Vitamin D is a fat-soluble vitamin responsible for enhancing gastrointestinal absorption of Ca, Mg, phosphate and Zn( Reference Holick 1 ). It has an important role in bone mineralization, as it increases the absorption of Ca in the small intestine, promotes osteoblastic activity and maintains serum concentration of Ca and P in the normal range( Reference Holick 2 ). Vitamin D may prevent insulin-dependent diabetes mellitus, hypertension and cancer( Reference Holick 1 ). Vitamin D deficiency results in rickets, osteomalacia and low bone density( Reference Rabbani, Alavian and Motlagh 3 ).
Vitamin D status varies widely between different countries in Europe, Asia and the Middle East( Reference Lips 4 ). It may be caused by differences in the amount of sunlight exposure, intake of dietary vitamin D and use of supplementary vitamin D( Reference Holick, Binkley and Bischoff-Ferrari 5 ). Vitamin D deficiency is very prevalent in children in Australia, the Middle East, Africa, India and South America( Reference Holick 2 , Reference Marwaha, Tandon and Reddy 6 , Reference Thacher, Fischer and Strand 7 ). In Iran, in spite of using supplementary vitamin D in breast-fed infants, vitamin D deficiency is common among children( Reference Neyestani, Hajifaraji and Omidvar 8 ). Prevalence of vitamin D deficiency in children under 12 years of age was reported as 38·3–46·0 % in the northern and central part of Iran in 2008( Reference Rabbani, Alavian and Motlagh 3 , Reference Neyestani, Hajifaraji and Omidvar 8 , Reference Ardestani, Salek and Keshteli 9 ), but no data are available about the prevalence of vitamin D deficiency in Iranian teenagers or in the south of Iran. Serum 25-hydroxyvitamin D (25(OH)D) concentration depends on many personal and environmental factors( Reference Willis, Laing and Hall 10 ). Understanding these factors helps to predict vitamin D deficiency in children. Most studies have studied only a few of these factors, such as sun exposure( Reference Rabbani, Alavian and Motlagh 3 , Reference Lips 4 , Reference Thacher, Fischer and Strand 7 , Reference Zhu, Zhan and Shao 11 , Reference Gordon, DePeter and Feldman 12 ). There is some confusion in the existing literature regarding the correlation between vitamin D deficiency and pubertal stage or BMI( Reference Zhu, Zhan and Shao 11 – Reference Fuleihan, Nabulsi and Choucair 18 ) and there are also limited data on the correlation of body composition (fat or lean mass) with vitamin D status( Reference Willis, Laing and Hall 10 ).
The present study was conducted to bridge the existing gap in knowledge on vitamin D status and Ca metabolism in children and teenagers living in southern Iran, the controversies about the association of vitamin D status with its various determinants and the lack of data about the associations of vitamin D status with body composition over a wide range of ages in children.
Materials and methods
To evaluate vitamin D status in Fars Province, we performed a cross-sectional study on healthy Iranian children aged 9–18 years in Kawar, an urban area located 50 km east of Shiraz, the capital city of Fars Province, in the autumn and winter of 2011. An age-stratified, randomly selected sample was drawn of 7·5 % of pupils from all the elementary, secondary and other schools in the area, to provide a representative group of 477 participants for this investigation. Children diagnosed with bone disease, disorders of Ca metabolism, malabsorptive disorders and chronic granulomatous disease, as well as those using certain medications (such as anticonvulsants or medically prescribed vitamin D preparations), were excluded from the study.
The Ethics Committee of Shiraz University of Medical Sciences approved our study. All parents and children who participated in our study provided signed informed consent.
Anthropometric measurements and pubertal assessment
A trained general physician performed the physical examination of children, including weight, height and pubertal stage. Weight was measured with a standard scale (Seca, Germany) while the children were wearing light clothing and without shoes, and rounded to the nearest 0·1 kg. Height was measured with a wall-mounted stadiometer on barefoot children and rounded to the nearest 0·5 cm. BMI was calculated by the standard method (weight/height2, kg/m2). Pubertal stage was determined according to Tanner’s five-stage classification( Reference Tanner and Whitehouse 19 ). BMI Z-score was calculated using the growth charts of the Centers for Disease Control and Prevention (www.cdc.gov/growthcharts).
Assessment of physical activity and sun exposure
Physical activity on at least 3 d/week is recommended for children and adolescents by the American College of Sports Medicine( Reference Pate, Pratt and Blair 20 ). Children and their parents were asked about the number of days per week they undertook physical activity (walking, recreational activity or sports) and placed in groups with acceptable or unacceptable physical activity (≥3 or <3 d/week). Children and their parents were also asked about their average exposure to sunshine per day and classified into three groups (those exposed for <15 min/d, 15–30 min/d and >30 min/d).
Body composition measurements
Total fat mass (grams), total lean mass (grams) and total body fat percentage were determined by dual-energy X-ray absorptiometry (Hologic Discovery QDR instrument, USA). Fat mass index was calculated as fat mass/height2 (kg/m2) and lean mass index as lean mass/height2 (kg/m2). Assessments were performed with the children wearing standardized clothing and without shoes. The CV in our laboratory was 0·7 % for fat mass and 1·9 % for lean mass and fat percentage.
Biochemical variables
A 5 ml venous blood sample was drawn from all participants in the hormonal research laboratory of Shiraz University of Medical Sciences. All samples were centrifuged, separated, and serum and plasma stored at −20°C until analysis. Serum 25(OH)D was assayed by HPLC (Young Lin 9100 system, South Korea; inter- and intra-assay CV 3·3 % and 5·1 %, respectively) according to current Endocrine Society clinical practice guidelines for the evaluation, treatment and prevention of vitamin D deficiency (vitamin D deficiency was defined as serum 25(OH)D <20 ng/ml (<50 nmol/l); serum 25(OH)D values between 21 and 29 ng/ml were defined as vitamin D insufficiency( Reference Holick, Binkley and Bischoff-Ferrari 5 )). Serum Ca, P and alkaline phosphatase were measured by colorimetric assays using an auto-analyser (Biosystems SA, Spain). Normal range for serum corrected Ca was 8·5–10·5 mg/dl, and for P was 3·7–5·4 mg/dl for children <16 years old and 2·5–4·5 mg/dl for those aged ≥16 years.
Statistical analysis
Data are presented as means and standard deviations. Normality of data distribution was evaluated with the Kolmogorov–Smirnov test. Student’s t test was used for the comparison of normally distributed data and the Mann–Whitney test for comparison of non-normally distributed data. Correlations between normally distributed parameters were determined using Pearson’s test and Spearman’s ranking test for non-normally distributed data. Comparison of qualitative data was carried out using the χ 2 test. Variables with significant correlations with serum concentration of 25(OH)D in univariate analysis were identified. Multivariate binary logistic regression analysis was then performed using these variables to assess their independent predictive effect on serum 25(OH)D concentration; collinearity was assessed by variance inflation factor, with a variance inflation factor value <5 considered as non-collinearity. The linear regression analyses used data that were normally distributed (assessed by Q–Q plots), or data normalized before use, with homogeneity of variance and reported independence of predictive effects. P<0·05 was considered significant. Statistical analysis was carried out using the statistical software package IBM SPSS Statistics 18·0.
Results
Four hundred and seventy-seven children aged 9–18 years were included in the present study; 49 % of them were female (236 girls and 241 boys). General characteristics and a summary of serum biochemical variables are shown in Table 1. Boys were heavier and taller, and had more sun exposure than girls (P=0·009, P<0·001 and P<0·001, respectively).
Table 1 General characteristics of the studied population: Iranian children aged 9–18 years, Fars Province, autumn and winter of 2011

ALP, alkaline phosphatase; 25(OH)D, 25-hydroxyvitamin D.
* P value refers to the comparison of each variable between sexes.
Vitamin D status and body composition
Mean serum 25(OH)D concentration of the children in autumn and winter was 15·2 (sd 5·6) ng/ml. Four per cent of the children had normal vitamin D status (serum 25(OH)D >30 ng/ml), 15 % had 25(OH)D insufficiency (serum 25(OH)D=20–30 ng/ml), 82 % of the children were vitamin D deficient (serum 25(OH)D <20 ng/ml) and 13 % had severe vitamin D deficiency (serum 25(OH)D <10 ng/ml; see Table 2). Mean total body fat percentage was 22·6 (sd 8·1), mean fat mass index was 4·2 (sd 2·9) kg/m2 and mean lean mass index was 14·4 (sd 10·6) kg/m2 in the study population (see Table 3).
Table 2 Vitamin D status (based on serum 25(OH)D) in the studied populationFootnote *: Iranian children aged 9–18 years, Fars Province, autumn and winter of 2011
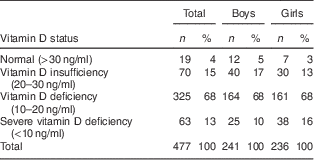
25(OH)D, 25-hydroxyvitamin D.
* Vitamin D status was not dependent on sex (P=0·145).
Table 3 Body composition of the studied population: Iranian children aged 9–18 years, Fars Province, autumn and winter of 2011

* P value refers to the comparison of each variable between sexes.
Factors associated with vitamin D status
Vitamin D status was associated with BMI (r=−0·10, P=0·02), pubertal status (r=−0·08, P=0·04) and sun exposure (r=0·10, P=0·04). Fat mass index was associated with 25(OH)D concentration (r=−0·13, P=0·03), but lean mass index showed no association with 25(OH)D concentration (P=0·86).
There was no significant difference in 25(OH)D concentration between boys and girls (P=0·3). Vitamin D status was not associated with age (r=−0·07, P=0·15) or physical activity (r=0·10, P=0·08). On multiple regression analysis, there were independent predictive effects of age, sun exposure, physical activity, pubertal state and fat mass index on 25(OH)D concentration (Table 4). All variance inflation factor values were ≤5 (range: 1·05–3·19). Age remained associated with 25(OH)D concentration after adjustment for sun exposure, physical activity, pubertal state and fat mass index (P=0·008). The association between puberty and 25(OH)D concentration persisted (P=0·006) after adjustment for age, physical activity, sun exposure and fat mass index, but the association between BMI and fat mass index and 25(OH)D concentration was abolished by adjustment for age, sun exposure, physical activity and puberty (P=0·06 and P=0·14, respectively). The association between exercise and 25(OH)D concentration remained significant after adjustment for BMI (P=0·01) and fat mass index (P=0·02).
Table 4 Factors associated with vitamin D status in the studied population: Iranian children aged 9–18 years, Fars Province, autumn and winter of 2011
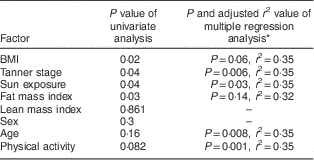
* All factors with P<0·2 in univariate analysis were included in multiple regression analysis (mode: Enter).
Discussion
Vitamin D status
The present study showed a remarkably high prevalence of vitamin D deficiency in children aged 9–18 years in southern Iran (83 % in boys and 86 % in girls). 25(OH)D concentration was independently associated with age, exercise and sun exposure, and with pubertal status after adjustment for age, physical activity, sun exposure and fat mass index.
To date, three cross-sectional studies have been conducted to evaluate the prevalence of vitamin D deficiency in the northern (Tehran) and central (Isfahan) parts of Iran( Reference Rabbani, Alavian and Motlagh 3 , Reference Neyestani, Hajifaraji and Omidvar 8 , Reference Ardestani, Salek and Keshteli 9 ). One showed an 86 % prevalence of vitamin D deficiency in Tehran in children aged 9–12 years( Reference Neyestani, Hajifaraji and Omidvar 8 ); another study in children aged 7–12 years in Tehran showed that 53·6 % of girls and 11·3 % of boys had serum 25(OH)D <20 ng/ml( Reference Rabbani, Alavian and Motlagh 3 ); and in children aged 6–7 years in Isfahan, the prevalence of serum 25(OH)D <20 ng/ml was 5 % in girls and 10 % in boys( Reference Ardestani, Salek and Keshteli 9 ).
The present study appears to be the first to have assessed vitamin D status in children aged 9–18 years in Fars Province, in the south of Iran, and shows a high prevalence of vitamin D deficiency in southern Iran, at 84 %. Differences in age of participants, in season of sampling for serum 25(OH)D and in methods used to measure serum 25(OH)D concentration (RIA v. HPLC) may explain the difference in findings between the present and previous studies in Iran. However, most of the reported data has indicated a high prevalence of vitamin D deficiency in Iran, which needs to be recognized and corrected, probably through suitable health ministry programmes.
Factors associated with serum 25-hydroxyvitamin D concentrations
Children’s age
Our study showed that children’s age was inversely related to serum 25(OH)D concentration after adjustment for physical activity, pubertal status, sun exposure and fat mass index, an association not previously recognized during childhood and adolescence. Previous reports studied children within a narrow age range, such as adolescents or toddlers, limiting the ability of such studies to reveal any negative effect of age( Reference Willis, Laing and Hall 10 , Reference Lee, Kim and Hong 21 – Reference Shin, Shin and Lee 26 ). Some reports in Korea and Europe have also shown that younger adolescents had higher serum 25(OH)D concentrations than older ones( Reference Holick, Binkley and Bischoff-Ferrari 5 , Reference Lee, Kim and Hong 21 – Reference Shin, Shin and Lee 26 ), while Willis et al. showed that age was negatively associated with 25(OH)D concentration in pre-pubertal females, although this was abolished by controlling for fat-free soft tissue mass( Reference Willis, Laing and Hall 10 ). The only study on a wider range of younger ages was that of Weng et al. in the USA( Reference Weng, Shults and Leonard 27 ), who found an inverse relationship between 25(OH)D concentration and age in American children and adolescents after adjustment for confounding factors such as puberty and vitamin D intake. However, they did not evaluate sun exposure or outdoor activity although they did suggest these should be evaluated in future studies( Reference Weng, Shults and Leonard 27 ), a suggestion supported by the fact that younger children tend to have more physical activity, spend more time outdoors (school and playground) and have a healthier diet (enriched by dairy foods under parental control) than older children( Reference Choi, Oh and Choi 22 , Reference Ginde, Liu and Camargo 23 ). Indeed, we showed that the inverse relationship between age and vitamin D status persists after adjusting for sun exposure and physical activity, while further work is needed to explain the reasons for decreases in serum 25(OH)D in older children, since this appears to be independent of the effects of physical activity, pubertal status, sun exposure or obesity.
Sun exposure
Shiraz is located at a latitude of 29·6°N, longitude of 52·5°E and 1506 m elevation above sea level, with relatively good sun exposure, and our study revealed that sun exposure was positively associated with serum 25(OH)D concentration (P=0·04). Exposure of skin to sunlight is a major source of vitamin D for man( Reference Holick, Binkley and Bischoff-Ferrari 5 ); although it is reduced by a variety of factors including increased skin pigmentation, topical usage of sunscreen and changes in latitude, not all these could be adjusted for in the present study( Reference Holick 2 , Reference Holick, Binkley and Bischoff-Ferrari 5 ). Differences in housing may be another factor influencing serum 25(OH)D concentration. Some children may have a yard or garden attached to the house where they may privately remove some of their outer clothes and get more sun( Reference Kumar, Muntner and Kaskel 24 ).
BMI and body composition
Our study showed that BMI and fat mass index had an inverse association with serum 25(OH)D concentration, but not lean mass index. Many studies have shown a negative correlation between BMI and vitamin D status( Reference Kumar, Muntner and Kaskel 24 – Reference Jeong 29 ); however, there is little information on the association between fat mass index and 25(OH)D concentration, which a previous study showed to be more consistent in its complications than BMI( Reference Horan, Gibney and Molloy 30 ). Fat mass index has also been shown to be associated with vitamin D status in 10-year-old urban South African children( Reference Poopedi, Norris and Pettifor 31 ), but in a narrow age range limited to pre-pubertal children. As originally suggested, vitamin D may well be sequestered in fat stores, reducing its bioavailability( Reference Wortsman, Matsuoka and Chen 32 ), as supported by the findings of large-scale Mendelian randomization studies( Reference Vimaleswaran, Berry and Lu 33 ). A further factor causing reduced 25(OH)D concentrations with increased fat mass could be the fact that increased leptin released from excess body fat can inhibit renal activation of vitamin D( Reference Tsuji, Maeda and Kawane 34 ). Some studies also suggest reverse confounding due to reduced outdoor activity in obesity and to lower dietary vitamin D intakes in obese children who are dieting to lose weight, which also reduces 25(OH)D values( Reference Harel, Flanagan and Forcier 35 , Reference Dong, Pollock and Stallmann-Jorgensen 36 ); and indeed our study does show that the association of BMI or fat mass index with 25(OH)D concentration disappears after adjustment for factors such as age, puberty, physical activity and sun exposure. Consistent with our results, a US study using multivariable models showed that neither lean mass nor fat mass index was associated with vitamin D status( Reference Weng, Shults and Leonard 27 ), a finding that may be due to the use of appropriate adjustments of the analyses for the confounding effects of age and puberty since prior studies reporting associations between fat mass and hypovitaminosis D may have been confounded by the effects of age, puberty and sunlight, that were not allowed for.
Puberty
Our study revealed a negative correlation between Tanner stage of puberty and 25(OH)D concentration in both boys and girls, which persisted after adjusting for age, sun exposure, physical activity and fat mass index, while some previous studies showed that serum 25(OH)D concentration decreased with increasing Tanner stage in boys (but not in girls), with the greatest proportion of vitamin D insufficiency being found during Tanner stages 4–5( Reference Ginty, Cavadini and Michaud 17 ). As in our results, some other studies have shown negative associations between pubertal status and serum 25(OH)D concentration in both sexes( Reference Aypak, Türedi and Yüce 15 , Reference Fuleihan, Nabulsi and Choucair 18 , Reference Guillemant, Cabrol and Allemandou 37 , Reference Cashman 38 ), although increasing age, lesser outdoor sport and greater demands on vitamin D stores for skeletal mineralization could contribute to this finding( Reference Ginty, Cavadini and Michaud 17 , Reference Cashman 39 ). It is necessary to consider pubertal stage as it is associated with a remarkable change in the lifestyle of children in terms of daily sun exposure and outdoor physical activity; clothing and percentage of body surface area exposed may contribute significantly to change in 25(OH)D concentration in this stage( Reference Gannagé-Yared, Chemali and Yaacoub 40 ).
Exercise
The present study showed that exercise and sun exposure have a positive association with vitamin D status after adjusting the analysis for BMI or fat mass index, as previously suggested by a study in Saudi adolescents that showed physical activity was directly associated with 25(OH)D concentration, independent of sunshine exposure( Reference Al-Musharaf, Al-Othman and Al-Daghri 41 ). Physical activity was shown to be associated with improved local bone mass, decreased Ca excretion and increased Ca absorption in a further study, while exercise could reduce body weight and BMI, thereby reducing vitamin D sequestration into fatty tissues allowing higher serum 25(OH)D values( Reference Michael, Smit and Seguin 42 ).
Study limitations
Our study was cross-sectional in nature and the various vitamin D–related genetic factor axes (known to contribute independently to growth and probably also to pubertal status) were not examined, so that these are possible relevant factors. Future studies in this area should therefore ensure that changes in vitamin D status during childhood and adolescence can be adjusted for the effects of all the other relevant factors, such as age, clothing, vitamin D–gene variant axes and leptin secretion.
Conclusion
The prevalence of vitamin D deficiency was 82 % among children in the south of Iran and 25(OH)D concentration was related to sun exposure, physical activity, age and pubertal stage of the children. Health improvement strategies should be developed to prevent vitamin D deficiency during this critical period, although further studies are needed to determine the pathophysiology of the decrease in serum 25(OH)D concentration seen during and after puberty, since it appears to be independent of the effects of physical activity, pubertal status, fat mass index or exposure to sunshine.
Acknowledgements
Acknowledgements: The authors would like to thank Dr Nasrin Shokrpour for editorial assistance and Mrs Sareh Roosta for statistical analysis at Center for Development of Clinical Research of Nemazee Hospital. Financial support: This work was supported by Shiraz University of Medical Sciences (grant number 89–5127). The funder had no role in the design, analysis or writing of this article. Shiraz University of Medical Sciences funded our research just as a thesis for graduate students. Conflict of interest: None. Authorship: F.S. formulated the research questions, designed the study, analysed the data and wrote the article. M.H.D. formulated the research questions, designed the study and analysed data. G.R.O. carried out the study. M.B. formulated the research question. Ethics of human subject participation: The study was approved by the Ethics Committee of Shiraz University of Medical Sciences. All parents and children who participated provided signed informed consent.







