INTRODUCTION
Globally, enteric infections rank third in all causes of infectious disease burden, being responsible for 1·7–2·5 million deaths per year, mostly in infants and young children in developing countries [Reference Girard1, Reference Rudan2]. Theoretically a number of time-proven intervention strategies, e.g. hand washing, provision of safe drinking water and sanitation are known to reduce morbidity caused by acute diarrhoea. However, the implementation of these tools in resource-poor settings continues to be a formidable challenge. Therefore, there is a need for the implementation of alternative strategies for prevention of acute diarrhoea, and strategies including the prophylactic use of zinc or probiotics are being considered [Reference Bhandari3, Reference Sazawal4].
Probiotics are commensal organisms that exert their effects by positively influencing normal microbe–microbe and host–microbe interactions and may augment the protection afforded by commensal flora through competitive interactions, direct anatagonism of pathogens, and/or production of antimicrobial factors [Reference Shanahan5]. The Food and Agriculture Organisation and World Health Organisation (WHO) have jointly proposed a new definition that describes probiotics as ‘microorganisms that exhibit beneficial health effects for hosts when a sufficient amount of them are ingested’ [Reference Reid6]. A meta-analysis of masked, randomized, placebo-controlled trials (of which only one was community-based in developing country) has shown probiotics to significantly reduce antibiotic-associated diarrhoea by 52% [95% confidence interval (CI) 35–65], reduce the risk of travellers' diarrhoea by 8% (95% CI 6–21) and that of acute diarrhoea of diverse causes by 34% (95% CI 8–53) [Reference Sazawal4]. Although there is some suggestion that probiotics may be efficacious in preventing acute diarrhoea, there is lack of sufficient data from community-based trials, especially from developing countries, to assess the effect on acute diarrhoea unrelated to antibiotic usage [Reference Sazawal4]. Therefore we conducted a double-blind, randomized, controlled field trial to examine whether the daily intake of a probiotic drink, containing 6·5 billion probiotic Lactobacillus casei strain Shirota (LcS), has a beneficial role in protecting children from acute diarrhoea in an urban slum community in Kolkata, India and to examine whether the preventive role, if any, is associated with specific aetiological agents of diarrhoea. We also attempted to study the impact of the probiotic on the nutritional status of the children.
METHODS
Study design
The study was a double-blind, randomized, controlled field trial involving ~4000 children aged between 1 and 5 years in an impoverished urban slum of Kolkata, India. Eligible children (aged 1–5 years with verbal consent of parents for participation of their children in the study) were enrolled through a demographic census conducted at the initiation of the study. The census was used to define 100 contiguous geographical clusters that served as the unit of randomization. About 40 children were included in each cluster. The clusters were randomized in a 1:1 ratio of study and control arms. Four codes were used (two codes for each agent) to strengthen the blinding effect for cluster randomization. All eligible children in each cluster received the same agent code.
STUDY METHODOLOGY
The whole slum area of ward 66 in Kolkata Municipal Corporation with ~60 000 individuals (~15 000 families) was selected to reach a sample size of 4000 children aged 1–5 years. Initial demographic census was conducted in the study area by community health workers (CHWs) with a pre-tested questionnaire supervised by field supervisors. Census data entry and cleaning was carried out in real time. A unique number was assigned to each child and individual identity cards were provided to them. Five health outposts were set up for each 12 000 population for case management and follow-up. Each health outpost was manned by medical officers, sample collectors, field supervisors and CHWs for regular surveillance activities. There were 100 CHWs recruited for the study and each was responsible for administering one agent code to a cluster of ~40 study children daily. Each day, in the morning, four different agent codes were delivered to the health outposts in carrier boxes each containing ~40 bottles of one code with an ice pack for maintaining the temperature at 4–10°C. Each CHW assigned to a cluster handled only one code during the entire period of administration of the drinks. CHWs visited all study subjects in their cluster each day and administered the drink (probiotic or nutrient) under direct supervision. All empty bottles were collected by CHWs after consumption of the drink and crushed with compression equipment. It was possible for each CHW to administer drinks to 40 children each day within 4–6 h after leaving each health outposts.
Diarrhoeal surveillance was conducted by CHWs during their daily household visits for 24 weeks (12 weeks intake and 12 weeks follow-up). Children with diarrhoea were referred to the nearest health outpost for management by medical officers who also completed a case report form which included details of the diarrhoeal episode. Diarrhoea was defined as ⩾3 abnormally loose or liquid stools within the last 24-h period. If there were at least three diarrhoea-free days between a first and a second episode of diarrhoea in the same individual, the second diarrhoea episode was considered as a new one.
Baseline anthropometric measurements which included height, weight and mid-upper-arm circumference (MUAC) of the study children were taken by CHWs at the same time the first drink was administered. The second round of anthropometric measurements started after completion of probiotic intake at the end of 12 weeks. The third round of anthropometric measurements were taken at the end of 24 weeks.
Study agent
The study agent, i.e. the probiotic drink, contained 6·5 billion LcS, and the nutrient drink (sugar, defatted milk, glucose-fructose, flavouring agents) and the control drink (in 65-ml seal-topped single-dose bottles), contained the nutrients without LcS. The taste and colour of both drinks were similar. The nutritional content of each bottle was 48·4 kcal energy, 0·9 g protein, ⩽0·1 g lipid and 11·2 g carbohydrate. The probiotic and nutrient drinks were stored at 4–10°C in temperature-controlled and monitored cold rooms. Bottles delivered to the community for the children were transported in carrier boxes and each had a single ice pack. Care was taken not to freeze the contents of the bottles. Each box was equipped with a thermal monitor and measurement was recorded daily upon return from the study area.
Randomization
An independent statistician prepared the randomized list. A controller not related to the study, assigned two codes each to probiotic (A and F) and nutrient (K and T) groups and instructed the manufacturer of the drinks likewise. The controller maintained strict confidentiality of the codes until completion of study analysis. A responsible investigator assigned the drinks to subjects under double-blind condition.
Sample size
The sample size estimates were based upon two-sided hypotheses of effect, a 95% level of confidence and a power of 90%. Based on the results of our previous study in which 30% of children suffered from diarrhoea in this season [Reference Sur7] and assuming that the occurrence of diarrhoea in the probiotic group would be 25% in the 24 weeks of the study, we needed a sample size of 1670 per group (with a difference of at least 5% for proportion of children suffering from diarrhoea). With 15% attrition assuming no design effect from cluster randomization, the sample size was 1964 per group. We thus included a total of ~4000 children for this study.
Microbiological analysis
Stool samples of children with diarrhoea were collected from their homes. The mothers were given containers and a box containing ice packs for preserving the samples until they were collected by the sample collector and transferred to the designated laboratory. Samples were processed for bacterial, viral and parasitic enteric pathogens following standard techniques [8]. Multiplex PCR was performed targeting genes specific for enterotoxigenic Escherichia coli (ETEC), enteropathogenic E. coli (EPEC) and enteroaggregative E. coli (EAEC) [Reference Nguyen9]. Enteric parasites such as Entamoeba histolytica, Cryptosporidium spp. and Giardia lamblia were identified directly from the stool samples using commercial kits (Tech Lab, USA). Rotaviruses and adenoviruses were detected using commercially available ELISA kits (Oxoid, UK). Other viruses, e.g. sapovirus, astrovirus and norovirus (GI and GII) were identified by multiplex RT–PCR after extraction of RNA from faecal specimens [Reference Yan10].
Data management and analyses
Data obtained from the demographic survey, daily reports and other proforma were entered in a PC using SQL server and Visual Basic software. Data were manually scrutinized for accuracy and consistency before entering into computers in a dedicated area. All programs incorporated range and consistency checks. Data management included error reports, exception lists and summary reports for each activity. The data were automatically backed-up at systematic intervals onto external hard drives and included an audit trail of all sequential changes. The data management system was augmented by automatic computer virus scanning at start-up of each data entry and data management session. All participant data were computerized using password protection without personal identifiers. Access to both electronic and hard-copy data were restricted to authorized study personnel only. The children who received the study agent for a minimum of 67 days, i.e. 80% of the total intake period of 12 weeks, were considered for the analysis. The primary outcome was the occurrence of first episode of diarrhoea of one individual during 12 weeks of intake of study agent and for 12 weeks of follow-up. Clusters were primarily assigned based on geographical area with ~40 children. Comparison of individual characteristics was done by use of generalized estimating equations to adjust the effect of clustering. For dichotomous variables, the logit link function was used and for continuous variables, the identity link function was used. We compared cluster level variables using Student's t test for continuous variable and χ2 test for categorical variable.
To estimate probiotic protection, we used survival analysis of time to the first episode of diarrhoea. In descriptive analyses, Kaplan–Meier curves were fitted. We fitted unadjusted and covariate-adjusted Cox proportional hazards regression model. Hazard ratios (HR) were estimated by exponentiation of the coefficient for the study agent variables in these models. The protective efficacy of the probiotic was estimated as: [1 – hazard ratio]×100%. In the model cluster size was taken into account for the design effect of cluster randomization. All P values and 95% confidence intervals were calculated with two-tailed tests. Statistical analyses were performed using statistical software SPSS Statistics 17.0 (SPSS Inc., USA). Moreover, comparison was made for nutritional gain and aetiological pathogens between the two groups.
Statutory clearances
The Scientific Advisory Committee and Institutional Ethics Committee of the National Institute of Cholera and Enteric Diseases as well as the Health Ministry Screening Committee of the Indian Council of Medical Research approved the study. Written informed consent was obtained from the parent/guardian of each participating child for administration of the probiotic or nutrient drink.
Role of funding source
This study was supported by Yakult Honsha, Co. Ltd (Tokyo, Japan). The funding agency had no role in design, data collection, data analysis, data interpretation or writing the report. However the microbiologists of Yakult Central Institute for Microbiological Research, which is a subdivision of Yakult Honsha, Co. Ltd, conducted external quality assurance of laboratory testing.
RESULTS
A total of 4767 of children aged <5 years were registered at the time of census from a population of ~60 000 in the slum of Kolkata Municipal area. At the time of randomization, 3758 children aged between 1 and 5 years were eligible (children aged <1 year were not eligible because of their difficulty in swallowing a 65-ml drink at one time). The details of the trial profile are shown in the Consort chart (Fig. 1). The two study groups were all balanced at both the individual and cluster levels with respect to a variety of demographic and socioeconomic variables (Table 1).
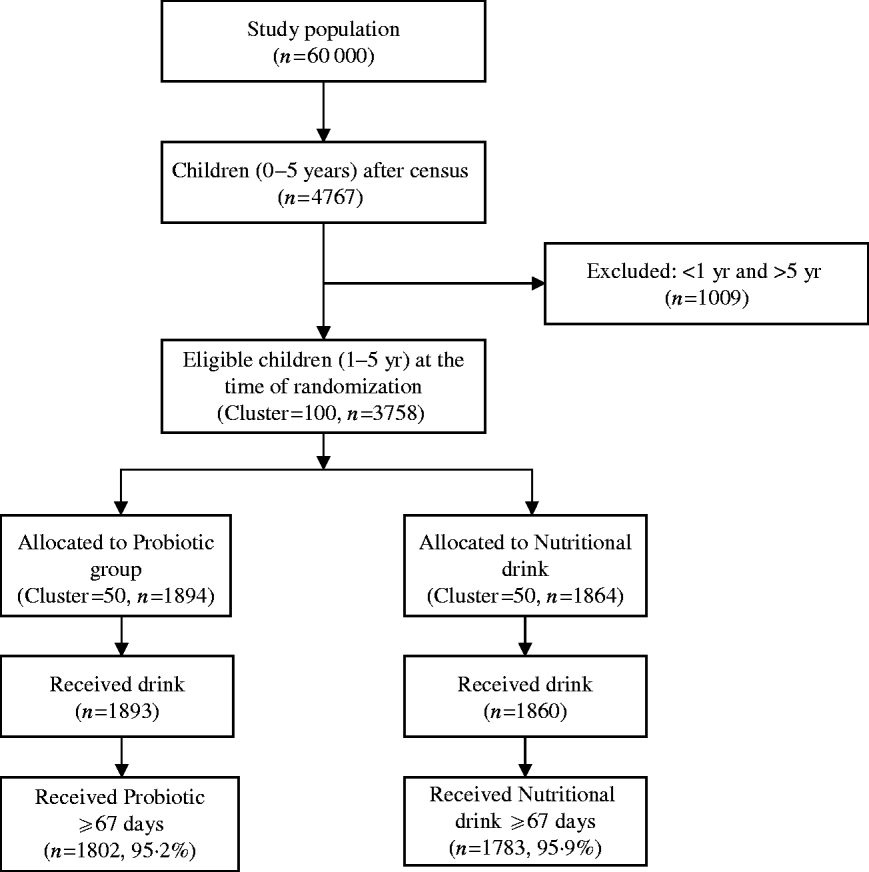
Fig. 1. Consolidated standards of reporting trials (Consort) chart showing grouping of children in the trial.
Table 1. Baseline characteristics of the subjects and clusters
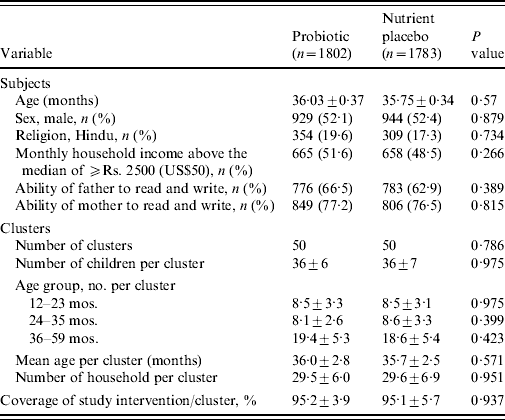
Values are means±s.d. unless otherwise specified.
More than 99% of participating children received the drinks. However, since the intake was on a daily basis for 84 days (12 weeks), there were some children who did not complete the total 84 days of intake. During analysis, a cut-off point of 80% days of intake was considered. Effectively, only those children who had consumed the drinks for ⩾67 days were included in the analysis. There was no loss of follow-up in these children during this study period of 24 weeks. With this cut-off value, 3585 (95·5%) of total enrolled children, 1802 (95·2%) in the probiotic group and 1783 (95·9%) in the nutrient group, received the drinks.
During the 24-week study period, there were 608 children with diarrhoea (0·88 cases/child per year) in the probiotic group and 674 children with diarrhoea (1·029 cases/child per year) in the nutrient group, resulting in a level of probiotic protective efficacy of 14% (95% CI 4–23, P<0·01). In Figure 2, the cumulative event-free survival curves were compared through Kaplan–Meier technique on a periodical time-interval from the point of intake of the drinks to the end of follow-up for the patients assigned to probiotic and nutrient groups. This shows that after 12 weeks of drink consumption the survival curve in the probiotic group was significantly higher (log rank test: χ2=7·6, P=0·01) than the nutrient group.
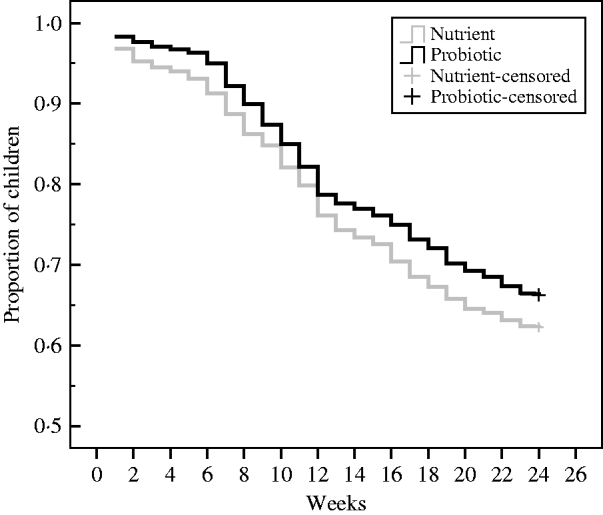
Fig. 2. Kaplan–Meier survival curves of the cumulative proportion of children without diarrhoea in the probiotic and nutrient groups. Log rank test: χ2=7·6, P=0·01.
Anthropometric indicator weight-for-age Z score was compared between probiotic and nutrient groups as shown in Table 2. Anthropometric analysis revealed that weight gain of children was similar at all three measurements. Similarly there was no statistically significant difference in height and MUAC between the two groups.
Table 2. Nutritional status: weight-for-age Z score
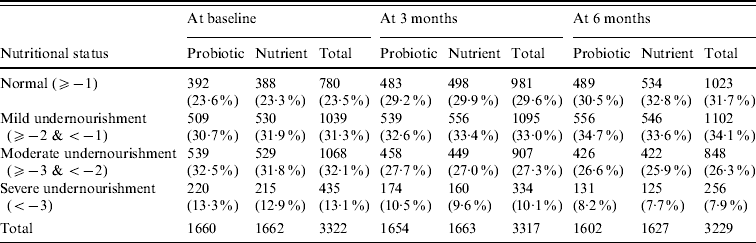
The differences in nutritional grade are not statistically significant (P>0·05) between probiotic and nutrient groups at baseline as well as at end of 3 months and 6 months.
The detection of different bacterial, viral and parasitic agents in children with diarrhoea in the probiotic and nutrient groups are shown in Table 3. The detection of different enteric pathogens was similar between the two groups, except for Aeromonas spp. and Cryptosporidium spp., which were significantly higher (P<0·05) in the nutrient group. In both groups, diarrhoeagenic E. coli was most frequently isolated followed by Vibrio spp., Campylobacter spp., and Shigella spp.
Table 3. Isolation of different enteric pathogens in the probiotic and nutrient groups
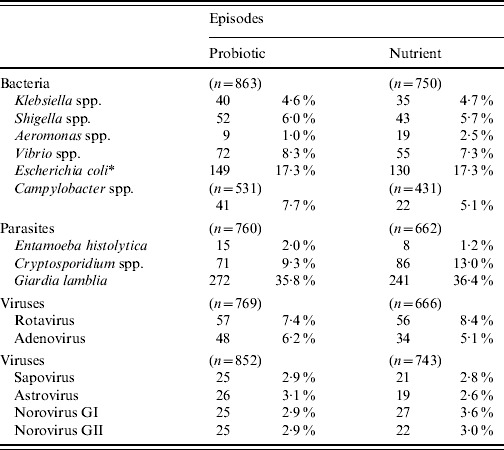
* Diarrhoeagenic E. coli.
DISCUSSION
Placebo-controlled trials provide sufficient evidence of the role of probiotics in the prevention of acute diarrhoea but there is insufficient evidence for extrapolation of these results for global recommendation, since trials in children in community settings in developing countries are lacking [Reference Sazawal4]. This study provides evidence of a significant preventive effect of a probiotic on acute diarrhoea in children aged between 1 and 5 years in an urban slum community in a developing country. We believe that this study is one of the largest of this kind.
It was not possible to assess herd protection in this study as there were not enough ‘non-recipient’ children in either group.
The probiotic used for the present study was LcS, which was cloned in 1930 as a strain tolerant to acid and bile and was used for the production of fermented milk products. LcS is reported to have several beneficial effects on gastrointestinal disturbances including some forms of diarrhoea and related diseases [Reference Kiwaki, Nomoto, Lee and Salminen11] and is reported to improve the balance of microflora as well as bowel movement frequency and stool consistency [Reference Matsumoto12]. It also promotes proliferation of phagocytes such as macrophages and neutrophils in the bone marrow and spleen thus activating the innate immune system which in turn is important in the infection-preventing effect of the probiotic [Reference Yokokura13].
In another community-based study on the prophylactic effect of a probiotic on diarrhoea in an impoverished developing country setting, Lactobacillus GG was found to be useful as a prophylactic measure to control diarrhoea in undernourished Peruvian children at increased risk, especially non-breastfed children in the toddler age group [Reference Oberhelman14]. The Peruvian study was performed on 204 undernourished children aged 6–24 months for a period of 15 months while the current study was on a larger group of children but for a shorter period of 6 months. Nonetheless, a significant decrease in the occurrence of diarrhoea in children in both community settings indicates that probiotics do, indeed, have a preventive role in reducing diarrhoea. The protective efficacy of probiotics was shown to be 14% in the current study which is somewhat lower than the 21% found in the meta-analysis conducted by Sazawal et al. [Reference Sazawal4].
Interestingly, although the probiotic drink was administered for 12 weeks, the proportion of the different aetiological agents in the diarrhoeal cases in the probiotic and nutrient groups did not show a remarkable difference. LcS has been shown to prevent enterohaemorrhagic E. coli O157 infection in an animal model and increase intestinal antibody titres against O157 and Shiga toxin produced by O157 [Reference Ogawa15]. Faecal shedding of Cryptosporidium oocysts was reduced by probiotic Lactobacillus strains [Reference Alak16–Reference Pickerd and Tuthill18]. When therapeutically used, various probiotics such as L. rhamnosus GG, L. reuteri SD 2222 and Bifidobacterium Bb12 have shown favourable results on rotaviral diarrhoea in healthcare settings [Reference Isolauri19–Reference Saavedra22]. Administering oral rehydration solution containing Lactobacillus GG to children with acute diarrhoea is safe and results in shorter duration of diarrhoea, less chance of protracted course, and faster discharge from hospital [Reference Guandalini21]. However, prophylactic use of probiotics as shown in this study as well as others, indicates that there may not be a variable effect in diarrhoea of different aetiologies.
Probiotic drinks containing LcS has been consumed for more than 70 years in Japan. At present, this product is safely consumed in more than 30 countries and regions by more than 25 million people every day. In clinical studies, safety of LcS has been investigated in critically ill children admitted for intensive care. There was no evidence of either tissue colonization or bacteraemia with LcS in bacteriological cultures obtained from 28 subjects [Reference Srinivasan23]. Furthermore, no adverse events were observed in the current study.
The Indian Academy of Paediatrics (IAP) Guidelines 2006 on management of acute diarrhoea [Reference Bhatnagar24] suggest that the effect of probiotics is strain related and there is paucity of data to establish the efficacy of the probiotic species (namely L. acidophilus, lactic acid bacteria) available in the Indian market. Moreover, randomized controlled trials in Indian children need to be conducted before recommending a particular species. The present randomized controlled study has demonstrated that the probiotic LcS is efficacious in preventing acute childhood diarrhoea.
ACKNOWLEDGEMENTS
This work received funding from Yakult Honsha Co. Ltd (Tokyo, Japan). [The trial was registered in Clinical Trials.gov, operated by the U.S. National Institute of Health, as NCT00534170.]
DECLARATION OF INTEREST
K.N., T.T., T.S., H.T. and T.K. are microbiologists of Yakult Central Institute for Microbiological Research, which is a subdivision of Yakult Honsha Co. Ltd.







