The WHO monograph on Endemic Goiter of 1960(Reference Kelly and Snedden1) characterized the Asian section of the former USSR as ‘one of the most notorious goiter areas of the world’. Under an Ordinance of the Ministry of Health in Moscow in 1956, entitled ‘On Improvement of Measures to Fight Endemic Goiter’, the targeted distribution of iodized salt to areas documented with endemic goitre was a matter of high Soviet concern. After a detailed geological chemistry survey of the USSR during the 1960s was completed, the areas with the lowest iodine content in soil and water, including the Kirghiz Republic, were put under even stricter control in terms of mandatory iodized salt supply. A large-scale population survey in 1969 encompassing more than 30 million people demonstrated that the goitre occurrence in the vast Soviet territory had decreased to a sporadic level and that big-size goitres and cretinism were no longer observed. In consequence, the Ministry of Health officially declared that endemic goitre had been overcome; it abolished the central and regional oversight, cancelled the obligatory registration and monitoring of goitre cases, and broadened the orientation of the widespread network of specialist endocrine centres from its singular focus on goitre prophylaxis(Reference Gerasimov2).
Researchers at the Academy of Science in Bishkek had made key contributions to studying the environmental origin and epidemiology of iodine deficiency in the former Kirghiz Soviet Republic. Analyses of foods, water sources and forages sampled in various parts of the mountainous country demonstrated that locally grown foods were not capable of providing sufficient dietary iodine(Reference Sultanalieva3). Detailed surveys of thyroid enlargement showed that 36 % of the population had goitre, with variations of 11–42 % in the northern and 55–61 % in the southern areas of the country. The investigations also demonstrated the existence of a close relationship between the iodine content in the local water and food supply, and the extent and severity of goitre in the population. At the end of the 1960s, population monitoring revealed drastic reductions of the prevalence and severity of goitre with the mandatory supplies of iodized salt. Consequently, in the early 1980s the goitre monitoring was suspended and the iodine prophylaxis such as free meat and milk supplies in schools and orphanages, and iodine supplement distribution among pre-school children and pregnant and nursing women, was abandoned(Reference Sultanalieva3).
Upon emerging as an independent nation in 1991, the Kyrgyz Republic started building its own policy making, oversight and supply systems. A major part of the salt supplies continued to be imported from Kazakhstan, but also a number of local enterprises started with edible salt processing, in part using salt from various unregulated natural deposits. The shortages of management expertise and material inputs for these new ventures and the relaxed food import inspections allowed a continuous increase in the market share of non-iodized or insufficiently iodized salt. In 1995–8, when investigators of the Kyrgyz Academy of Medical Sciences adopted modern survey techniques, new evidence was uncovered of moderate and severe iodine deficiency(Reference Sultanalieva3). The investigations were indicative of a serious public health problem of iodine-deficiency disorders (IDD) that had made a comeback after the previous success of the Soviet-directed approach.
Official concern about the deteriorating iodine situation became first evident in a decree by the Kyrgyz Government in 1994, which initiated a ‘National program for preventing conditions related to iodine deficiency, 1994–2000’. International agencies, including UNICEF, started assisting local salt processors in improving their methods of iodized salt manufacture and a campaign was initiated to inform the public of the dangers of IDD and the benefits of iodized salt. Also, the sanitary/epidemiological services under the Ministry of Health adopted salt inspections at production and retail, and the capacities of the research workers and the salt manufacturers were strengthened in a number of training workshops. While this helped boost the production capacity of the Kyrgyz enterprises(Reference Aliev, Denislamova and Schüth4), the overall progress to reach universal, good-quality salt iodization was uneven(Reference Schüth, Jamangulova and Janikeeva5) and the aim of protecting the population against IDD fell short of expectations(Reference Sultanalieva3). By 2000, when iodized salt was consumed in nearly 70 % of the households in the world(6), only 27 % of the salt consumed by the Kyrgyz population was adequately iodized(Reference Van der Haar7).
Cognizant of the shortcomings, the Kyrgyz Government enacted a national law on IDD prevention in January 2001, specifically prohibiting the import and sale of non-iodized salt for human and animal consumption and stipulating the use of potassium iodate at 25–55 mg iodine/kg salt. During the same year, the State Committee on Standardization and Metrology issued a normative standard for iodized salt and included the product in the list of foods for compulsory certification prior to its release on the markets. A multi-sector Kyrgyz delegation at the regional joint UNICEF–Asian Development Bank Almaty Forum in October 2001(Reference Van der Haar7) developed an action plan to further develop the national capacities for salt iodization, law enforcement and monitoring of the salt supplies and population status, together with actions to raise the public’s acceptance and improve accountability by periodic public reporting. The plan was officially adopted by the Government in 2002, followed by the launch of the ‘National program for decreasing IDD in Kyrgyz Republic, 2003–2007’. In March 2003, the salt processing enterprises coalesced in a Kyrgyz Association of Salt Producers, partially in defence against the still ongoing non-iodized salt imports, and the previous import tariffs on fortificant and equipment were lifted. Nevertheless, illegal imports by domestic trading firms continued to pose an obstacle by the persistent availability of non-iodized salt on the Kyrgyz markets, thereby maintaining a price difference for consumers and undercutting the sales of the legitimate local processors. In view of the lenient enforcement practices, the Kyrgyz–Swiss–Swedish Health Project started supporting the village health committees and primary health-care workers of Naryn province in their use of rapid salt test kits as a tool to persuade traders and retailers to accept only iodized salt from their suppliers(Reference Schüth, Jamangulova and Janikeeva5). During a 2-year campaign in households and retail outlets, a quantum increase occurred in the supply of iodized salt in the province. By using only test kits specific for potassium iodate, the salt testing campaign also helped shift the share in the trade channels away from salt iodized with potassium iodide, the less stable fortificant. These campaigns have subsequently been extended to other provinces in Kyrgyzstan with similar success in raising the iodized salt supplies. In 2006, the Multiple Indicator Cluster Survey by the Kyrgyz National Statistical Committee found adequately iodized salt (≥15 ppm iodine) in 76 % of households(8).
By 2000, three salt enterprises had started processing salt in the Kyrgyz Republic, together supplying 5300 tons of iodized salt(Reference Varghese9). Imported salt, mostly from Kazakhstan, represented approximately 70 % of the national salt requirement at that time. Since then, the technical, material and training support funded by the Asian Development Bank and UNICEF have accompanied a gradual growth of the local processing industries and, by 2006–7, twelve small and medium-scale local salt enterprises supplied 13 000 tons of iodized salt per annum, or approximately 65 % of the estimated national needs. Under the Kyrgyz Association of Salt Producers, the local salt enterprises have adopted self-reliant input procurement and quality assurance practices, and they are participating in a national food fortification coalition(Reference Muzafarov10).
The end of the second national programme in 2007 was the main rationale for the present survey, which aimed at assessing the effects of the universal salt iodization (USI) strategy on the iodine consumption, iodine nutrition status and IDD burden in the Kyrgyz population. The results of the survey would inform future policy making to strengthen the USI strategy for IDD elimination in Kyrgyzstan.
Materials and methods
A representative survey of the 5·2 million population in Kyrgyzstan was conducted from October to December 2007, using recommended standard methods and approaches(11). The survey obtained quantitative laboratory measurements of the iodine content in household salt to approximate the iodine consumption; the iodine nutrition status was assessed by measuring urinary iodine concentrations (UIC); and thyroid volume was measured by ultrasound to estimate the burden of IDD in the population. The survey protocol was reviewed and approved by the ethical committee of the Kyrgyz–Russian Slavonic University.
Subjects and sampling
Thirty primary schools were selected proportionate-to-population-size(Reference Gorstein, Sullivan and Parvanta12) from the national database maintained by the Ministry of Education, and thirty children aged 8–10 years were sampled at random in each school. In view of growing international concern about the iodine status of pregnant women(Reference Morreale de Escobar, Obregon and Escobar del Rey13), the standard school-based design was extended by enrolling also twenty pregnant women in each cluster location. Pregnant women were sampled opportunistically in one or more prenatal clinics located at closest distance to each selected primary school. The selected clusters covered each of the seven provinces of Kyrgyzstan, plus the capital Bishkek and the second largest city, Osh.Footnote * A three-person team of experienced field personnel (one lead endocrinologist, an ultrasound technician and a clinically certified laboratory assistant) coordinated closely with the provincial, district and local authorities to ensure their consent and obtain their support in mobilizing and proper sampling of the target groups. On consultation with the field team, local authorities (in most cases a village health committee member) verbally contacted the selected children and women and conveyed the purpose of the survey and their role in data collection. Verbal consent was obtained from the parents of participating children, while the pregnant women gave verbal consent for themselves. Most of the interviews and sample collections were carried out in a primary school, but in some settlements pregnant women were investigated in a family health centre. Pre-tested forms were used for registration and data recording and the field team verified the completeness of the data collection each evening and prior to leaving for the next cluster.
Iodine consumption
All participants were requested to write the salt’s brand name, firm, manufacturer and stated shelf-life on a small survey-provided envelope, and place two or three tablespoons of household salt inside. On arrival at the measurement site, a small portion of the salt was tested with a rapid test kit for demonstration and the envelope with the remainder of the salt was marked with the subject’s unique code. Salt samples were delivered to the laboratory of the Endocrinology Dispensary for measuring the iodine content by titration(11).
Iodine nutrition status
Each participating child and woman was asked to provide a urine sample in a 20 ml wide-mouth glass vial, which was tightly capped and marked with the person’s unique code. The urine samples were transported separate from the salt samples to the Endocrinology Dispensary in Bishkek, where the UIC was assayed in random order with the Sandell–Kolthoff reaction after digestion of urine with perchloric acid(11) in a dedicated laboratory room. The laboratory analysts had been thoroughly trained in the method and maintenance of quality procedures(Reference Ivanova and Timmer14), and the laboratory participates with success in the EQUIP urinary iodine quality assurance programme provided by the Centers for Disease Control and Prevention in Atlanta, GA, USA(Reference Caldwell, Makhmodov and Jones15). Control urine sample duplicates in the analytical runs showed a variation of 1·4 to 8·4 %, independent of the iodine concentration.
Evidence of iodine-deficiency disorders
The size of the thyroid gland of each participant was measured following international guidelines(11, Reference Zimmermann, Hess and Molinari16) with an Aloka model 500 portable scanner (Aloka Co., Tokyo, Japan) equipped with a 7·5 MHz linear transducer and the subject sitting upright with the neck extended. Thyroid gland volume was calculated by adding the products of the measured width, depth and length of each lobe, and multiplying this sum with 0·479(Reference Zimmermann, Hess and Molinari16). From previous surveys in Kyrgyzstan, the lead endocrinologist has extensive thyroid ultrasound experience and the differences for her blind duplicate measurements under routine circumstances vary by 10 to 15 %.
Data analysis
Data were entered on a personal computer and verified by generating frequencies and cross-tabulations, while rectifying entry errors against the original data forms. Data processing and analysis were performed mostly in Excel™ (Microsoft Corp., Seattle, WA, USA), enhanced with the Analyse-it® software plug-in (Analyse-it Software Ltd, Leeds, UK). Mainly, the statistical analysis produced data distributions, non-parametric group comparisons and linear regression. Relative risks of exposure(Reference Morris and Gardner17) were calculated for levels of salt iodine content. Associations between parameters were examined with the Mann–Whitney and Kruskal–Wallis non-parametric tests, using CIA software(Reference Bryant18). Criteria for classifying iodine indicators were as recommended(11) except for the classification of UIC in pregnant women, where the publication of a recent WHO consultation(Reference Andersson, De Benoist and Delange19) was used.
Results
The survey collected twenty-seven different salt brands, twenty-one of which were of domestic origin. Four hundred and fifty-three (50·3 %) of the schoolchildren were male. The 580 women enrolled in the survey were on average 22 weeks pregnant; 146 (25 %) women were in their first, 223 (38 %) were in their second and 211 (36 %) were in their third trimester of pregnancy.
Iodine nutrition situation
Because the distributions of salt iodine contents, UIC and thyroid volumes did not differ significantly between the male and female children, their results were combined in the present analysis. Table 1 shows the basic survey findings. All of the indicators had a significantly non-normal distribution. The iodine content in salt brought by the children and the women did not differ significantly. The median iodine content in 1480 household salt samples was 11·2 mg/kg (95 % CI 10·5, 12·0 mg/kg). Also, no significant difference was found in the median UIC between the 900 schoolchildren, 114 μg/l (95 % CI 106, 124 μg/l), and the 580 pregnant women, 111 μg/l (95 % CI 102, 121 μg/l). However, the thyroid volume in pregnant women was significantly larger (P < 0·001) than in children. The goitre prevalence in children was 5·2 % (95 % CI 3·9, 6·9 %), based on the age reference(Reference Zimmermann, Hess and Molinari16).
Table 1 Iodine content in household salt, iodine excretion in urine and thyroid volume in schoolchildren and pregnant women, Kyrgyzstan, 2008
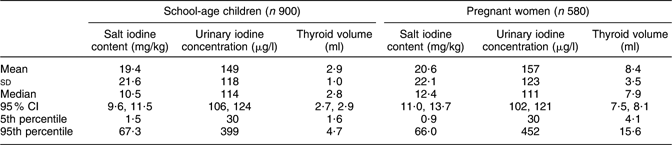
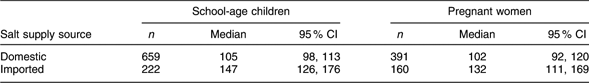
Non-iodized salt constituted 2·1 % of the samples, but iodine level ≥15 mg/kg was found in only 39·5 % of all the household salt. Twenty-seven per cent of the salt samples with a discernible brand name were imported brands. The median iodine content in imported salt was 22·7 mg/kg (95 % CI 16·5, 26·5 mg/kg), which is more than twice (P < 0·001) the median iodine content of 10·2 mg/kg (95 % CI 9·2, 10·8 mg/kg) in domestic salt. Salt iodine content <15 mg/kg was measured in 43 % of the imported salt, as compared with 66 % of the domestic samples (P < 0·001).
Among the schoolchildren, 338 (38 %) had UIC below 100 μg/l and 224 (25 %) had UIC of 200 μg/l or above. UIC < 150 μg/l was observed in 355 (61 %) of the pregnant women while 125 (22 %) women had UIC ≥ 250 μg/l. In both the children and the women, the lowest median UIC levels were observed in Chui and Naryn provinces. Children in Batken and Talas provinces had the highest median UIC, while the highest UIC in women was observed in Batken and Osh provinces.
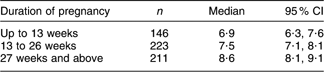
Analysis of the thyroid volume among pregnant women by trimester of pregnancy (Table 2) revealed thyroid enlargement with the pregnancy duration (P < 0·001). This relationship was not confounded by the household salt iodine content or the UIC of the women.
Table 2 Thyroid volume (ml) in pregnant women by trimester, Kyrgyzstan, 2008
Relationships among iodine indicators
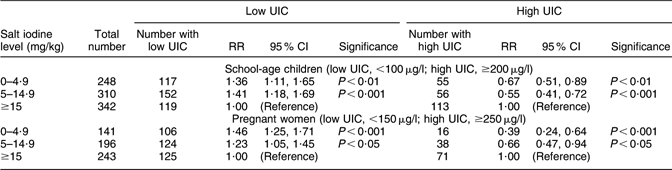
The wide range of iodine content in household salt (difference between the 5th and 95th percentiles in Table 1) suggested a considerable variation in dietary iodine consumption among the households. Table 3 tests the assumption that the UIC of the participants is associated with their exposure to iodine consumption from household salt. Compared with households with adequately iodized salt (≥15 mg/kg), the risks of low UIC were significantly higher among the children and women in households using salt with less than 15 mg iodine/kg, while their risks of high UIC were significantly lower. Similar analyses of the relationships between the thyroid volume among the children and the women, and their salt iodine or UIC levels, did not produce significant relationships (results not shown).
Table 3 Relative risks (RR) of low or high urinary iodine concentration (UIC) among children and pregnant women exposed to different levels of iodine content in household salt, Kyrgyzstan, 2008
The participating children and women in the present survey did not live together in the same households, but they were enrolled from the same settlements with access to the same salt supplies. The coefficient of the linear regression line between the UIC medians among the schoolchildren and the pregnant women by cluster (Fig. 1) was not different from unity and the UIC medians were strongly correlated (r = 0·63, P < 0·001).
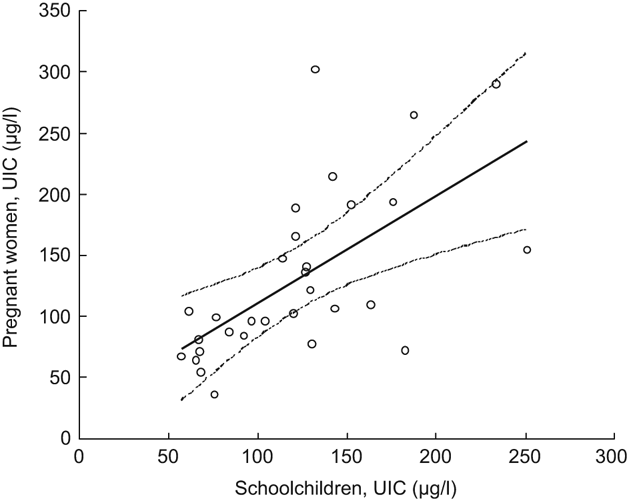
Fig. 1 Median urinary iodine concentrations (UIC) in pregnant women and school-age children across clusters, Kyrgyzstan, 2008: ○, group medians of each cluster; ———, slope; - - - -, 95 % confidence interval
Supply source of salt
The UIC in both the children and the women was significantly associated (P < 0·001 in each case) with the supply source of household salt (Table 4). Compared with households using domestic salt, the median UIC was higher by 30–40 μg/l among the survey participants using imported salt.
Table 4 Urinary iodine concentrations (μg/l) in children and women by supply source of household salt, Kyrgyzstan, 2008
Discussion
Provincial iodine surveys among schoolchildren aged 10–12 years in Bishkek, Issyk Kul, Jalalabad and Osh in 1985–8 had found median UIC values of 25–45 μg/l and goitre prevalence varying between 33 and 86 %(Reference Sultanalieva3), while spot tests of household salt showed that iodine was absent in the majority of the salt consumed by the population at that time. Therefore, the Government of the newly independent nation issued a decree in 1994 to promote salt iodization. For several years thereafter, however, the national salt situation remained characterized by resilience against aligning the stated norms with effective control, relaxed application of the rules in the domestic salt supplies, a fledgling national salt industry and high market dependence on salt imports. It took until the turn of the century for the experiences to coalesce into an official resolve to mandate USI. After enacting the law in 2001, acceptance among the general public has improved(Reference Schüth, Jamangulova and Janikeeva5), the local iodized salt processing capacity has been expanded, salt quality inspections in the trade channels have increased, and border control on a certificate of conformity has been tightened(Reference Muzafarov10). The present iodine survey was the first detailed assessment of the iodine nutrition situation since the enactment of the USI law. Therefore, the present survey offered a rare occasion to explore the programmatic factors leading to success or failure in accomplishing the USI strategy in Kyrgyzstan.
Compared with the situation in the late 1980s, the present results give evidence of the significant progress that has been made in reaching towards USI, alleviating iodine deficiency and eliminating IDD. Non-iodized salt constituted a minimal proportion of the salt consumed in the households, illustrating that the ongoing salt testing campaigns have been successful in signalling to salt traders that they will suffer loss of market share if there is no effort to iodize the product. At the same time, however, the low percentage of salt iodized according to official standards indicates that many salt enterprises add only a minimal amount of the fortificant during salt processing. The finding of a sizeably higher iodine content in imported than in domestic salt brands suggests that the insistence by customs officers on a certificate of conformity(20) at the borders may have been more effective in promoting adequate iodized salt supplies in the marketplace than the official oversight of the domestic iodized salt producers. Compared with the compulsory iodization range of 25–55 mg iodine/kg agreed in the Commonwealth of Independent States(21), more than half of the imported salt brands still fell short of the minimum, however, which indicates the need to further improve the quality of the iodized salt of domestic and foreign manufacturers alike. An attempt was made to identify also other factors, such as salt brand and province of residence, that would explain the variations in the salt iodine content and associated UIC levels among the survey participants, but none were found significant for guiding a focused effort to improve the iodized salt supply. Thus, improved assurance of the quality of the entire iodized salt supply is needed, irrespective of salt source, brand or geographical area. This calls for better performance by the salt industries in the quality assurance of their iodized salt processing, assisted by a genuine effort by the authorities in verifying the conformity of the salt sales with the standards.
Despite the imperfections in the iodized salt market, the median UIC of 114 μg/l in schoolchildren indicated adequate iodine nutrition. Also the median UIC among children in the households using domestic salt was above the minimum for iodine deficiency. Although the goitre prevalence among the children was slightly elevated, the goitre rates in Kyrgyzstan have been decreasing steadily for more than a decade(Reference Schüth and Sultanalieva22) and the current goitre occurrence in children is more likely the remainder of previous iodine deficiency(Reference Jooste, Weight and Lombart23). A recent WHO Expert Group(Reference Andersson, De Benoist and Delange19) recommended a median UIC of 150–249 μg/l as indicative for adequate iodine intake in pregnancy. The median UIC of 111 μg/l in the pregnant women in the present survey does not attain this range, indicating that their iodine consumption is insufficient. This conclusion is supported by finding an increasing thyroid volume with the duration of pregnancy, since an increase in thyroid size during pregnancy has been a classical indicator of iodine deficiency(Reference Glinoer, De Nayer and Delange24, Reference Berghout and Wiersinga25). The finding in a population-representative survey that pregnant women, while consuming the same diet, have lower UIC than school-age children is not unique for Kyrgyzstan(Reference Delange26–Reference Ategbo, Sankar and Schultink28). The low median UIC among pregnant women in the current survey should caution against a conclusion that the threat of iodine deficiency in Kyrgyzstan has been overcome. Rather than setting pregnant women apart from the general population, however, and devising a special effort to provide iodine supplements for them in addition to the USI strategy(Reference Andersson, De Benoist and Delange19, Reference Zimmermann, Jooste and Pandav29), the more cost-effective and potentially sustainable course of action would be to improve the steady supplies of adequately iodized salt.
To analyse whether the current norms for salt iodization in Kyrgyzstan are adequate for attaining optimum iodine nutrition throughout the population, the survey data of the schoolchildren and pregnant women were used to examine their iodine status in relation to the differing levels of salt iodine content (Table 5). The improvements in the UIC medians among the survey participants along with the stepwise higher salt iodine levels illustrate the significant responses of the iodine status in both groups (P < 0·001), and in the pregnant women, the iodine status attained the level considered adequate for their dietary needs in those households with salt iodine content ≥25 mg/kg. Table 5 also illustrates the shifts at the margins of the UIC distributions that took place with the stepwise increases in salt iodine content. In children, the majority of low (<100 μg/l) UIC values in the households using salt with low iodine levels changed to evenly balanced proportions of low and high UIC values in the households with adequately iodized salt. Similarly, the sizeable predominance of low (<150 μg/l) UIC values in the pregnant women consuming salt iodized at low levels was attenuated to 50 % low values among the women in the households using salt with ≥25 mg iodine/kg. It would seem therefore that the mandatory level of salt iodization in Kyrgyzstan is adequate for reaching optimum iodine nutrition in the population.
Table 5 Urinary iodine concentration (μg/l) of schoolchildren and pregnant women by the salt iodine content in their household, Kyrgyzstan, 2008
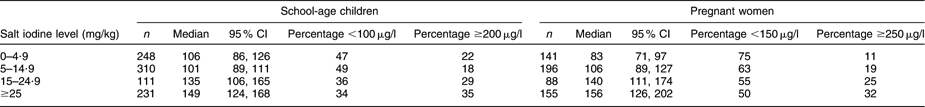
A weakness in the present survey was that the data collection excluded salt sampling in food manufacturing industries. In Kyrgyzstan, bread is of major importance in the daily food basket of the population. Because the cities, including the capital Bishkek, are served by large bread factories, it was anticipated that the use of iodized salt by food industries may lead to evidence of better iodine status in the urban population. An analysis by province of the UIC distributions (results not shown) found, however, that the UIC medians of the children and women in Bishkek City were lower than in most other provinces, which suggests that the bread bakeries were unlikely to be using iodized salt at the time of the survey. This shortfall in the USI mandate also needs to be addressed in the food fortification coalition when it considers measures to improve the quality of the iodized salt supply. The moderate median UIC levels in the schoolchildren, even when limiting the analysis to only those children who lived in households with salt iodized at ≥25 mg/kg (Table 5), are confirming the ample room for flexibility that still exists for raising the iodine consumption of the population in Kyrgyzstan before excess iodine consumption would become an issue of public health concern.
Acknowledgements
Funding for the survey was provided by UNICEF, Bishkek. The authors state no conflict of interest. R.B.S. was responsible for the design and execution of the survey. S.M. supervised the laboratory analyses, and R.B.S. and S.M. wrote the first report of the survey. F.v.d.H. conducted the in-depth statistical analysis and wrote a draft manuscript to which the other authors made substantial contributions during revisions. All authors approved the final report. We thankfully acknowledge Gulnara Risbekova, Aigul Musambetova and Aigul Abikova for their support in carrying out the survey. Inputs and comments on a draft manuscript were provided by Tobias Schüth and Amir Makhmodov, for which we are grateful. We are also grateful to Cholpon Imanalieva for assisting with translations.








