from previous studies revealed that malnutrition rates were more serious in northwestern China, where geographical environments are more extreme and economic levels are lower, especially in rural areas( Reference Yang, Li and Li 36 ). Therefore, our findings emphasised that interventions aimed at promoting infant growth should be promoted in developing countries, especially in areas where the childhood undernutrition rate is still high.
The important health benefits of exclusive breast-feeding and protein intake on childhood growth have been suggested by many previous studies( Reference Kramer, Guo and Platt 37 – Reference Bray, Smith and de Jonge 40 ). For example, a previous study reported that prolonged and exclusive breast-feeding may accelerate weight gain in the first few months( Reference Kramer, Guo and Platt 37 ). In detail, the mean weight was higher in the breast-feeding group by 1 month of age. The difference in mean weight increased through 3 months of age and declined slowly thereafter( Reference Kramer, Guo and Platt 37 ). Although higher energy intake was positively associated with increased weight gain during infancy, a study in the UK reported higher energy intake at 4 months was associated with greater weight gain and a higher risk of childhood obesity, but breast-feeding was not associated with rapid weight gain or further obesity risk( Reference Yang, Li and Li 36 ). Evidence from a study conducted in Germany indicated an association between exclusive breast-feeding and preventing increased weight gain during infancy( Reference Kalies, Heinrich and Borte 39 ). In addition, another study conducted in the USA emphasised the important health benefits of protein intake on weight gain. Protein contributed to changes in lean body mass and energy expenditure but not to increased body fat( Reference Bray, Smith and de Jonge 40 ). Therefore, exclusive breast-feeding and protein supplementation for infants should help to improve weight gain, and it would not cause rapid weight gain during infancy or increase the risk of obesity in the future. However, the association between exclusive breast-feeding, protein intake and weight gain during infancy is still unknown in rural areas of China. In this study, our results indicated a positive association between weight gain during infancy and exclusive breast-feeding and protein intake in low-income rural areas of China.
The prospective cohort design and standardised intelligence scales (WISC-IV) that are widely used in various cultures and settings were used to evaluate the intellectual development of children, which was the strength of this study. Our study has several limitations. One limitation was the relatively small sample size and our inability to examine all children from the original cohort. With the rapid development of China, mass migration out of the original study field took place, making it impossible to track the participants. The results shown in online Supplementary Table S4 demonstrate that there was no difference in baseline information between the study participants and those who did not take part in the present study. In addition, given that the mothers from this study participated in the RCT, the ability to extrapolate the present results to the general population may be limited. In addition, the questionnaire used in the present study has not been validated. Another limitation is that some of the data were missing for weight and height measurements at different time points during infancy. We used multiple imputation methods to estimate the missing data, then reanalysed the data and found that the results were robust after comparing the results with the different data sets. In addition, we did not include all possible confounders. In detail, parental intellectual function is a strong predictor of the intellectual function of offspring( Reference Deary, Strand and Smith 41 ), and it may also affect children’s development during infancy via feeding practice and physical activity. We did not collect data on maternal psychological distress that may be associated with parenting behaviour. However, a large birth cohort study showed that the association between behavioural problems and birth weight is not affected by adjusting for maternal anxiety and depression( Reference Alati, Najman and O’Callaghan 42 ).
The biological mechanisms linking growth with intellectual development have not been identified. A possible mechanism underlying the association observed in the present study is that insulin-like growth factor (IGF)-1 mediates growth regulation and organ development( Reference Gunnell, Miller and Rogers 43 , Reference van Dam, Aleman and de Vries 44 ). Previous studies have reported that IGF-1 levels have been found to be associated with intellectual development in healthy children with normal growth and in children who were born small for gestational age and who were treated with growth hormone( Reference Gunnell, Miller and Rogers 43 , Reference van Pareren, Duivenvoorden and Slijper 45 ).
In conclusion, we identified an association between weight gain from 6 to 12 months of age and improved intellectual development and a decreased occurrence of malnourished status in early school-aged children in low-income areas of China. Reduced weight gain from 18 to 24 months of age and reduced height gain from 0 to 6 months of age were also found to be associated with malnourished status. However, further research is required to clearly delineate populations in other areas that may find different effects of weight gain.
Acknowledgements
Thanks to the Health Departments of each project county, the local health bureaus and education bureaus for their cooperation and organisation of the field data collection and the staff of Xi’an Jiaotong University for their participation in the field of data collection.
The National Natural Science Foundation of China (no. 81230016), China Postdoctoral Science Foundation (no. 2016M592804) and the New Century Excellent Talents in University grant (no. NCET-11-0417) supported this work.
C. L. conceptualised and designed the research, drafted the initial manuscript and approved the final manuscript as submitted. L. Z., D. W., S. A., S. J., J. Z., T. C. and V. W. carried out the initial data analyses, reviewed and revised the manuscript and approved the final manuscript as submitted. H. Y. was the principal investigator, designed the data collection instruments, coordinated and supervised data collection at the study sites, critically reviewed the manuscript and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
There are no conflicts of interest relevant to this article to disclose.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519000060








