Introduction
Biosensors prepared using magnetic nanomaterials are favored in biosensing applications due to their high sensitivity, rapid response, strong selectivity, large specific surface area, and strong electrical conductivity (Sleutels et al. Reference Sleutels, TerHeijne, Buisman and Hamelers2012; Turner Reference Turner2013; Cortina et al. Reference Cortina, Melli, Roberti, Mass, Longinotti and Tropea2016; Wei et al. Reference Wei, Ma, Dong, Krause, Zhang and Willbold2016). More importantly, in electrochemical sensor applications, the modification or incorporation of magnetic nanomaterials can significantly increase the surface area, accelerate electron transfer, and increase response speed, electrode stability, and selective detection (Magro et al. Reference Magro, Baratella, Pianca, Toninello, Grancara and Zboril2013; Galović et al. Reference Galović, Samardžić and Sak-Bosnar2015; Marin et al. Reference Marin, Montoya, Arnache and Calderon2016). However, in complex applications of magnetic nanomaterials, the inherent small size and the spin-canting effect of the particle surface cause defects such as low saturation magnetization, slow magnetic response, and poor physical and chemical stability. To make up for these deficiencies and achieve better biomedical application performance, the search for new kinds of low-toxicity and efficient magnetic nanoparticles has become a focus in current research. The nanorings/nanodisks with vortex magnetic domains avoid particle agglomeration due to the closed distribution of magnetic moments, which can form a good suspension and, at the same time, have a larger particle size than that of superparamagnetic particles. Magnetic susceptibility and saturation magnetization provide a new means of improving magnetic properties (Liu et al. Reference Liu, Yang, Ng, Zhao, Zhang and Bay2015; Goiriena-Goikoetxea et al. Reference Goiriena-Goikoetxea, Guslienko, Rouco, Orue, Berganza and Jaafar2017; Vigo-Cotrina and Guimarães Reference Vigo-Cotrina and Guimarães2018). To achieve a stable vortex magnetic domain structure, the size and structure of the nanorings/nanodisks, such as the outer diameter, height, and inner/outer diameter ratio, must be strictly controlled. Therefore, the realization of controllable preparation of structurally stable nanorings/nanodisks has become a hot topic in the research of vortex magnetic nanomaterials.
Magnetic nanoparticles can make the separation of proteins more efficient and rapid. In protein research, the use of magnetic nanoparticles in the separation of proteins has increased significantly (Kamioka et al. Reference Kamioka, Agatsuma, Kajikawa, Ueda, Furuse and Fuchino2014; Fuchino et al. Reference Fuchino, Furuse, Agatsuma, Kamioka, Iitsuka and Nakamura2014; Sharafeldin et al. Reference Sharafeldin, Bishop, Bhakta, El-Sawy, Suib and Rusling2017), and most of the studies have used superparamagnetic nanoparticles. However, the research on vortex magnetic nanorings used in protein separation and detection has been limited. Previous studies have found that magnetic nanorings are less stable in protein detection due to their poor stability in strong magnetic fields (Takahashi et al. Reference Takahashi, Akiyama, Ikezumi, Nagata, Yoshino and Iizuka2009), and, hence, the application performance needs to be improved. The soluble protein contained in plasma is mainly serum protein (Anirudhan et al. Reference Anirudhan, Rejeena and Tharun2012). Among serum proteins, bovine serum albumin (BSA) and human serum proteins are very similar; they have good water solubility, are stable, cheap, and are easy to obtain (Simard et al. Reference Simard, Zunszain, Ha, Yang, Bhagavan and Petitpas2005; Ravikumar et al. Reference Ravikumar, Chen, Jayaraman, Poh and Chan2017; Nosrati et al. Reference Nosrati, Sefidi, Sharafi, Danafar and Kheiri2018). Thus, BSA is commonly selected for separation experiments.
Clay minerals, as conventional adsorbent materials, have been used to remove metals and organic pollutants from water bodies. Magnetite (Fe3O4) is a common mineral in soil, weathered soils, and clays (Chen et al. Reference Chen, Chen, Xie, Xu, Liu and Zhou2018), and the existence of magnetite can affect the biological activity of the clay minerals (Roh et al. Reference Roh, Zhang, Vali, Lauf, Zhou and Phelps2003; Khare et al. Reference Khare, Eggleston and Lovelace2005). The adsorption of BSA protein on Fe3O4 nanorings can, therefore, be used as a reference for the removal of soil organic pollutants (Yu et al. Reference Yu, Li, Tong, Zhou, Lin and Xu2013; Zhou and Keeling Reference Zhou and Keeling2013; An et al. Reference An, Zhou, Zhuang, Tong and Yu2015; Chen et al. Reference Chen, Zhou, Fiore, Tong, Zhang, Li, Ji and Yu2016; Zhou et al. Reference Zhou, Zhao, Wang, Chen and He2016). The objective of the present study was to use a hydrothermal method combined with calcination in a mixed hydrogen-argon atmosphere to obtain ordered mono-crystalline magnetic Fe3O4 nanorings with controllable size and uniform structure, and to investigate the application of the nanorings in the adsorption of BSA protein.
Materials and Methods
Materials and Equipment
FeCl3∙6H2O, NaH2PO4, and Na2SO4 were purchased from Kelon Reagent (Chengdu, Sichuan, China). The beakers used in the experiment were from Sichuan Shubo Group Co., Ltd. (Chengdu, Sichuan, China). The Coomassie brilliant blue was purchased from Aladdin (Shanghai, China).
Synthesis and Characterization of Fe3O4 Nanorings
Three solutions, 0.02 M FeCl3∙6H2O, 1.8×10-4 M NaH2PO4, and 5.5×10-4 M Na2SO4, were mixed and dispensed into a 1 L volumetric flask. The mixed solution was transferred to a 100 mL reaction vessel and kept in an autoclave at 220°C for 48 h. After the autoclave was cooled to room temperature, the reactor was opened, the sample was centrifuged at 4,000 rpm (~1,790 ×g) for 10 min (TGL-16, Shuke Technology Co., Ltd, Chengdu, China), and the supernatant was discarded. The remaining product was α-Fe2O3 nanorings. Samples were washed with double-distilled water and centrifuged at 4,000 rpm (~1,790 ×g) for 5 min and then filtered and dried at 70°C. The powder obtained was crushed and then transferred to a tubular atmosphere furnace for heat treatment at 360°C for 5 h under a mixed hydrogen-argon atmosphere (H2/(H2 + Ar) = 8%) to obtain black Fe3O4 nanorings (Liu et al., Reference Liu, Yin, Yi and Duan2016a, Reference Liu, Zhu and Wangb). γ-Fe2O3 nanorings were obtained by heat treatment at 240°C for 2 h in a muffle furnace.
The nanostructures of the products were characterized by field emission scanning electron microscopy (FESEM) (Carl Zeiss, Jena, Germany), transmission electron microscopy (TEM) (Carl Zeiss, Jena, Germany), and high-resolution transmission electron microscopy (HRTEM). X-ray diffraction (XRD) (PANalytical B.V., Almelo, Netherlands) with Cu Kα radiation was used to identify the mineral phase. Energy-dispersive X-ray spectroscopy (EDS) was used to detect the elemental composition. The magnetic measurements (using a BKT-4500Z, Vibrating Magnetometer Zetian Weiye Technology Co., Ltd. Beijing, China) were performed at room temperature (magnetic field range: 0–2.0 T; step accuracy: 1 Oe; magnetic moment range: 10–3–100 emu; and test temperature range: room temperature to 500°C).
Preparation of BSA Standard Solution
The BSA standard solution, deionized water, and Coomassie brilliant blue solution were added to a small weighing bottle, shaken gently, allowed to stand for 5 min, and then subjected to ultraviolet-visible (UV-Vis) spectrophotometry. The concentration of BSA in the standard curve ranges from 0 to 0.1 mg mL–1 (Fig. 4). Next, a standard curve was drawn using a UV-Vis spectrophotometer UV-1800 (Shimadzu, Kyoto, Japan), measuring wavelength range: ~190–1100 nm and spectral bandwidth (resolution): 1 nm. Coomassie brilliant blue is a staining agent, which is red in the free state, and has a maximum light absorption of 465 nm. When Coomassie brilliant blue combines with protein, the protein-pigment conjugate turns into cyan and has maximum light absorption at a wavelength of 595 nm. The Coomassie brilliant blue method can thus be used for the quantitative determination of proteins according to Beer-Lambert law.
Adsorption of BSA on Fe3O4 Nanorings at various pH Values
In this experiment, a phosphate buffer solution was used to configure the various pH values of BSA solution. 100 g of sodium dihydrogen phosphate was added to 800 mL of distilled water in a beaker, and the pH was measured. Dilute hydrochloric acid was added slowly to the beaker and, simultaneously, the pH was measured. When the pH reached the required value (pH =3, 4, 5, 6, 7), the addition of hydrochloric acid was stopped and the solution was transferred to a 1000 mL volumetric flask.
First, 0.225 g of BSA was weighed into a flask, then 75.0 mL of a phosphate buffer solution at pH 3.0, 4.0, 5.0, 6.0, or 7.0 was added to prepare a BSA solution with a concentration of 3 mg·mL-1. The above steps were repeated to prepare a sufficient amount of adsorption solution. Then, 100 mL aliquots of the BSA solutions with the five different pH values were placed in separate conical flasks, and 0.02 g of Fe3O4 nanorings was added to each conical flask. In addition, a blank control phosphate buffer solution was prepared, and 0.02 g of Fe3O4 nanoring was also added to measure the absorbance as a reference control group. The Erlenmeyer flask containing ring-shaped Fe3O4 was placed in an ultrasonic cleaning apparatus for 10 min of ultrasonication. The flasks were left to stand for 1 h (including shaking for 10 min), and each group was moved to the previously prepared solution using a 1000 μL pipette gun. In numbered weighing bottles, 5 mL Coomassie brilliant blue solution was added for each UV-Vis absorbance test. Each group was tested three times.
Adsorption of BSA at various Concentrations of Fe3O4 Nanorings
The pH 5.0 phosphate buffer solution prepared was used as a solvent to prepare five bottles of BSA solutions: 1.75 mg·mL-1, 2.0 mg·mL-1, 2.25 mg·mL-1, 2.5 mg·mL-1, and 2.75 mg·mL-1. 100 mL of each BSA solution was added to six Erlenmeyer flasks and labeled sequentially. Next, 0.02 g Fe3O4 nanoring particles was added. A blank control with 100 mL of phosphoric acid (pH 5) and 0.02 of g of Fe3O4 nanorings was also prepared. All seven suspensions in the Erlenmeyer flasks were dispersed ultrasonically for 10 min and allowed to stand for 230 min. After standing, seven weighing bottles marked with the corresponding number were taken, and a 1,000 μL pipette was used to transfer 1 mL of BSA solution from the conical flask to the corresponding numbered weighing bottle. 5 mL of Coomassie brilliant blue solution was added, and a UV-Vis measurement was carried out. Each set was tested three times.
Results and Discussion
Morphology Analysis
The scanning and transmission electron micrographs of the Fe3O4 nanorings prepared by the hydrothermal method coupled with calcination in a mixed hydrogen-argon gas were compared (Fig. 1). The Fe3O4 nanorings prepared exhibited a ring structure with uniform size, having an outer diameter of ~160–180 nm, an inner diameter of ~80–110 nm, and a height of ~70–110 nm. HRTEM imaging revealed lattice fringes, giving the outer edge (111) crystal dimension of the Fe3O4 nanorings as ~0.48 nm (Fig. 1e).

Fig. 1 SEM (a, b), TEM (c, d), and HRTEM (e) images of nanorings
Particle-size Analysis
The particle-size distribution of monodisperse magnetic Fe3O4 nanorings was between 80 and 100 nm (Fig. 2).

Fig. 2 The distribution of nanoring sizes
XRD Analysis
The XRD pattern (Fig. 3) of the prepared nanorings showed diffraction peaks at 24.2, 33.1, 35.6, 40.9, 49.5, 54.1, 57.6, 62.5, 64.0, and 72.3°2θ, corresponding to the (012), (104), (110), (113), (024), (116), (018), (214), (300), and (119) crystal planes of α-Fe2O3, respectively, as marked by ♠ (JCPDS Card No. 33-664). The diffraction peaks at 18.3, 30.1, 35.4, 37.1, 43.1, 47.1, 53.4, 56.9, and 62.5°2θ corresponded to the (111), (220), (311), (222), (400), (331), (442), (511), and (440) crystal planes of Fe3O4 nanorings, respectively, as marked by ♣ (JCPDS Card No. 87-2334). The result indicated that the nanorings prepared by the hydrothermal method were α-Fe2O3, and the nanorings prepared by heating treatment under mixed hydrogen-argon atmosphere were Fe3O4. The peaks in the XRD patterns were strong, indicating the good crystallinity of these two types of nanoring.

Fig. 3 XRD patterns of α-Fe2O3 and Fe3O4 nanorings
Magnetic Analysis
The UV-Vis standard curve (Fig. 4) for the BSA in solution yielded a linear equation, y = 5.2377x + 0.0331, and a correlation coefficient R2 = 0.99293, where x stands for BSA concentration, and y stands for absorbance value. This result showed that the standard curve of BSA measured by UV-Vis spectrophotometry had a good linear relationship.

Fig. 4 UV-vis standard curve of bovine serum albumin (BSA)
Magnetization measurements revealed a hysteresis loop for the Fe3O4 nanorings with increasing and decreasing magnetic field at 300 K (Fig. 5). The coercive force was 120 Oe at room temperature, and the saturation magnetization of the magnetic nanoring was 78.2 emu·g-1, which was smaller than the saturation magnetization of the bulk Fe3O4 reported in the literature (92 emu·g-1) (Pankhurst et al. Reference Pankhurst, Connolly, Jones and Dobson2003; Yang et al. Reference Yang, Zhu, Yang, Ma, Wu and Lan2016). (Coercive force means that after the magnetic material is saturated and magnetized, its magnetic induction does not fall back to zero when the external magnetic field returns to zero. Only when a magnetic field of a certain magnitude is added in the opposite direction to the original magnetization field can the magnetic induction be returned zero; this magnetic field is referred to as a 'coercive force.') The decrease in magnetization might be due to the surface effect caused by the small particle size and the magnetic domain characteristic of magnetic Fe3O4. In the M-H diagram (Fig. 5) (Magnetization- coercive force, H), two opposite values of Mr (Magnetic resonance) appear near the zero point, indicating that the residual magnetization is not zero.

Fig. 5 Hysteresis loop of Fe3O4 nanorings magnetization measured at 300 K
Effect of Magnetic Fe3O4 Nanorings on the Adsorption Properties of BSA Protein Under Various Conditions
The amount of BSA adsorbed to 0.02 g of Fe3O4 nanorings was tested at various pH values (Fig. 6a). BSA adsorption increased with increasing pH until pH 5, then decreased. The isoelectric point of the Fe3O4 nanorings was 4.8, indicating that maximum adsorption occurred when the charge was neutral. This is contrary to previous studies which argued that the adsorption model of BSA to magnetic Fe3O4 nanorings is electrostatic and can be characterized by electrical double layer theory (Sheng et al. Reference Sheng, Liu, Xie and Liu2016).

Fig. 6 (a) Effect of pH on BSA adsorption capacity (m/V = 0.02 g/100 mL, initial BSA concentration: 3.0 mg·mL-1, contact time: 1 h). (b) Effect of time on BSA adsorption (m/V = 0.02 g/100 mL, pH = 5, initial BSA concentration: 2.6 mg·mL-1, contact time: 1 h). (c) Effect of the initial concentration on BSA adsorption (pH = 5, contact time: 3 h)
The length of adsorption time is an important parameter to study adsorption kinetics. The time required for adsorption to occur on 0.02 g of magnetic Fe3O4 nanorings was measured at 2 h, 3 h, 4 h, and 5 h (Fig. 6b). In the first 3 h, the extent of adsorption increased steadily with time, and then leveled off after 3 h, indicating that surface adsorption sites became saturated and no more adsorption occurred (Fig 7).

Fig. 7 The adsorption-desorption curve of the Fe3O4 nanorings
The influence of the initial concentration of BSA at pH 5 on the amount adsorbed (Fig. 6c) over a period of 3 h revealed that the maximum adsorption capacity was not quite achieved under these conditions (even at a BSA concentration of 2.8 mg·mL-1) but the result is close enough to estimate the adsorption capacity (Q e, mg·g-1) of the magnetic Fe3O4 nanorings for BSA at pH 5 according to Eq. 1:
where C 0 represents the initial concentration of BSA in the solution before adsorption in mg·mL-1; C 1 indicates the BSA concentration in the solution after adsorption in mg·g-1; V indicates the volume of BSA solution in mL; and W indicates the amount of Fe3O4 nanorings present. After calculation, the maximum adsorption capacity of magnetic Fe3O4 nanorings for BSA at pH 5 was 325.2 mg·g-1. This result is greater than the value of 250 mg·g-1 reported by Zhu et al. (Reference Zhu, Shen, Xu, Wang and Han2007) and may be because the special annular cavity structure of magnetic Fe3O4 nano-rings has a large specific surface area and so adsorbs and immobilizes BSA more easily.
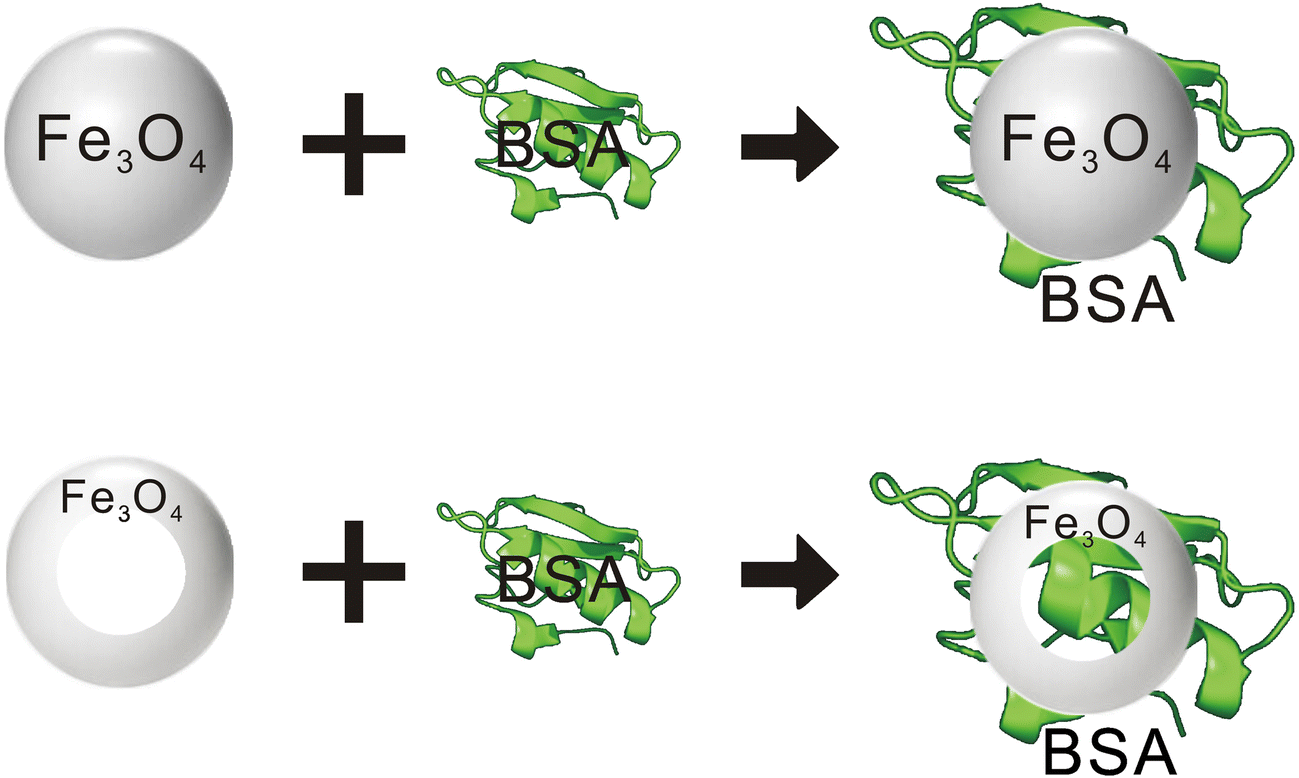
The magnetic Fe3O4 nanorings seem to perform better than Fe3O4 magnetic nanoparticles. As illustrated in the sketch in Fig. 8, the difference may be related to the special ring-shaped cavity structure of the magnetic Fe3O4 nanorings, which enabled some of the protein molecules to enter the ring cavity and was more likely to adsorb and immobilize BSA, even though the magnetic Fe3O4 had a greater specific surface area. Furthermore, the unique vortex domain characteristics of the nanorings enhance effectively the adsorption effect.

Fig. 8 Sketch map of the Fe3O4 nanoparticles and Fe3O4 nanorings combined with BSA
Conclusion
In this work, the preparation of magnetic Fe3O4 nanorings with a regular morphology through the combination of hydrothermal and annealing methods under a mixed hydrogen-argon atmosphere was reported. Its ability to sequester BSA was then tested. The magnetic Fe3O4 nanorings have a coercive force of 120 Oe and a saturation magnetization of 78.2 emu·g-1 at room temperature. The maximum adsorption capacity of magnetic Fe3O4 nanorings for BSA was calculated to be 325.2 mg·g-1 at an optimum pH of 5. The special ring-shaped cavity of the magnetic Fe3O4 nanoring was credited for enhancing the adsorption of the protein molecules by their entering the ring cavity, thus making BSA adsorption and fixation more complete. Moreover, the characteristic vortex magnetic domain characteristics enhanced the adsorption effect.
Acknowledgements
This work was supported by the Scientific Research Fund of Sichuan Provincial Education Department (17ZA0401), Fundamental Science on Nuclear Wastes and Environmental Safety Laboratory (15kffk05), Sichuan Science and Technology Program (2018JY0315), China Postdoctoral Science Foundation (2018M633411) and Research Support Program for Longshan Academic Talents (18LZX673), National Natural Science Foundation of China (41872039 and 41831285), and the One-Thousand-Talents Scheme in Sichuan Province, Sichuan Science and Technology Program(2018JY0462).