Introduction
The Rhynchocephalia is an ancient group of reptiles that originated in the early Mesozoic. Currently this group has low diversity, being represented by a single species, the famous ‘living fossil’ Sphenodon punctatus (Gray, Reference Gray1842) from New Zealand (Jones et al., Reference Jones, Anderson, Hipsley, Müller, Evans and Schoch2013; Cree, Reference Cree2014; Herrera-Flores et al., Reference Herrera‐Flores, Stubbs and Benton2017). In contrast to their current low diversity, Mesozoic rhynchocephalians were diverse, showing varied morphologies and a wide geographical distribution (Jones, Reference Jones2006a, Reference Jones2009; Rauhut et al., Reference Rauhut, Heyng, López-Arbarello and Hecker2012; Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Herrera-Flores et al., Reference Herrera‐Flores, Stubbs and Benton2017). Among the earliest rhynchocephalians, species of the genus Clevosaurus Swinton, Reference Swinton1939 were the most diverse and widely distributed in the early Mesozoic. Clevosaurus hudsoni Swinton, Reference Swinton1939 was the first described species of the genus; it was named after F. G. Hudson, who discovered the fossil remains at Cromhall Quarry, England (Fraser, Reference Fraser1988). Since the description of C. hudsoni, nine species of Clevosaurus have been erected—C. bairdi Sues, Shubin, and Olsen, Reference Sues, Shubin and Olsen1994, C. brasiliensis Bonaparte and Sues, Reference Bonaparte and Sues2006, C. convallis Säilä, Reference Säilä2005, C. latidens Fraser, Reference Fraser1993, C. mcgilli Wu, Reference Wu1994, C. minor Fraser, Reference Fraser1988, C. petilus Young, Reference Young1982, C. sectumsemper Klein et al., Reference Klein, Whiteside, de Lucas, Viegas and Benton2015, and C. wangi Wu, Reference Wu1994—and new records have been reported from localities in Belgium, Brazil, Canada, China, Great Britain, Mexico, and South Africa (Fraser, Reference Fraser1988, Reference Fraser1993; Wu, Reference Wu1994; Sues et al., Reference Sues, Shubin and Olsen1994; Duffin, Reference Duffin1995; Sues and Reisz, Reference Sues and Reisz1995; Säilä, Reference Säilä2005; Bonaparte and Sues, Reference Bonaparte and Sues2006; Reynoso and Cruz, Reference Reynoso and Cruz2014; Klein et al., Reference Klein, Whiteside, de Lucas, Viegas and Benton2015).
The anatomy of Clevosaurus is well known and the monograph of Fraser (Reference Fraser1988) offers a very thorough review of the general morphology of this genus. It is recognized that the genus Clevosaurus is highly diverse, but the taxonomic validity of some Clevosaurus species has been questioned (Jones, Reference Jones2006a). Hsiou et al. (Reference Hsiou, De França and Ferigolo2015) presented a review of C. brasiliensis that included a phylogenetic analysis of almost all known Clevosaurus species. Their study demonstrated that some species might not be valid taxa or are perhaps not directly referable to this genus. One of these conflicting taxa is C. latidens, a species described by Fraser (Reference Fraser1993) from the Late Triassic fissure deposits of Cromhall Quarry, England. The uncertain taxonomic affinity of C. latidens and its dubious relationship with Clevosaurus have been noted in many previous studies (Jones, Reference Jones2006a, Reference Jones2009; Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Hsiou et al., Reference Hsiou, De França and Ferigolo2015; Klein et al., Reference Klein, Whiteside, de Lucas, Viegas and Benton2015), and some phylogenetic analyses even suggested a closer relationship with opisthodontians, but no taxonomic revision of this taxon has been carried out.
For a long time, the relationships among rhynchocephalians were poorly known, and most taxa were assessed by overall morphological similarities. The first phylogenetic analysis of the group was performed by Fraser and Benton (Reference Fraser and Benton1989), followed by many different analyses, including new descriptions or redescriptions of taxa (e.g., Wu, Reference Wu1994; Reynoso, Reference Reynoso1996, Reference Reynoso1997, Reference Reynoso2000, Reference Reynoso2005; Reynoso and Clark, Reference Reynoso and Clark1998; Apesteguía and Novas, Reference Apesteguía and Novas2003; Rauhut et al., Reference Rauhut, Heyng, López-Arbarello and Hecker2012; Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Apesteguía and Carballido, Reference Apesteguía and Carballido2014; Apesteguía et al., Reference Apesteguía, Gómez and Rougier2012, Reference Apesteguía, Gómez and Rougier2014; Cau et al., Reference Cau, Baiano and Raia2014; Hsiou et al., Reference Hsiou, De França and Ferigolo2015). So far, all phylogenetic studies of the Rhynchocephalia have only used parsimony analysis, recovering a few distinct clades. More recently, Bayesian inference methods have been employed for phylogenetic analyses based on morphological characters (e.g., Parry et al., Reference Parry, Edgecombe, Eibye-Jacobsen and Vinther2016; Wright, Reference Wright2017), and recent studies suggest that Bayesian methods outperform parsimony for morphological data (O’Reilly et al., Reference O’Reilly, Puttick, Parry, Tanner, Tarver, Fleming, Pisani and Donoghue2016; Puttick et al., Reference Puttick, O’Reilly, Tanner, Fleming, Clark, Holloway, Lozano-Fernandez, Parry, Tarver, Pisani and Donoghue2017), recovering more accurate, but less precise results.
To clarify the doubtful taxonomic affinity of Clevosaurus latidens, we re-examined the type specimens and other material described by Fraser (Reference Fraser1993). We updated the character matrix of a recent phylogenetic analysis of the Rhynchocephalia (Hsiou et al., Reference Hsiou, De França and Ferigolo2015), recoded morphological characters for C. latidens, and performed both parsimony and Bayesian analyses. Our results confirm that C. latidens is not related to Clevosaurus, but represents a new genus. Our phylogenetic analyses recover similar topologies using both parsimony and Bayesian approaches. We employ the new phylogeny to propose formal names for two higher clades within Rhynchocephalia.
Material and methods
We re-examined the type material and other material described by Fraser (Reference Fraser1993) as Clevosaurus latidens. All specimens of C. latidens consist of fragments of dentary, maxilla, and premaxilla housed in the collections of the Virginia Museum of Natural History and the University of Aberdeen. For anatomical comparisons, we reviewed several specimens of Clevosaurus from the paleontological collections of the University of Bristol and the University Museum of Zoology in Cambridge.
To explore the phylogenetic relationships of rhynchocephalians and the position of Clevosaurus latidens, we used the largest and most up-to-date data matrix of Rhynchocephalia (Hsiou et al., Reference Hsiou, De França and Ferigolo2015). We added three taxa—C. sectumsemper Klein et al., 2015, Derasmosaurus pietraroiae Barbera and Macuglia, Reference Barbera and Macuglia1988, and Priosphenodon minimus Apesteguía and Carballido, Reference Apesteguía and Carballido2014—and recoded some characters for C. latidens and Pelecymala robustus Fraser, Reference Fraser1986 after examination of the type specimens. The new matrix comprises 47 operational taxonomic units scored for 74 characters. We rooted the trees with the lepidosauromorph Sophineta cracoviensis Evans and Borsuk-Bialynicka, Reference Evans and Borsuk-Białynicka2009. Two squamates, the Late Jurassic–Early Cretaceous Eichstaettisaurus Kuhn, Reference Kuhn1958 and the extant Pristidactylus Gray, Reference Gray1845, were also used as outgroups.
The revised taxon-character data matrix was analyzed using both equally weighted maximum parsimony and Bayesian inference. Parsimony analysis was performed in TNT v. 1.5 (Goloboff et al., Reference Goloboff, Farris and Nixon2008; Goloboff and Catalano, Reference Goloboff and Catalano2016), first using the ‘New Technology’ search options. The initial tree search used multiple replications with sectorial searches, four rounds of tree fusing, 10 rounds of drifting, and 200 ratcheting iterations. Following this, the generated most parsimonious trees (MPTs) were analyzed using traditional tree bisection and reconnection branch swapping. All recovered MPTs were then summarized in a 50% majority rule consensus tree, and clade robustness was assessed with Bremer decay indices (Bremer, Reference Bremer1994). Bayesian inference trees were estimated using MrBayes v. 3.2 (Huelsenbeck and Ronquist, Reference Huelsenbeck and Ronquist2001; Ronquist et al., Reference Ronquist, Teslenko, Van Der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). The standard Mk model (Lewis, Reference Lewis2001) with gamma distribution priors for site rate variation was specified. The analysis was performed with four runs of four chains, run for 108 generations, sampling parameters every 1000 generations. The first 25% of sampled trees were discarded as burn-in. Convergence was assessed based on effective sample size (ESS) values >200. Results from the Bayesian analysis were summarized using a 50% majority consensus tree, revealing clades that have posterior probability values of ≥ 50%. The data matrix and analytical scripts are included in the Supplementary Data Set.
Repositories and institutional abbreviations
AUP=University of Aberdeen Paleontology Collection; BRSUG=Bristol University, School of Earth Sciences Collection; NMS=National Museums Scotland; SAMK=South African Museum; UMZC=University Museum of Zoology, Cambridge; VMNH=Virginia Museum of Natural History.
Systematic paleontology
Superorder Lepidosauria Haeckel, Reference Haeckel1866
Order Rhynchocephalia Günther, Reference Günther1867
Suborder Sphenodontia Williston, Reference Williston1925
Infraorder Eusphenodontia new infraorder
Remarks
See Discussion.
Clade Neosphenodontia new clade
Remarks
See Discussion.
Clade Opisthodontia Apesteguía and Novas, Reference Apesteguía and Novas2003
Genus Fraserosphenodon new genus
urn:lsid:zoobank.org:act:6C14E307-718C-47C8-AC8F-C658A048289B
Type species
Clevosaurus latidens Fraser, Reference Fraser1993.
Diagnosis for the genus and only known species
Moderate-sized rhynchocephalian. Maxillary teeth with relatively short crowns with transversely broadened posterolabial flanges without grooved facets on the labial surface. Robust dentary with a wide mandibular symphysis. Dentary with three generations of teeth. Front of dentary with two rounded successional teeth followed by a series of six or seven very small rounded hatchling teeth. Additional teeth in dentary transversely broadened distinctly triangular in labial view and rounded and bulbous in occlusal view.
Etymology
The genus epithet is in honor of the British paleontologist Nicholas ‘Nick’ Fraser, for his outstanding contributions to the knowledge of the British Triassic fauna, especially for his exceptional work on early rhynchocephalians.
Occurrence
Cromhall Quarry, Avon, England, site 5A of Late Triassic fissure deposit.
Remarks
All Fraserosphenodon specimens are quite fragmentary, but their tooth morphology, based on wide and robust teeth for grinding, clearly differs from the tooth shape for cutting and slicing characteristic of the genus Clevosaurus, and, indeed, is more similar to that of opisthodontians.
Fraserosphenodon latidens (Fraser, Reference Fraser1993) new combination
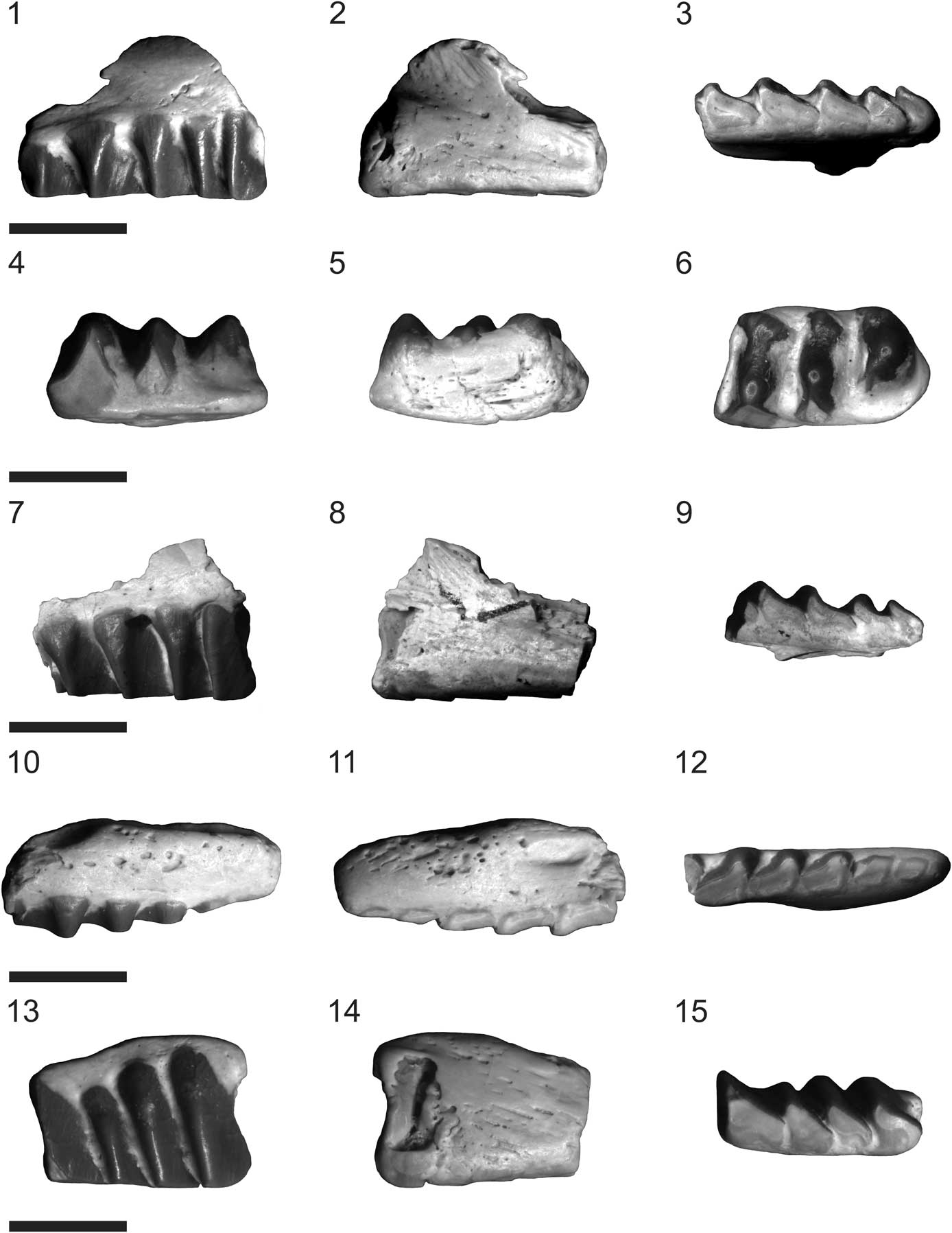
Figure 1 Fraserosphenodon latidens n. comb.; all specimens shown in labial, lingual, and occlusal views: (1–3) VMNH 524, holotype, left maxilla; (4-6) VMNH 525, paratype, right dentary; (7–9) VMNH 526, paratype, left maxilla; (10–12) VMNH 527, paratype, right maxilla; (13–15) VMNH 528, paratype, left maxilla. Scale bars=5 mm (1–3, 7–9, 10–12); 3.5 mm (4–6, 13–15).

Figure 2 Fraserosphenodon latidens n. comb.: (1, 2) AUP 11191, right premaxilla, shown in labial (1) and lingual (2) views; (3–5) AUP 11192, right dentary, shown in labial (3), lingual (4), and occlusal (5) views. Scale bars=6 mm (1, 2); 3.5 mm (3–5).
1986 aff. Pelecymala; Reference FraserFraser, p. 176, pl. 20, figs. 8, 9.
1988 Clevosaurus sp.; Reference FraserFraser, p. 163, fig. 43.
1993 Clevosaurus latidens Reference FraserFraser, p. 137, fig. 2.
Additional specimens
VMNH 525–528, AUP 11191–11192.
Remarks
The systematic paleontology section of Fraser’s original work referred to the holotype of Fraserosphenodon latidens (VMNH 524) as a dentary fragment (Fraser, Reference Fraser1993), but the description of this element treated it as a maxillary fragment. Our review of VMNH 524 confirms that it is a fragment of the posterior part of the left maxilla (Fig. 1.1–1.3). This element includes five well-preserved, complete teeth. The maxillary teeth have relatively short crowns with transversely broadened posterolabial flanges without grooved facets on the labial surface and heavily worn occlusal surfaces.
We agree with Fraser (Reference Fraser1993) that paratype specimen VMNH 525 is a dentary fragment that possibly belongs to the right dentary (Fig. 1.4–1.6). This element has three teeth that are also transversely broadened. In labial view, all teeth appear distinctly triangular. Only the second and third teeth are heavily worn, and the wear is especially pronounced on the third tooth. In occlusal view, the teeth of VMNH 525 appear round with a bulbous swelling developed medially on each tooth, as was described by Fraser (Reference Fraser1993) for specimen VMNH 543. The overall shape of both VMNH 525 and 543 is also quite similar. Note that Fraser (Reference Fraser1993) did not mention specimen VMNH 543 in the systematic paleontology section of his paper, and there is also no specimen in the VMNH collection assigned to Fraserosphenodon (C. latidens) with that catalog number. It might be that specimen VMNH 543 illustrated and described by Fraser (Reference Fraser1993, fig. 2C–E) is indeed specimen VMNH 525.
Paratypes VMNH 526–528 are maxillary fragments (Fig. 1.7–1.15). Specimens VMNH 526 and 528 (Fig. 1.7–1.9, 1.13–1.15) belong to the distal part of the left maxilla, whereas VMNH 527 (Fig. 1.10–1.12) belongs to the mesialmost part of the right maxilla. VMNH 526 and 528 include a series of four complete teeth, which are heavily worn on the occlusal surface, and have a morphology comparable to that of the holotype. The crowns of VMNH 528 are a little higher than in the other specimens (Fig. 1.13–1.15). VMNH 527 includes six complete teeth and a very small fragment of a broken tooth in the distal part of the element (Fig.1.10–1.12). The mesialmost tooth of this specimen is very small and rounded; the following tooth is also very small and of a semioval shape. The third to sixth teeth are all transversely broadened, with a right-angled triangular shape in labial view and a heavily worn occlusal surface. Paratype VMNH 529, a maxillary fragment according to Fraser (Reference Fraser1993), could not be located within the VMNH collection.
The heavily worn occlusal tooth surfaces in all type specimens suggest that they might belong to adult individuals (Fig. 1). A recent study of ontogenetic variation of the dentary in rhynchocephalians (Romo de Vivar-Martínez and Bento-Soares, Reference Romo de Vivar-Martínez and Bento-Soares2015) demonstrates that the occlusal surface of teeth shows high wear in mature specimens.
Additionally, another six specimens from the AUP collection can be referred to Fraserosphenodon. However, apart from AUP 11191 and 11192 (premaxilla and dentary, respectively), the other four specimens attributable to Fraserosphenodon are all fragmentary maxillary elements. All of these maxillary elements were stored in containers with other rhynchocephalian specimens without being labeled individually, making it impossible to associate the specimens with unique catalog numbers. These specimens all clearly exhibit the characteristic transversely broadened tooth morphology without grooved facets on the labial tooth surfaces, with heavy wear on the occlusal surface. The first specimen is a fragment of a right maxilla. It has four heavily worn teeth that include a small rounded tooth between the second and third tooth, which might represent a dental pathology. The second specimen is a fragment of a right maxilla that includes two isolated but complete teeth. The third specimen is a fragment of a right maxilla and includes four teeth. The mesialmost tooth of this specimen is heavily eroded and the tooth enamel of the third tooth is slightly damaged. The fourth specimen is a fragment of the distal end of a left maxilla; it includes two teeth with very short crowns due to the heavy wear of the occlusal surface. Among all rhynchocephalian specimens in the AUP collection, we did not identify any dentary specimens attributable to Fraserosphenodon with preserved coronoid processes (contra Fraser, Reference Fraser1993).
Specimen AUP 11191, a right premaxilla (Fig. 2.1, 2.2), was originally identified as Clevosaurus sp. by Fraser (Reference Fraser1988) and was subsequently reassigned to C. latidens by Fraser (Reference Fraser1993). The nasal process is broken in AUP 11191, but the ventral and dorsal maxillary processes are well preserved. The distal end of the ventral maxillary process has a clearly flattened oval shape; the dorsal maxillary process is relatively long and is angled at ~60o relative to the ventral maxillary process. On the convex dorsal surface of the premaxilla, between the dorsal maxillary process and the nasal process, it is possible to observe the premaxillary fossa, which is semicircular in shape. AUP 11191 exhibits three complete teeth, of which the distalmost tooth is very small, considerably shorter in relation to the other two teeth. In contrast, the two mesialmost teeth are of regular size and partially fused, and both have a rounded semicircular shape with minor signs of wear. The semifused condition of the two mesialmost teeth of AUP 11191 suggests that this specimen is a juvenile: as seen in other derived rhynchocephalians (e.g., Clevosaurus and Sphenodon spp.) these teeth fuse over time in mature individuals to form the characteristic chisel-like structure seen in late-diverging rhynchocephalians (Robinson, Reference Robinson1973).
Specimen AUP 11192, an anterior fragment of a right dentary (Fig. 2.3–2.5), was tentatively assigned to Pelecymala Fraser, Reference Fraser1986 by Fraser (Reference Fraser1986), but as in the case of AUP 11191, it was later referred to C. latidens by Fraser (Reference Fraser1993). In the description of AUP 11192, Fraser (Reference Fraser1986) noticed that the length of this specimen appeared quite similar to that of C. hudsoni, but was noticeably deeper in height. AUP 11192 has a robust and deep structure, similar to that of opisthodontians (e.g., Priosphenodon Apesteguía and Novas, Reference Apesteguía and Novas2003, Toxolophosaurus Olson, Reference Olson1960). The mandibular symphysis in AUP 11192 is quite wide; the Meckelian canal runs along the midline of the jaw. The specimen includes three generations of teeth, but caniniform teeth are lacking. The front of AUP 11192 has two rounded successional teeth similar to those of the premaxilla. These teeth are followed by a series of six or seven small semicircular remnants of hatchling teeth with minor signs of wear on the occlusal surfaces. On the distal end of this element, we found three or four additional teeth that in both labial and lingual view show the same triangular shape seen in VMNH 525. In occlusal view, the teeth of AUP 11192 show heavy signs of wear and the round, bulbous shape seen in VMNH 525. This round, bulbous shape is more pronounced in the distalmost additional tooth of AUP 11192. Additionally, AUP 11192 includes three mental foramina of relatively large size (Fig. 2.3), which suggests that this specimen comes from a juvenile. The length and height of AUP 11192, as preserved, are 10.5 mm and 5.4 mm, respectively.
Phylogenetic analyses
The parsimony analysis found 7176 MPTs of 265 steps, and the 50% majority rule consensus tree shows good resolution for most clades (Fig. 3.1). The consistency (CI) and retention indices (RI) for the 50% majority rule consensus tree are 0.38628 and 0.66403, respectively. No clade had a Bremer support score>1 (complete statistics and associated files for both phylogenetic analyses can be found in the Supplemental Data). Generally, our results agree with those of other recent studies (Rauhut et al., Reference Rauhut, Heyng, López-Arbarello and Hecker2012; Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Apesteguía et al., Reference Apesteguía, Gómez and Rougier2014; Cau et al., Reference Cau, Baiano and Raia2014; Hsiou et al., Reference Hsiou, De França and Ferigolo2015). One of the major differences is that our analysis recovered Pleurosauridae as the sister group of Sphenodontidae. The terrestrial Pamizinsaurus Reynoso, Reference Reynoso1997 is the earliest diverging taxon within the Sphenodontidae, which includes two major clades. The first clade includes Ankylosphenodon Reynoso, Reference Reynoso2000, Derasmosaurus Barbera and Macuglia, Reference Barbera and Macuglia1988, Oenosaurus Rauhut et al., Reference Rauhut, Heyng, López-Arbarello and Hecker2012, and Zapatadon Reynoso and Clark, Reference Reynoso and Clark1998 in a polytomy, whereas the second clade is well resolved, recovering the Early Jurassic Cynosphenodon Reynoso, Reference Reynoso1996 and the modern Sphenodon Gray, Reference Gray1831 as successive sister taxa to the clade comprising Theretairus Simpson, Reference Simpson1926 and Sphenovipera Reynoso, Reference Reynoso2005. The strict consensus tree of the second analysis of Cau et al. (Reference Cau, Baiano and Raia2014) also found Derasmosaurus, Oenosaurus, and Zapatadon in a similar polytomy, and forming the sister group of the clade comprising Sphenodon, Cynosphenodon, Sphenovipera, Kawasphenodon Apesteguía, Reference Apesteguía2005, and Theretairus. The close relationship of Sphenovipera and Theretairus has been constantly recovered in previous analyses (e.g., Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Apesteguía et al., Reference Apesteguía, Gómez and Rougier2014; Hsiou et al., Reference Hsiou, De França and Ferigolo2015).
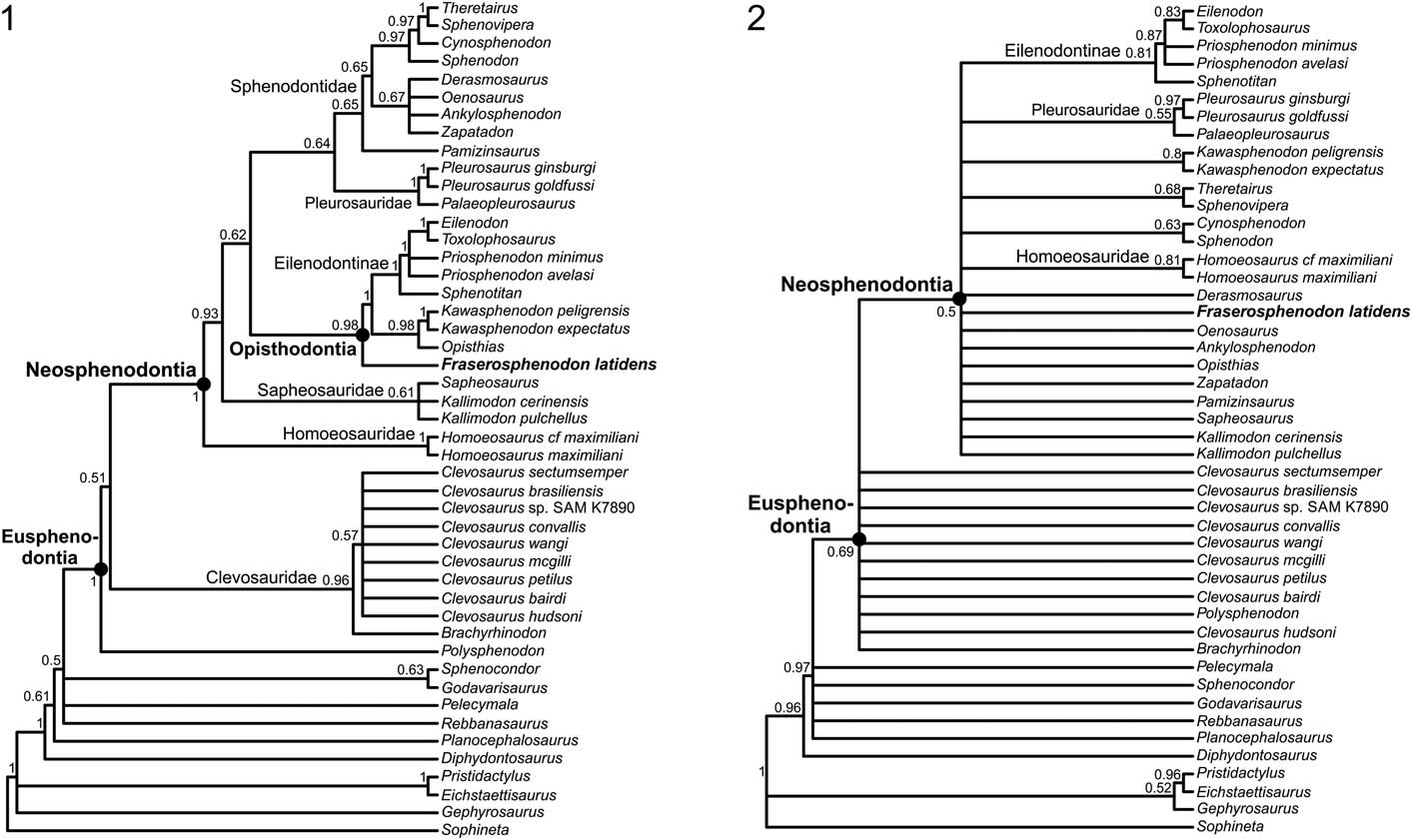
Figure 3 Consensus trees recovered from the phylogenetic analyses: (1) maximum parsimony 50% majority rule consensus tree; CI=0.38628, RI=0.66403; node labels denote the proportion of MPTs that recover that node; (2) 50% majority rule consensus tree from the Bayesian-inference analysis, with clade credibility values (decimal proportions) labeled on the nodes.
Within clevosaurs, Brachyrhinodon Huene, Reference Huene1910 was recovered as the earliest diverging taxon. All Clevosaurus species are grouped in a polytomy, which obscures the relationships between the species. The results for clevosaurs are quite similar to those recovered by the strict consensus tree of Hsiou et al. (Reference Hsiou, De França and Ferigolo2015). The only difference is that in their analysis, Polysphenodon Jaekel, Reference Jaekel1911 appears as the earliest diverging taxon within Clevosauridae, but all other taxa were recovered in a polytomy. A similar polytomy for clevosaurs was also shown in the strict consensus tree of Rauhut et al. (Reference Rauhut, Heyng, López-Arbarello and Hecker2012). Our results agree with the work of Martínez et al. (Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013) and Hsiou et al. (Reference Hsiou, De França and Ferigolo2015) in recovering Fraserosphenodon latidens as an early diverging opisthodontian. Indeed, we recovered F. latidens as the earliest diverging taxon within Opisthodontia. This clearly confirms that F. latidens is not referable to the genus Clevosaurus, and supports the erection of a new opisthodontian genus, as previously suggested (Jones, Reference Jones2006a, Reference Jones2009; Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Hsiou et al., Reference Hsiou, De França and Ferigolo2015; Klein et al., Reference Klein, Whiteside, de Lucas, Viegas and Benton2015). Within Opisthodontia, the relationships of eilenodontines are quite well resolved; our results only differ from the works of Martínez et al. (Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013) and Cau et al. (Reference Cau, Baiano and Raia2014) in finding Ankylosphenodon outside of Opisthodontia.
Another major difference compared to the previous analyses of Martínez et al. (Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013) and Hsiou et al. (Reference Hsiou, De França and Ferigolo2015) is that the Triassic taxon Pelecymala was no longer recovered as closely related to Opisthodontia, but was found in a polytomy with early-diverging rhynchocephalians such as Rebbanasaurus Evans, Prasad, and Manhas, Reference Evans, Prasad and Manhas2001, the clade of Sphenocondor Apesteguía, Gomez, and Rougier, Reference Apesteguía, Gómez and Rougier2012 and Godavarisaurus Evans, Prasad, and Manhas, Reference Evans, Prasad and Manhas2001, and the clade Eusphenodontia.
Overall, the results of the Bayesian analysis (Fig. 3.2) resemble those of the parsimony analysis, but with considerably less resolution. Several large polytomies were recovered, but where clades are resolved, the clade credibility values are often moderately high. The Bayesian 50% majority rule consensus tree also recovered Pelecymala in a polytomy with early diverging rhynchocephalians, which confirms that this taxon is not related to opisthodontians as previously assumed (Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Hsiou et al., Reference Hsiou, De França and Ferigolo2015). The Bayesian tree did not recover clevosaurs as a monophyletic group; all of them were recovered in a large polytomy that obscures the relationships between the taxa. Relationships among other, later-diverging rhynchocephalians are unclear; many of them are part of a polytomy that includes Fraserosphenodon, but no clevosaurs. This result confirms that Fraserosphenodon is not closely related to Clevosaurus.
It should be noted that the Bayesian tree recovered a close relationship between the extant Sphenodon and the Jurassic Cynosphenodon, a close relationship between Theretairus and Sphenovipera, and pleurosaurs as a monophyletic group. The Bayesian tree did not recover Opisthodontia as a monophyletic group, but completely agrees with the parsimony tree for the interrelationships of eilenodontines, which are quite robust and well resolved.
Discussion
Among Mesozoic rhynchocephalians, clevosaurs were one of the most diverse groups. Clevosaurs are represented by three genera: Polysphenodon, Brachyrhinodon, and Clevosaurus. The first two genera are monospecific, whereas Clevosaurus currently has nine formally recognized species. The high diversity of the genus Clevosaurus, however, is debatable because of the doubtful validity/referral of some of the species, especially those based on poorly preserved or very fragmentary material, such as the three Chinese species (C. mcgilli, C. petilus, and C. wangi) or ‘C. latidens’ from Great Britain. According to Jones (Reference Jones2006a), the Chinese specimens are too poorly preserved to diagnose them as three distinct species, but clearly all of them belong to Clevosaurus. In contrast to the Chinese specimens, the referral of ‘C. latidens’ to Clevosaurus has been widely questioned (Jones, Reference Jones2006a, Reference Jones2009; Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013; Hsiou et al., Reference Hsiou, De França and Ferigolo2015; Klein et al., Reference Klein, Whiteside, de Lucas, Viegas and Benton2015).
Before the description of ‘Clevosaurus latidens,’ specimen AUP 11192, a dentary fragment, was tentatively related to Pelecymala based on its transversely wide teeth (Fraser, Reference Fraser1986). When Fraser (Reference Fraser1993) formally described ‘C. latidens,’ he noted that the tooth morphology of the new ‘Clevosaurus’ species was quite similar to that of other taxa with transversely wide teeth such as P. robustus and Toxolophosaurus cloudi Olson, Reference Olson1960 (Fraser, Reference Fraser1993). Some of the diagnostic characters of the genus Clevosaurus based on features of the skull could not be observed in ‘C. latidens’ for obvious reasons. However, at least the dentition of ‘C. latidens’ did not match that of Clevosaurus, which consists of larger, blade-like teeth with lateral flanges. It has been suggested that the tooth morphology of Clevosaurus was very specialized for a possible omnivorous or carnivorous diet (Jones Reference Jones2006b, Reference Jones2009; Rauhut et al., Reference Rauhut, Heyng, López-Arbarello and Hecker2012; Martínez et al., Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013), whereas the dentary and maxillary teeth ‘C. latidens’ were more like those of herbivorous taxa. Fraser (Reference Fraser1993) also pointed out that the wear facets on the teeth of ‘C. latidens’ suggested a propalinal movement of the lower jaw, which contrasts with the orthal jaw movement seen in Clevosaurus.
Based on dentary, maxillary, and premaxillary tooth morphology, as well as the suggested propalinal movement of the lower jaw, our review of ‘C. latidens’ specimens confirms that this taxon is not referable to Clevosaurus. Our phylogenetic analyses, including both parsimony and Bayesian approaches, confirm its position outside Clevosaurus. We rename ‘C. latidens’ as Fraserosphenodon latidens n. comb. The parsimony tree (Fig. 3.1) suggests that F. latidens is an early-diverging opisthodontian, but not closely related to Pelecymala as was previously suggested by Fraser (Reference Fraser1986, Reference Fraser1993), Martínez et al. (Reference Martínez, Apaldetti, Colombi, Praderio, Fernandez, Malnis, Correa, Abelin and Alcober2013), and Hsiou et al. (Reference Hsiou, De França and Ferigolo2015). While reviewing the type specimens of Pelecymala (AUP 11140, 11214–11215), we noticed that the teeth of Pelecymala are not transversely broadened as had been described by Fraser (Reference Fraser1986); in contrast, their shape is more conical, slightly curved, and labiolingually flattened. The tooth morphology of Pelecymala is actually more similar to that of some of the earliest diverging rhynchocephalians, which is also confirmed by our phylogenetic analyses (Fig. 3). A complete taxonomic redescription of Pelecymala appears necessary, but is beyond the scope of this study. The Bayesian tree (Fig. 3.2) could not recover the exact relationships of F. latidens, because this taxon is found in a polytomy that includes many other species. Like the parsimony analysis, however, the Bayesian approach recovered F. latidens as a genus distinct from Clevosaurus and not closely related to clevosaurs. Following the parsimony analysis, we consider F. latidens as an early diverging opisthodontian.
The parsimony analysis of Rhynchocephalia showed better resolution than the Bayesian approach. This result is not unexpected, because studies have shown that Bayesian methods are more accurate but less precise than parsimony-based analyses (O’Reilly et al., Reference O’Reilly, Puttick, Parry, Tanner, Tarver, Fleming, Pisani and Donoghue2016). There are some minor differences between the internal branches in both trees, but several higher clades were recognized by both phylogenetic methods (Fig. 3). Some of these higher clades within Rhynchocephalia have been frequently recovered in other recent phylogenetic analyses, and have been informally named as ‘crown-sphenodontians,’ ‘derived-sphenodontians,’ or ‘eupropalinals’ (e.g., Apesteguía et al., Reference Apesteguía, Gómez and Rougier2012, Reference Apesteguía, Gómez and Rougier2014; Apesteguía and Carballido, Reference Apesteguía and Carballido2014).
We propose formal names for two well-supported clades: Eusphenodontia and Neosphenodontia (Fig. 3). We define Eusphenodontia as the least inclusive clade containing Polysphenodon muelleri Jaekel, Reference Jaekel1911, Clevosaurus hudsoni, and Sphenodon punctatus. In the 50% majority rule consensus tree, three unambiguous character transitions were recovered for Eusphenodontia under both ACCTRAN and DELTRAN optimization: (1) wear facets on marginal teeth of the dentary and/or on marginal teeth of the maxilla are clearly visible (character 46: 0 to 1), (2) the premaxillary teeth are merged into a chisel-like structure (character 49: 0 to 1), and (3) the palatine teeth are reduced to a single tooth row with an additional isolated tooth (character 52: 0 to 1). Neosphenodontia is defined as the most inclusive clade containing S. punctatus but not C. hudsoni. In the 50% majority rule consensus tree, Neosphenodontia is supported by the following six unambiguous character changes that are recovered under both ACCTRAN and DELTRAN optimization: (1) the relative length of the antorbital region is increased, reaching one-quarter to one-third of the complete skull length (character 1: 2 to 1), (2) the posterior edge of the parietal is only slightly incurved inward (character 18: 0 to 1), (3) the parietal foramen is found at the same level or anterior of the anterior border of the supratemporal fenestra (character 19: 0 to 1), (4) the palatine teeth are further reduced to a single lateral row (character 52: 1 to 2), (5) the number of pterygoid tooth rows is reduced to one or none (character 55: 1 to 2), and (6) the ischium is characterized by a prominent process on its posterior border (character 60: 1 to 2). The families Homoeosauridae, Pleurosauridae, and Sphenodontidae form in our analyses, as in others, the content of the clade Neosphenodontia. Levels of homoplasy in Eusphenodontia and Neosphenodontia are generally high, with individual character consistency indices (CI) often <0.5. For both clades, no individual character has a CI of 1 in the 50% majority rule consensus tree (for the complete list of characters, apomorphies, and other tree statistics, see the Supplemental Data). We consider the formal naming of these higher clades necessary to facilitate future discussion about the phylogenetic relationships of rhynchocephalians.
Conclusion
This study confirms previous doubts about the referral of ‘C. latidens’ to Clevosaurus. The recognition of ‘C. latidens’ belonging to a new genus now formally named Fraserosphenodon emphasizes the high generic diversity of Rhynchocephalia in the Mesozoic, especially among herbivorous taxa. Furthermore, our study demonstrates that the use of Bayesian approaches can be useful to contrast and validate phylogenies that were previously based only on parsimony methods. Bayesian inference exhibits generally lower resolution in some parts of the tree, but a few higher clades are strongly supported and are consistently recovered by both Bayesian and parsimony analyses.
Acknowledgments
We thank A. Hastings (VMNH) for the loan of type specimens of Fraserosphenodon. We also thank T. Colin (AUP), M. Lowe (UMZC), and J. Hanson (BRSUG) for access provided to their collections. We thank N. Fraser (NMS) for his assistance to JAH-F during his visit to Aberdeen, and D. Whiteside (University of Bristol) for his helpful comments and discussions on Clevosaurus. This work was funded by a Ph.D. scholarship from CONACYT, Mexico, and Bob Savage Memorial Fund from University of Bristol to JAH-F, NERC grant NE/I027630/1 to MJB and TLS, and NERC grant NE/L002434/1 to AE.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.9n153





