CHD is the most common congenital defect, presenting in approximately 8 per 1000 live births. Reference van der Linde, Konings and Slager1 Of all children with this condition, approximately 25% have a critical form (i.e., critical CHD); for these children, survival is dependent on early complex surgical interventions. Reference Oster, Lee, Honein, Riehle-Colarusso, Shin and Correa2 While advances in the surgical and medical care of children with critical CHD have led to increased survival rates, children with critical CHD, and particularly those with univentricular CHD, are at high risk for neurodevelopmental impairments. The common developmental profile of children with critical CHD includes mild to moderate difficulties in motor, cognitive, attention, and language skills, which have all been linked to different prenatal, perioperative, and post-surgical factors. Reference Yoshida, Hiraiwa and Ibuki36
Until now, most studies examining the neurodevelopmental outcomes of children with critical CHD have concentrated on the motor and/or cognitive development, resulting in the synthesis of the literature on cognition and motor domains. Reference Bolduc, Dionne, Gagnon, Rennick, Majnemer and Brossard-Racine7–Reference Feldmann, Ullrich and Bataillard10 Despite the critical role language skills play in social connection and academic performance Reference Barre, Morgan, Doyle and Anderson11 , there has been notably less attention on the receptive (comprehension of language) Reference McLaughlin12 and expressive (communication of language) Reference McLaughlin12 language outcomes of children with critical CHD; to date, no study has synthesised the literature regarding language abilities in children with this condition.
As a result, this systematic review and meta-analysis aimed to determine the language abilities of preschool children with critical CHD, including a comparison of language outcomes between those with univentricular versus biventricular CHD.
Materials and methods
Search strategy
An initial search (11 January, 1990-1 July, 2020) was completed on 1 July, 2020 to identify relevant literature on expressive and receptive language outcomes in MEDLINE, Embase, Scopus, Child Development and Adolescent Studies, ERIC, PsycINFO, and CINAHL databases. In July of 2021, the same search strategy was repeated to identify any subsequently published studies. The search strategies were performed using the constructs of preschool children, critical CHD, and language outcomes to formulate the search, with adaptations to the search strategy according to each database. The search strategy is available from the authors upon request.
Inclusion criteria
This review included studies published in English from 1990–2021 that examined the receptive and expressive language outcomes of children aged 5 years or younger with critical CHD who required a complex cardiac procedure within the first year of life. Complex cardiac procedure was defined as having undergone surgery with cardiopulmonary bypass or catheter-based intervention. Studies had to involve direct assessment of a child’s expressive and receptive language ability through standardised testing using a validated tool to be included in the review. Study designs included in the review were cross-sectional, case–control, cohort, as well as randomised controlled trial.
We excluded studies of children: (1) who did not require surgery or (2) who had their initial heart surgery after one year of age or (3) non-bypass surgeries. Studies that assessed language abilities using screening tools or parent-completed questionnaires were also excluded.
The protocol was registered and submitted to Prospero, 13 an international prospective register for systematic reviews (CRD42020192505).
Study selection
The study selection was completed through a two-step process. Two reviewers (Reviewer 1, Reviewer 2) independently screened titles and, where available, abstracts. The reviewers categorised each study as “include,” “unsure,” or “exclude.” The full text of potentially relevant studies, the “include” or “unsure” categories, was obtained. The formal a priori inclusion criteria were independently applied to each potentially relevant study by Reviewer 1 and Reviewer 2 Discrepancies were resolved by a third reviewer (Reviewer 3).
The authors of articles were contacted if the expressive and receptive outcomes were assessed but the results were not reported. If the author was able to provide the required data, the study was included.
Data extraction
A standardised form to facilitate data extraction was developed based on the Cochrane Handbook for Systematic Reviews of Interventions, Systematic Reviews: CRD’s guidance for undertaking reviews in health care, and clinical acumen by healthcare professionals and researchers. Reference Li, Higgins and Deeks14,15 General and demographic information extracted included article title, author names, date of publication, country of study, study design, single or multicentre study, sample size, population age, population sex, and cardiac diagnoses. Extraction of perioperative variables included procedures performed, number of cardiac surgeries under cardiopulmonary bypass, length of hospital stay, and comorbidities. Language data included age at language assessment, language tool used, and language outcome results for children with critical CHD and control data, if available. Data extraction was first performed independently by two reviewers (Reviewer 1, Reviewer 4) and then reviewed together to resolve any discrepancies.
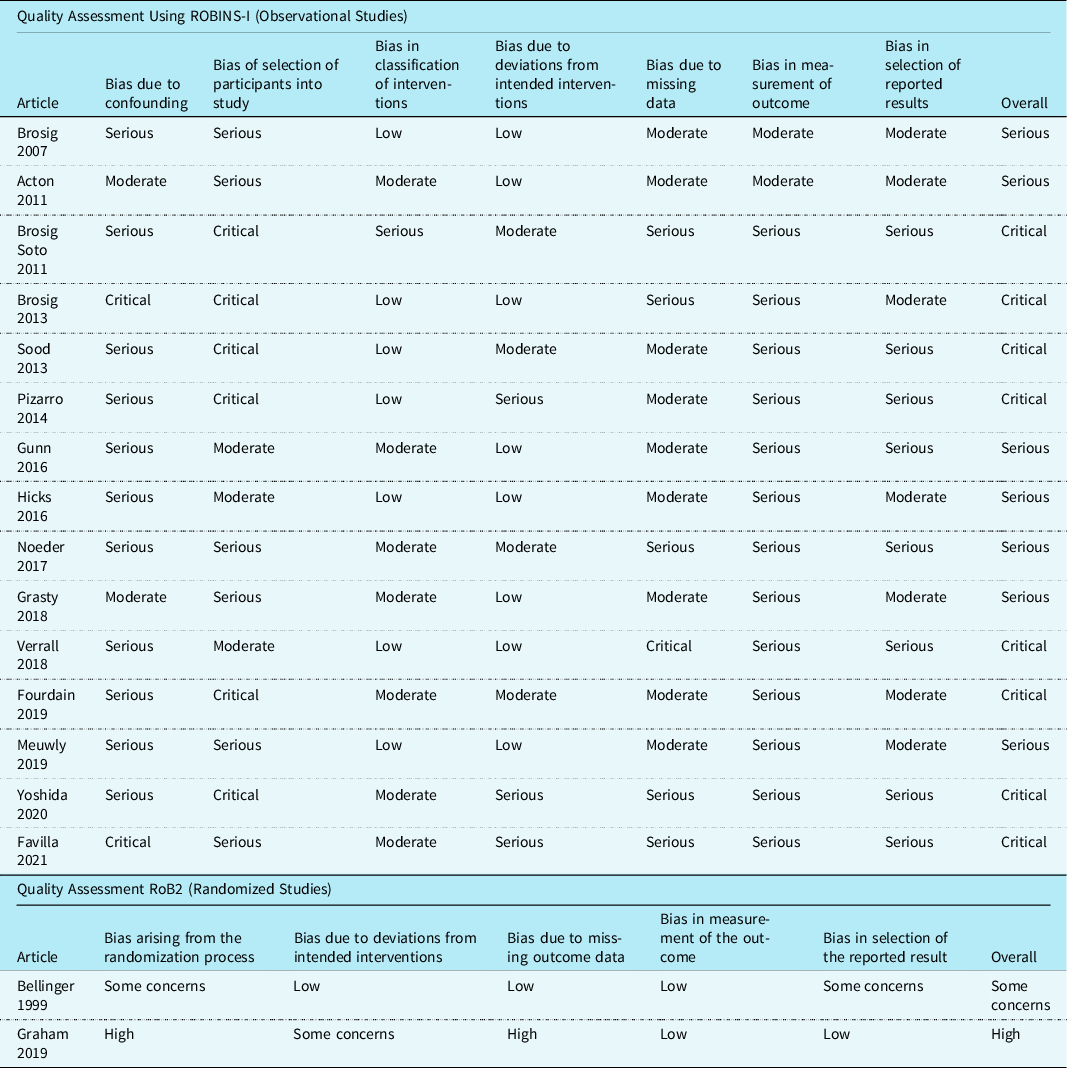
Quality assessment
Quality assessment was completed using the Revised Cochrane Risk-of-Bias tool for randomised trials Reference Sterne, Savović and Page16 and Risk Of Bias In Non-randomised Studies of Interventions Reference Sterne, Hernán and Reeves17 assessment tool and template, as per the study design. The Revised Cochrane Risk-of-Bias tool for randomised trials tool assesses randomised studies through 5 domains for potential bias: randomisation process, deviations from intended interventions, missing outcome data, measurements of the outcome, and the selection of reported results. The Revised Cochrane Risk-of-Bias tool for randomised trials tool then classifies the randomised studies as low, some concerns, or high risk of bias. Reference Sterne, Savović and Page16 The Risk Of Bias In Non-randomised Studies of Interventions tool assesses non-randomised studies through 7 different domains of potential bias: confounding, selection of study participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcome, and the selection of reported results. The Risk Of Bias In Non-randomised Studies of Interventions tool then classifies each non-randomised study as low, moderate, serious, or critical risk of bias. Reference Sterne, Higgins, Elbers and Reeves18
Two reviewers (Reviewer 1 and Reviewer 2) independently assessed each article. The reviewers first pilot-tested 3 articles to ensure they operationalised each domain similarly based on the detailed guide and tool provided. Any disagreements were resolved by discussion or a third reviewer (Reviewer 3).
Statistical analyses
Review Manager 19 software (version 5.4) was used to pool the study results into a standardised mean difference for overall, expressive, and receptive language outcomes when individual study results provided the mean and standard deviation, and it was statistically and clinically appropriate. Both fixed and random effects meta-analyses were performed. A standardised mean difference pooled result was calculated and displayed as a random effects model with a 95% confidence interval since different language outcome tools were used by different studies. Four studies Reference Brosig, Mussatto, Kuhn and Tweddell24,Reference Pizarro, Sood, Kerins, Duncan, Davies and Woodford31,Reference Verrall, Walker and Loughran-Fowlds35,Reference Favilla, Faerber and Hampton37 reported results using the median, interquartile range, or range as described in Table 1. If studies reported critical CHD subgroups without a score for the entire critical CHD cohort, such as children with normal hearing as compared to those with hearing loss, Reference Grasty, Ittenbach and Knightly20 a combined summary statistic was calculated through the formulae provided by the Cochrane Handbook for Systematic Reviews of Interventions. Reference Li, Higgins and Deeks14,21 For studies that compared univentricular to biventricular critical CHD, a subgroup analysis compared language scores. Statistical heterogeneity between studies was measured using the I2 statistic as suggested by the Cochrane Collaboration, in which values of 0–40% may be considered unimportant, 30–60% as moderate, 50–90% as representing substantial heterogeneity, and 75–100% as considerable heterogeneity (overlapping proportions are intentional). Reference Li, Higgins and Deeks14,21 If the fixed and random effects results were similar, the random effects models were reported. Reference Li, Higgins and Deeks14 Publication bias was assessed through visual interpretation of funnel plot symmetry and formally with the Egger test Reference Jin, Zhou and He22 using STATA software 23 where p < 0.05 indicated likely publication bias.
Results
Literature search results
A total of 5001 articles were identified through the initial database literature search, and an additional 268 articles were identified in the follow-up year of 2021. Ultimately, 17 articles (15 observational, 2 randomised) met the inclusion criteria (Fig 1), with thirteen articles published in the last ten years. Reference Grasty, Ittenbach and Knightly20,Reference Noeder, Logan and Struemph25,Reference Hicks, Sauve and Robertson26,Reference Meuwly, Feldmann and Knirsch28,Reference Verrall, Walker and Loughran-Fowlds35–Reference Favilla, Faerber and Hampton37,Reference Graham, Martin and Atz39

Figure 1. PRISMA flow diagram.
Characteristics of the seventeen included studies are provided in Table 1. To summarise, 13 (77%) Reference Grasty, Ittenbach and Knightly20,Reference Brosig, Mussatto, Kuhn and Tweddell24–Reference Acton, Biggs and Creighton27,Reference Gunn, Beca and Hunt29–Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36–Reference Bellinger, Wypij and Kuban38 included language outcomes for preschool children aged 2 to 5 years; six Reference Noeder, Logan and Struemph25,Reference Meuwly, Feldmann and Knirsch28,Reference Fourdain, St-Denis and Harvey33,Reference Verrall, Walker and Loughran-Fowlds35,Reference Favilla, Faerber and Hampton37,Reference Graham, Martin and Atz39 assessed language outcomes at approximately 12 months of age, and two Reference Noeder, Logan and Struemph25,Reference Brosig Soto, Olude and Hoffman34 reported language outcomes at approximately 6 months of age. Thirteen studies (76%) Reference Grasty, Ittenbach and Knightly20,Reference Brosig, Mussatto, Kuhn and Tweddell24–Reference Acton, Biggs and Creighton27,Reference Sood, Benzaquen, Davies, Woodford and Pizarro30–Reference Brosig Soto, Olude and Hoffman34,Reference Favilla, Faerber and Hampton37–Reference Graham, Martin and Atz39 were conducted in North America, two (12%) Reference Gunn, Beca and Hunt29,Reference Verrall, Walker and Loughran-Fowlds35 in Australia, one (6%) Reference Meuwly, Feldmann and Knirsch28 was in Switzerland, and one (8%) Reference Yoshida, Hiraiwa and Ibuki36 in Japan. Study designs included cohort (8; 47%), Reference Noeder, Logan and Struemph25,Reference Hicks, Sauve and Robertson26,Reference Meuwly, Feldmann and Knirsch28,Reference Gunn, Beca and Hunt29,Reference Brosig Soto, Olude and Hoffman34–Reference Favilla, Faerber and Hampton37 prospective case series (4; 24%), Reference Grasty, Ittenbach and Knightly20,Reference Acton, Biggs and Creighton27,Reference Brosig, Mussatto and Hoffman32,Reference Fourdain, St-Denis and Harvey33 cross-sectional (3; 18%), Reference Brosig, Mussatto, Kuhn and Tweddell24,Reference Sood, Benzaquen, Davies, Woodford and Pizarro30,Reference Pizarro, Sood, Kerins, Duncan, Davies and Woodford31 and randomised controlled trial (2; 12%) Reference Bellinger, Wypij and Kuban38,Reference Graham, Martin and Atz39 . Most studies included both cardiac pathologies (13; 76%), Reference Grasty, Ittenbach and Knightly20,Reference Brosig, Mussatto, Kuhn and Tweddell24,Reference Noeder, Logan and Struemph25,Reference Acton, Biggs and Creighton27–Reference Pizarro, Sood, Kerins, Duncan, Davies and Woodford31,Reference Fourdain, St-Denis and Harvey33–Reference Yoshida, Hiraiwa and Ibuki36,Reference Graham, Martin and Atz39 , one study examined only univentricular cardiac physiology (6%), Reference Brosig, Mussatto and Hoffman32 and three examined only biventricular physiology (18%). Reference Hicks, Sauve and Robertson26,Reference Favilla, Faerber and Hampton37,Reference Bellinger, Wypij and Kuban38
Table 1. Description of included studies and language results summary

Bayley-III = Bayley Scales of Infant and Toddler Development, Third Edition; CA = cardiac arrest; CELF-P2 = Clinical Evaluation of Language Fundamentals Preschool-2; CHD = congenital heart disease; EOWPVT = Expressive One-Word Picture Vocabulary Test; EOWPVT (3rd ed.) = Expressive One-Word Picture Vocabulary Test, Third Edition; IP-DHCA = Total body intermittent perfusion-Deep hypothermic circulatory arrest group; IQR = interquartile range; IVS = interventricular septum; LFB = low flow bypass; Mo = months; ROWPVT = Receptive One-Word Picture Vocabulary Test; ROWPVT (2nd ed.) = Receptive One-Word Picture Vocabulary Test, Second Edition; SV = single ventricle; TGA = transposition of the great arteries; U-DHCA = uninterrupted-deep hypothermic circulatory arrest group; VLBW = very low birth weight; VSD = ventricular septal defect; 2V = two ventricle.
*Unpublished data provided by author.
Of the 13 studies focused on preschool children, 13 (76%) Reference Noeder, Logan and Struemph25–Reference Pizarro, Sood, Kerins, Duncan, Davies and Woodford31,Reference Fourdain, St-Denis and Harvey33–Reference Favilla, Faerber and Hampton37,Reference Graham, Martin and Atz39 used the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III), a standardised assessment tool that measures cognitive, motor, and language (expressive and receptive) development of children up to the age of 42 months (general population mean of 100 and a standard deviation of 15; expressive and receptive subscales: mean of 10 and a standard deviation of 3). Reference Bayley40,Reference Bayley41 The other 4 studies completed language assessment using either the Receptive and Expressive One-Word Picture Vocabulary tests (mean of 100, standard deviation of 15), Reference Gardner42–Reference Brownell45 the Preschool Language Scale, Fourth Edition (mean of 100, standard deviation of 15), Reference Zimmerman, Steiner and Pond46 or the Clinical Evaluation of Language Fundamentals Preschool-2 Word Sentence and Sentence Structure Subtests (mean of 10, standard deviation of 3). Reference Wiig, Semel and Secord47
Methodological quality of included studies
Of the 15 observational studies using the Risk Of Bias In Non-randomised Studies of Interventions tool, 7 (47%) Reference Grasty, Ittenbach and Knightly20,Reference Brosig, Mussatto, Kuhn and Tweddell24–Reference Gunn, Beca and Hunt29 studies were at serious risk of bias, and eight (53%) Reference Sood, Benzaquen, Davies, Woodford and Pizarro30–Reference Favilla, Faerber and Hampton37 had a critical risk of bias. Sources of bias included confounding, the selection of study participants, and the measurement of outcome due to lack of blinding. Of the 2 randomised studies that were assessed using the Revised Cochrane Risk-of-Bias tool for randomised trials tool, one study Reference Bellinger, Wypij and Kuban38 had some concerns and the other Reference Graham, Martin and Atz39 was rated as high risk of bias. Sources of bias included the randomisation process and the selection of the reported results. Specific details are provided in Table 2.
Table 2. Methodological quality of included studies
Language outcomes at 2–5 year of age
Thirteen studies examined language outcomes for children aged 2–5 years of age. Reference Grasty, Ittenbach and Knightly20,Reference Brosig, Mussatto, Kuhn and Tweddell24–Reference Acton, Biggs and Creighton27,Reference Gunn, Beca and Hunt29–Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36–Reference Bellinger, Wypij and Kuban38
Expressive language outcomes
Ten studies (77%) Reference Grasty, Ittenbach and Knightly20,Reference Noeder, Logan and Struemph25–Reference Acton, Biggs and Creighton27,Reference Gunn, Beca and Hunt29,Reference Sood, Benzaquen, Davies, Woodford and Pizarro30,Reference Brosig, Mussatto and Hoffman32,Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36,Reference Bellinger, Wypij and Kuban38 reported data on expressive language outcomes that could be used for the meta-analysis. The standardised mean difference for expressive language score was statistically significantly lower for children with critical CHD compared to those without this condition (standardised mean difference: –0.45; 95 % confidence interval: –0.54, –0.37; Fig 2a).
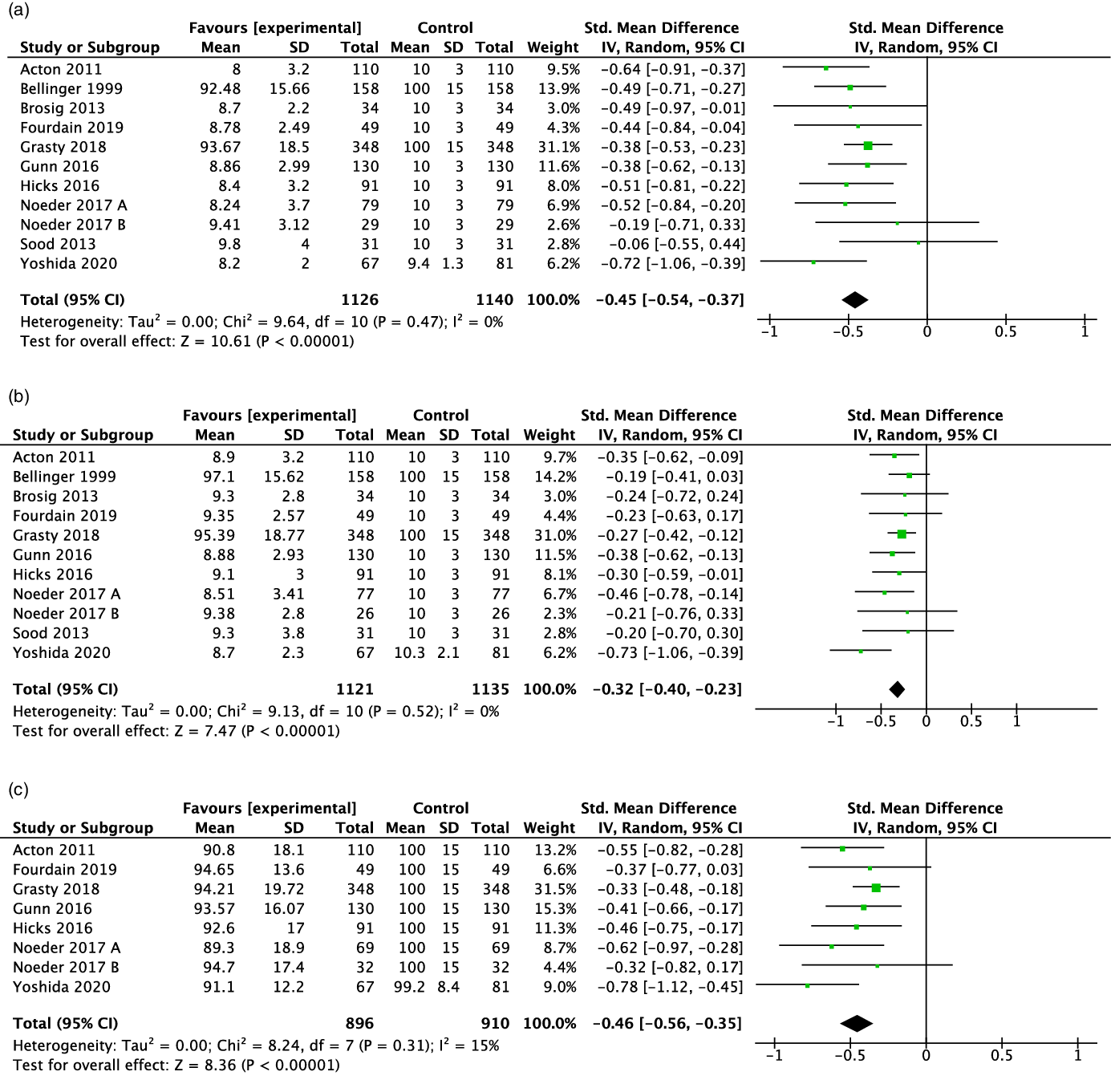
Figure 2. Forest plot and standard mean difference for expressive (2a), receptive (2b) and overall (2c) language scores of children 2–5y with critical congenital heart disease The standard mean difference for each study is represented by a square with confidence interval bars. The size of the box indicates the relative weight of the study, The total meta–analysis result is represented by the diamond. Negative values indicate lower scores for the critical congenital heart disease.
Four studies (31%) Reference Brosig, Mussatto and Hoffman32,Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36,Reference Bellinger, Wypij and Kuban38 reported an expressive language score statistically significantly lower than the normative population data (but still within one standard deviation of the population mean).
Receptive language outcomes
In the meta-analysis, 10 studies (77%) Reference Grasty, Ittenbach and Knightly20,Reference Noeder, Logan and Struemph25–Reference Acton, Biggs and Creighton27,Reference Gunn, Beca and Hunt29,Reference Sood, Benzaquen, Davies, Woodford and Pizarro30,Reference Brosig, Mussatto and Hoffman32,Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36,Reference Bellinger, Wypij and Kuban38 reported receptive language outcomes that could be pooled. The standardised mean difference for receptive language was statistically significantly lower for children with critical CHD compared to those without this condition (standardised mean difference: –0.32; 95 % confidence interval: –0.40, –0.23; Fig 2b).
Three studies (23%) Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36,Reference Bellinger, Wypij and Kuban38 provided a receptive language outcome score statistically significantly lower than the normative population data and within one standard deviation of the population mean.
Overall language outcomes
The 7 studies (78%) Reference Grasty, Ittenbach and Knightly20,Reference Noeder, Logan and Struemph25–Reference Acton, Biggs and Creighton27,Reference Gunn, Beca and Hunt29,Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36 with data available for overall language resulted in a pooled estimate that showed that the overall language standardised mean difference was statistically significantly lower for children with than without critical CHD (standardised mean difference: –0.46; 95 % confidence interval: –0.56, –0.35; Fig 2c).
Nine studies Reference Grasty, Ittenbach and Knightly20,Reference Noeder, Logan and Struemph25–Reference Acton, Biggs and Creighton27,Reference Gunn, Beca and Hunt29,Reference Pizarro, Sood, Kerins, Duncan, Davies and Woodford31,Reference Fourdain, St-Denis and Harvey33,Reference Yoshida, Hiraiwa and Ibuki36,Reference Favilla, Faerber and Hampton37 reported an overall language score: 2 (22%) Reference Gunn, Beca and Hunt29,Reference Yoshida, Hiraiwa and Ibuki36 were statistically significantly lower than the normative mean (but still within one standard deviation of the general population mean).
Subgroup analysis based on cardiac physiology
Only 4 studies (24%) Reference Brosig, Mussatto, Kuhn and Tweddell24,Reference Acton, Biggs and Creighton27,Reference Gunn, Beca and Hunt29,Reference Yoshida, Hiraiwa and Ibuki36 described language outcomes for children with univentricular as compared to biventricular cardiac physiologies. The I2 statistic assessing statistical heterogeneity within the 2 studies was substantial (I2 = 64%) for expressive language scores, precluding a formal meta-analysis. Reference Li, Higgins and Deeks14 Two studies reported significantly lower overall language scores for children with univentricular critical CHD as compared to biventricular physiology. Reference Gunn, Beca and Hunt29,Reference Sood, Benzaquen, Davies, Woodford and Pizarro30,Reference Yoshida, Hiraiwa and Ibuki36 One study Reference Brosig, Mussatto, Kuhn and Tweddell24 found expressive language values to be significantly lower for children with univentricular versus biventricular physiology. Two studies Reference Brosig, Mussatto, Kuhn and Tweddell24,Reference Yoshida, Hiraiwa and Ibuki36 reported significantly lower receptive language values for univentricular physiology as compared to biventricular physiology.
Language outcomes at 12 months
The I2 statistic assessing statistical heterogeneity for all language outcomes was significant, therefore, precluding a meta-analysis. However, all 6 studies Reference Noeder, Logan and Struemph25,Reference Meuwly, Feldmann and Knirsch28,Reference Fourdain, St-Denis and Harvey33,Reference Verrall, Walker and Loughran-Fowlds35,Reference Favilla, Faerber and Hampton37,Reference Graham, Martin and Atz39 reported overall, expressive, and receptive language scores on the Bayley-III language tool within one standard deviation of the normative mean. One study Reference Fourdain, St-Denis and Harvey33 reported statistically significantly lower expressive and receptive language scores for children with critical CHD when compared to the general population mean. Overall language was not determined to be statistically significantly different in any study. Reference Noeder, Logan and Struemph25,Reference Meuwly, Feldmann and Knirsch28,Reference Fourdain, St-Denis and Harvey33,Reference Favilla, Faerber and Hampton37,Reference Graham, Martin and Atz39
Language outcomes at 6 months
The I2 statistic assessing statistical heterogeneity for all language outcomes was significant; therefore, a meta-analysis was not performed. Both studies Reference Noeder, Logan and Struemph25,Reference Brosig Soto, Olude and Hoffman34 reported expressive and receptive language scores below the mean. Brosig Soto et al (2011) Reference Brosig Soto, Olude and Hoffman34 reported a language outcome significantly lower than the normative population mean at 96.8 (SD: 12.7, p = .005), while Noeder et al (2017) Reference Noeder, Logan and Struemph25 reported a language score greater than one standard deviation below the population mean at 84.1 (SD: 15.0).
Publication bias
Publication bias could only be assessed for language outcomes for children 2 to 5 years of age. Funnel plots showed little evidence of publication bias for overall (p = 0.14), expressive (p = 0.89), and receptive (p = 0.62) language outcomes.
Discussion
Findings of this systematic review and meta-analysis indicate that preschool children with critical CHD have statistically significantly lower expressive, receptive, and overall language abilities when compared to their peers and that they struggle more with expressive than with receptive language skills. Additionally, although statistical heterogeneity precluded determining a pooled overall effect to quantify the difference, children with univentricular physiology appear to have higher rates of language delay than children with biventricular physiology. The importance of studying language outcomes relies on the significant role language plays in a child’s development; language is essential for communication and is a key component of academic functioning. Even modest deficits in language abilities are known to significantly impact a child’s day-to-day function, communication, and to negatively influence social interaction. Reference van Agt, Essink-Bot, van der Stege, de Ridder Sluiter and de Koning48
The results of this study are consistent with previous reports and recommendations. The Cardiac Neurodevelopmental Outcome Collaborative indicates expressive language delays are a common concern for children with critical CHD and should be monitored. Reference Ware, Butcher and Latal49 The guidelines developed by American Heart Association also highlight the need to assess language development and to refer to speech-language pathology when language deficits are identified. Reference Ware, Butcher and Latal49,Reference Marino, Lipkin and Newburger50 Findings that children with univentricular critical CHD struggle more in certain areas of neurodevelopment than children with biventricular critical CHD have been previously reported and determined to be statistically significant . Reference Puosi, Korkman and Sarajuuri51–Reference Huisenga, La Bastide-Van Gemert and Van Bergen54
This review found similar findings to those of studies looking at the longer-term language outcomes of school-aged children with critical CHD. In an article by Bellinger et al (2003), Reference Bellinger, Wypij and duPlessis55 school-aged children within the critical CHD cohort were found to have significantly lower scores than the expected mean. Similarly, in an article by Mahle et al (2000), Reference Mahle, Clancy, Moss, Gerdes, Jobes and Wernovsky56 children with hypoplastic left heart syndrome at school age had expressive and receptive skills that were statistically significant and below the norm. In a study by Hövels-Gürich (2006), Reference Hovels-Gurich, Konrad and Skorzenski57 expressive and receptive language values in children at school age were found to be within one standard deviation of the normative mean within the cohort studied and were statistically significant findings as compared to the normative population mean. In a second study by Hövels-Gürich (2008), Reference Hovels-Gurich, Bauer and Schnitker58 school-aged children were more impaired in expressive language testing than overall or receptive language testing. Such studies then highlight the importance of long-term follow-up as children with critical CHD appear to continue to be at high risk for language delays (and particularly expressive language delays). A lack of significant language difference found at 12 months compared to those differences found at 2–5 years suggests a need for continuous follow-up that is supported by the literature. Reference Marino, Lipkin and Newburger50
The results of this language-focused systematic review are also consistent with reviews of studies in motor and cognitive neurodevelopmental delay in children with critical CHD. Reference Bolduc, Dionne, Gagnon, Rennick, Majnemer and Brossard-Racine7–Reference Sterken, Lemiere, Van den Berghe and Mesotten9 Reviews of both motor and cognitive abilities of preschool children with critical CHD have shown that children with this condition score significantly lower than their non-critical CHD peers. Reference Bolduc, Dionne, Gagnon, Rennick, Majnemer and Brossard-Racine7–Reference Sterken, Lemiere, Van den Berghe and Mesotten9 Proposed explanations of such delays in multiple developmental domains include chronic brain hypoxia, increased incidences of pre- and post-natal brain injury, brain immaturity, and other clinical and environmental factors. Reference Morton, Ishibashi and Jonas59–Reference Limperopoulos, Majnemer and Shevell63
Importantly, some of the literature suggests the Bayley-III overestimates language ability for both healthy developing and children with critical CHD; Reference Acton, Biggs and Creighton27,Reference Anderson, De Luca, Hutchinson, Roberts and Doyle64–Reference Goldstone, Baiocchi and Wypij66 which could mean that children with critical CHD have even worse language skills than this systematic review and meta-analysis reports. Anderson et al (2017) Reference Anderson and Burnett65 found an increase in language scores on the most recent edition of the Bayley-III compared to the Bayley-II. Moreover, Goldstone et al (2020) Reference Goldstone, Baiocchi and Wypij66 found this increase to be significant in children with critical CHD. Notably, our review included publications that used other language assessment tools and determined that those findings were consistent with the results of studies that utilised the Bayley-III. This consistency suggests that the language abilities of preschool children with critical CHD are typically below average.
Limitations
There are several limitations to our systematic review and meta-analysis. First, the methodological quality of the included studies was rated quite poorly. This rating is unsurprising given that the detailed guide of the Risk Of Bias In Non-randomised Studies of Interventions tool indicates that “…it will be rare that an NRSI [non-randomised studies of the effects of interventions] is judged as at low risk of bias due to confounding, we anticipate that most NRSI will be judged as at least at moderate overall risk of bias.” Reference Sterne, Higgins, Elbers and Reeves18 Often, the overall risk of bias was rated as serious or critical and was typically due to the first domain regarding confounders, missing statements of possible confounders, selection of participants, and lack of blinding. However, the results of the two randomised controlled trials that focused on 2 to 5 year-old-children, which should have a balance of known and unknown confounders between the 2 groups, were similar to the non-randomised controlled trial results. The expressive and receptive scores led to the consistent conclusion that children with critical CHD at 2–5 years of age are below the normative means. While many studies were classified at critical risk, basing the use of articles solely on the quality assessment of studies that are not amenable to randomised control trials may prevent the inclusion of critical results in many different areas of research. Reference Thomson, Craig, Hilton-Boon, Campbell and Katikireddi67
Comparing outcomes to normative population data without further adjustments may introduce bias into the results as any difference found between children with critical CHD and the normative population may seem to be causal, when in reality differences such as socioeconomic status, support interventions, or other differences may have large effects in children with critical CHD that may not be accounted for.
Finally, only two studies Reference Acton, Biggs and Creighton27,Reference Yoshida, Hiraiwa and Ibuki36 reported language outcome data comparing univentricular and biventricular cardiac physiologies. Although heterogeneity precluded pooling the individual results, reports of lower language scores and other neurodevelopmental domains support the findings of our review. Reference Puosi, Korkman and Sarajuuri51,Reference Reich, Heye and Tuura52,Reference Huisenga, La Bastide-Van Gemert and Van Bergen54 Likewise, although the presence of a genetic anomaly among children with critical CHD is known to impact developmental outcomes, this review was unable to examine language outcomes based on the presence or absence of genetic anomalies as the included studies did not consistently stratify their results by this variable. Future research to determine the exact clinical and statistical significance of differences in language outcomes between these two cardiac physiologies and the stratification of language abilities in children with additional genetic anomalies is recommended.
Conclusion
This systematic review and meta-analysis is the first to review and assess the results from the literature on overall, expressive, and receptive language abilities in preschool children with critical CHD. The findings indicate that preschool children with critical CHD have significantly lower language abilities when compared to the general population and may be more affected in the expressive language domain than in their receptive language skills. Future research should focus on determining language outcomes among older children with critical CHD as well as on testing interventions to improve language skills in this population.
Acknowledgements
Thank you to the medical librarian, Janet Rothney, for her assistance in the search strategy, the Excellence in Neurodevelopmental Rehabilitation Research in Child Health group for their trainee programme feedback and to Advanced Degrees in Medicine University of Manitoba for the opportunity to complete this review as a Bachelor of Science in Medicine degree Project.
Financial support
Ms. Turner was supported by the Bachelor of Science in Medicine degree programme.
Conflict of interest
None.







